TO THE EDITOR:
Verma et al1 recently demonstrated that vitamin K antagonist (VKA) treatment compromises hematopoiesis in mice, and they raised the concern that VKA use might increase the risk of myelodysplastic syndrome (MDS) in humans. To clarify this issue, we conducted a study to assess the effect of VKA exposure on the incidence of MDS compared with direct oral anticoagulant (DOAC) exposure, focusing on patients with nonvalvular atrial fibrillation (NVAF).
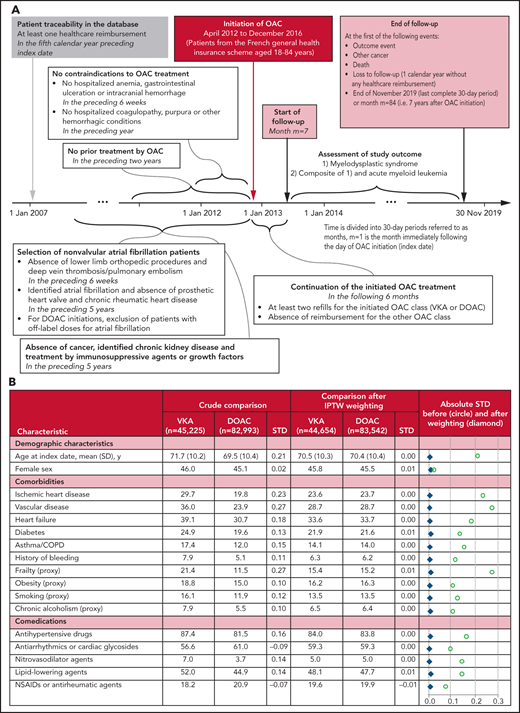
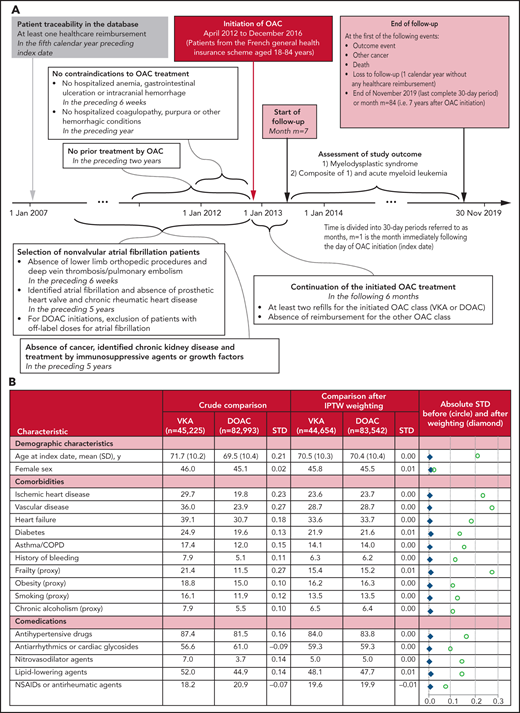
This observational study used a new-user cohort design with an active comparator.2 It is based on data from the French National Healthcare Database (Système National des Données de Santé).3,4 The construction of the study population is depicted in Figure 1A. First, all patients initiating oral anticoagulants (OACs) between April 2012 and December 2016 and aged 18 to 84 years were identified. Then, patients with NVAF at OAC initiation were retained, and patients with contraindications were excluded, as well as patients with prior cancer, identified chronic kidney disease, or treatment by immunosuppressive agents or growth factors. Finally, only patients with at least 2 refills for the initiated OAC class (VKA or DOAC) in the first 6 months after OAC initiation were retained, and patients who changed the OAC class were excluded.
Study design and resulting study population. (A) Diagram of the study design. All time periods that were considered to determine patient conditions prior to OAC initiation included the date of OAC initiation itself. (B) Patient characteristics according to OAC treatment. Data are expressed as column %, unless otherwise specified. An absolute standardized difference <0.1 was considered to indicate a negligible between-group difference. Additional characteristics are given in supplemental Table 1: age at index date, year, in classes (18 to 49, 50 to 59, 60 to 64, 65 to 69, 70 to 74, 75 to 79, 80 to 84), deprivation index of the area of residence, arrhythmias (other than AF), history of ATE, dementia or Parkinson disease, epilepsy or mental illness, history of DVT/PE, opioid-related disorders, HIV infection, other chronic and debilitating diseases, thyroid disease, oral corticosteroids, opioids and other analgesics, antiulcer agents, hypnotics or anxiolytics, polymedication at index date (<5, 5 to 9, ≥10 ATC classes), number of general practitioner visits during the year before OAC initiation (0, 1 to 5, 6 to 11, ≥12), influenza vaccination during the vaccination campaign directly preceding OAC initiation, first OAC prescriber’s specialty (hospital practitioner, general practitioner, private cardiologist, other private practitioners), calendar year of OAC initiation. ATC, anatomical therapeutic chemical; ATE, arterial thromboembolic events (ischemic stroke, arterial systemic embolism or transient ischemic attack); COPD, chronic obstructive pulmonary disease; DVT/PE, deep vein thrombosis/pulmonary embolism; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation; STD. standardized difference.
Study design and resulting study population. (A) Diagram of the study design. All time periods that were considered to determine patient conditions prior to OAC initiation included the date of OAC initiation itself. (B) Patient characteristics according to OAC treatment. Data are expressed as column %, unless otherwise specified. An absolute standardized difference <0.1 was considered to indicate a negligible between-group difference. Additional characteristics are given in supplemental Table 1: age at index date, year, in classes (18 to 49, 50 to 59, 60 to 64, 65 to 69, 70 to 74, 75 to 79, 80 to 84), deprivation index of the area of residence, arrhythmias (other than AF), history of ATE, dementia or Parkinson disease, epilepsy or mental illness, history of DVT/PE, opioid-related disorders, HIV infection, other chronic and debilitating diseases, thyroid disease, oral corticosteroids, opioids and other analgesics, antiulcer agents, hypnotics or anxiolytics, polymedication at index date (<5, 5 to 9, ≥10 ATC classes), number of general practitioner visits during the year before OAC initiation (0, 1 to 5, 6 to 11, ≥12), influenza vaccination during the vaccination campaign directly preceding OAC initiation, first OAC prescriber’s specialty (hospital practitioner, general practitioner, private cardiologist, other private practitioners), calendar year of OAC initiation. ATC, anatomical therapeutic chemical; ATE, arterial thromboembolic events (ischemic stroke, arterial systemic embolism or transient ischemic attack); COPD, chronic obstructive pulmonary disease; DVT/PE, deep vein thrombosis/pulmonary embolism; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation; STD. standardized difference.
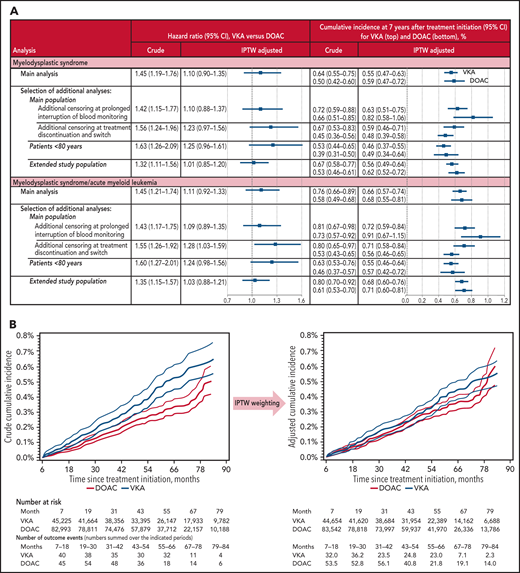
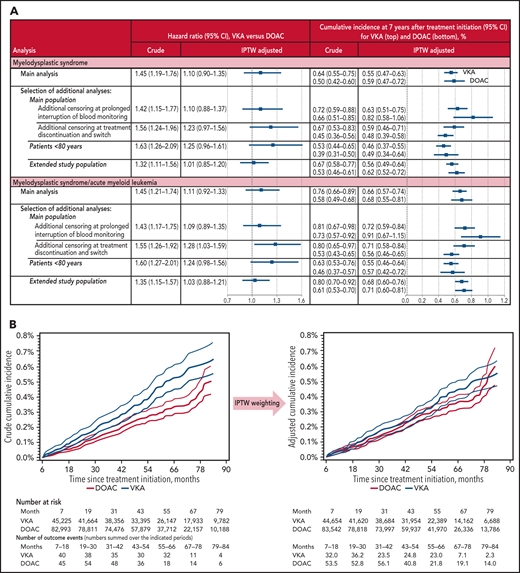
Covariates were assessed at baseline and comprised demographic characteristics, cardiovascular conditions and other comorbidities, proxies for smoking and frailty, comedications, and health care utilization proxies (Figure 1B). Two outcomes were considered: (1) MDS, (2) the composite outcome of MDS and acute myeloid leukemia (AML), as MDS is known to progress to AML in a third of patients with MDS.5 Follow-up started after the 6-month observation period following OAC initiation and continued until (1) outcome event, (2) censoring for other cancer, loss to follow-up, or death, or (3) end of November 2019 or month 84, whichever occurred first.
All statistical analyses were performed for each of the 2 considered outcomes. A Cox proportional hazard model was used to estimate the hazard ratio (HR), comparing VKA to DOAC use. The HR was adjusted by means of a propensity score approach, namely, inverse probability of treatment weighting (IPTW), including weight stabilization and truncation.6 Nonparametric cumulative incidence curves were also estimated.7 Their 95% confidence intervals were obtained by bootstrap with 200 replications. Two additional analyses implied further censoring: (1) censoring at prolonged interruption of blood monitoring, that is, a 15-month period with neither outpatient complete blood count nor hospitalization, (2) censoring at treatment switch or discontinuation of OAC therapy, that is, a 6-month period without OAC fills (per-protocol analysis). The first additional analysis was performed because we initially expected differential blood monitoring. The second one completes the intention-to-treat approach in the main analysis, which was selected to better capture potential long-term effects of OAC exposure on MDS, and because MDS diagnosis is often delayed. Two other analyses were performed on modified study populations: (1) the subgroup of patients <80 years, (2) an extended study population also including patients without clearly identified atrial fibrillation (AF) in the database, but with probable AF according to the algorithm by Billionnet et al.8 Sensitivity analyses focused on the impact of weight truncation and the role of the various covariates in adjustment, as well as alternative ways to handle age, a highly important MDS risk factor. For details on the method, including a description of further additional analyses and their results, please refer to supplemental methods and results, available on the Blood Web site.
Overall, 128 248 patients were included in the study (supplemental Figure 1), of whom 45 225 initiated VKAs and 82 993 initiated DOACs. The baseline characteristics are indicated in Figure 1B and supplemental Table 1. The mean duration from OAC initiation to end of follow-up was 4.5 years (maximum, 7 years), whereby 26.9% of the patients experienced a censoring event (12.6% developed another cancer, 13.1% others died, and 1.3% others were lost to follow-up) (supplemental Table 2). MDS was observed for 411 patients, corresponding to a crude incidence rate of 79.7 per 100 000 person-years, and MDS/AML for 479 patients.
The crude and adjusted HRs of MDS comparing VKA with DOAC were 1.45 (1.19 to 1.76) and 1.10 (0.90 to 1.35), respectively (Figure 2). For MDS/AML, they were 1.45 (1.21 to 1.74) and 1.11 (0.92 to 1.33), respectively. With additional censoring at prolonged interruption of blood monitoring, another 43 411 (33.9%) patients were censored (supplemental Table 2). The crude incidence rate of prolonged interruption of blood monitoring did, in fact, not differ between VKA and DOAC users (13.8 per 100 person-years in both groups). In the per-protocol analysis, another 12 908 (10.1%) patients switched from VKA to DOAC or vice versa, and 33 710 (26.3%) discontinued OAC treatment. The mean treatment duration was thus 3.4 and 3.6 years in patients with VKA and DOAC , respectively (see also supplemental Figure 3). The subgroup of patients <80 years comprised 100 169 (78.1%) patients (245 with MDS), and 44 385 additional patients (+34.6%) with probable AF were included in the extended study population (supplemental Figure 4). In summary, all analyses showed consistent results.
Study results. Adjustment included all patient characteristics listed in Figure 1B and supplemental Table 1. (A) Estimated effect of VKA exposure on the incidence of MDS and the composite outcome of MDS and AML, respectively, compared with DOAC exposure. The extended study population also included patients with probable AF. (B) Crude and adjusted cumulative incidence of MDS according to OAC treatment. Follow-up started at month 7 after OAC initiation. 95% confidence intervals (CIs) are indicated by thin curves.
Study results. Adjustment included all patient characteristics listed in Figure 1B and supplemental Table 1. (A) Estimated effect of VKA exposure on the incidence of MDS and the composite outcome of MDS and AML, respectively, compared with DOAC exposure. The extended study population also included patients with probable AF. (B) Crude and adjusted cumulative incidence of MDS according to OAC treatment. Follow-up started at month 7 after OAC initiation. 95% confidence intervals (CIs) are indicated by thin curves.
Verma et al1 addressed the potential clinical impact of their murine findings by a first simple analysis: the age- and sex-adjusted odds ratio of MDS diagnosis comparing patients with and without VKA use was 2.49 (95% CI: 2.3 to 2.7) and 8.2 (95% CI: 6.6 to 10.1) in patients aged 70 to 79 and 50 to 60 years, respectively (French National Healthcare Database, 2015). With this approach, temporality between VKA use and MDS diagnosis was not considered. Because of the absence of an active comparator and any further adjustment, it is difficult to draw clinical conclusions. In particular, cardiovascular conditions were not taken into account, whereas an MDS precursor state, clonal hematopoiesis, causes cardiovascular diseases.9,10 These limitations were overcome in our real-life study (however, clonal hematopoiesis status itself was also unknown).
Besides the limited duration of VKA exposure (3.4 years on average up to switch or discontinuation; maximum 7 years), the absence of external clinical validation of the MDS diagnosis remains a potential limitation of this study. Nevertheless, the MDS incidence by age and sex in our study showed the same age dependence as those reported in registries.11 Overall, MDS incidence in our study was somewhat higher, but this might be explained by the high portion of cardiovascular patients. Within 1 year of MDS diagnosis, 56.2% of the patients received a blood transfusion, 37.0% of the patients received growth factors, 2.4% of the patients received immunosuppressive agents, and 10.9% of the patients received chemotherapy (overall, 68.6% had at least 1 treatment). Residual confounding cannot be excluded, although a rich set of covariates was taken into account for adjustment. Among the recognized MDS risk factors,5,12 some risk factors are not a concern in our study (patients with a history of chemotherapy and radiotherapy were excluded; agricultural workers were not included), one was included via a proxy (smoking) and some others were not available (exposure to benzene and its derivates; industrial workers). In an extended discussion in the supplemental data, we provide detailed arguments for the following further points: exposure misclassification should be very rare;12 departures from the proportional hazards assumption underlying the Cox model remained limited; all IPTW assumptions not yet discussed above seem plausible; potential limitations to the generalizability of the study result are listed, for example, concerning patient’s age and the different VKA drugs.
In summary, the results of this large-scale population-based study do not suggest an increased risk of MDS with VKA compared with DOAC in patients with NVAF in the 7 years following OAC initiation.
The authors try to share publication-related data as much as possible: algorithms and other additional information are provided in the supplemental data; aggregated data can be supplied upon request by contacting the corresponding author at anke.neumann@assurance-maladie.fr.
The online version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Géric Maura, Cécile Billionnet, and Jérôme Drouin for their previous work, in particular, for the elaboration of numerous definitions used to identify the study population and to calculate the covariates, and for providing part of the data used in our analyses.
Authorship
Contribution: M.Z. conceived the study idea; A.N., M.-J.J., and M.Z. designed the study; A.N. collected data and performed statistical analyses; A.N., M.-J.J., and M.Z. analyzed and interpreted data; A.N. drafted the manuscript; and A.N., M.-J.J., and M.Z. revised the manuscript and contributed to its preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.