Abstract
Despite advances in the genetic characterization of chronic myelomonocytic leukemia (CMML), the molecular mechanisms that drive the disease during its distinct phases remain unclear. To uncover vulnerabilities in CMML that could be therapeutically targeted to halt its evolution, we sought to dissect at the single-cell level the cellular and transcriptomic changes that occur in the hematopoietic system at the time of CMML's initiation and its progression after hypomethylating agent (HMA) therapy.
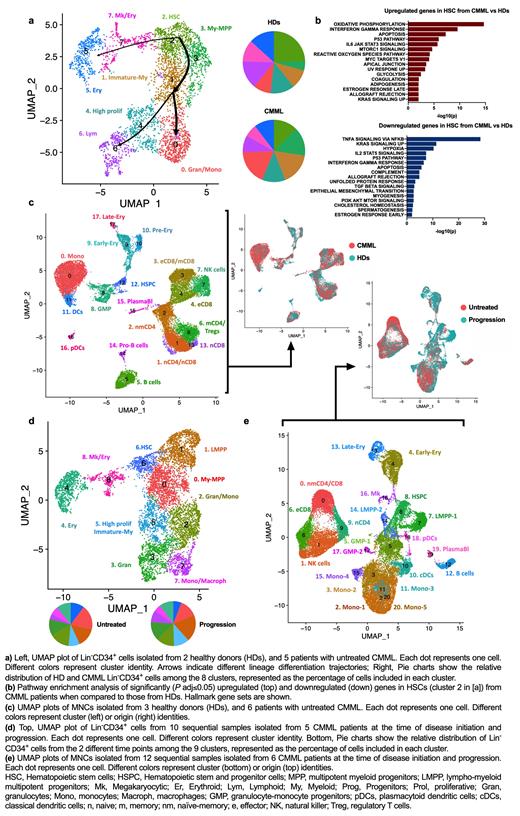
To evaluate the molecular mechanisms underlying CMML maintenance, we performed single-cell RNA sequencing (scRNA-seq) analysis of lineage-negative (Lin -)CD34 + hematopoietic stem and progenitor cells (HSPCs) and bone marrow (BM) mononuclear cells (MNCs) isolated from untreated CMML patients (n=5 and 6, respectively) and age-matched healthy donors (HDs; n=2 and 3, respectively). Our integrated analysis revealed that CMML Lin -CD34 + HSPCs had a predominant granulomonocytic differentiation route with an increased frequency of early and committed myeloid-monocytic progenitors at the expense of HSCs and megakaryocyte/erythroid progenitors (Fig. 1a). Differential expression analysis among the clusters revealed that most transcriptomic differences occurred in CMML HSCs, which were characterized by the upregulation of genes involved in oxidative phosphorylation, type I interferon (IFN) and IFNγ response, myeloid development, and inflammatory signaling and had downregulated expression of genes involved in TNFα-mediated NF-κB activation (Fig. 1b). These data suggest that CMML HSCs undergo metabolic reprogramming and demand a higher level of mitochondrial activity to maintain activated monocytic differentiation in response to inflammatory signaling.
Consistent with these results, scRNA-seq analysis of MNCs isolated from the same HD and CMML BM samples showed that monocytes were significantly increased at the expense of erythroid precursors and B cells in CMML (Fig. 1c). CMML monocytes had upregulated genes involved in IFNγ response, oxidative phosphorylation, MYC targets, NF-κB activation, and inflammation (e.g., S100A9, CCL3, IL1B). Interestingly, among the anti-apoptotic BCL2 family members, only the NF-κB transcriptional target BCL2A1 was significantly overexpressed.
To investigate the mechanisms of resistance to HMA therapy, we performed integrated scRNA-seq analysis of sequential Lin -CD34 + cells and BM MNCs isolated from CMML patients at the time of disease initiation and progression after HMA therapy failure. CMML progression was driven by a significant expansion of lympho-myeloid progenitors (LMPPs) at the expense of earlier HSCs , which exacerbated myelomonocytic differentiation in the HSPC compartment (Fig. 1d). Expanded LMPPs were characterized by higher levels of IFNγ response, NF-κB survival signaling, and cell cycle regulators. Accordingly, scRNA-seq analysis of MNCs cells from the same patients showed significantly increased frequencies of monocytes and a reduction of naïve CD4 +/CD8 + T cells and effector memory CD8 + T cells. Differential expression analysis of the 2 sample groups in the monocyte population identified five different cellular clusters, one of which emerged only at progression (Fig. 1e). This population was characterized by high expression levels of inflammatory cytokines and the anti-apoptotic modulators MCL1 and BCL2A1. Together, these data suggest that CMML progression arises from immature myeloid progenitors at the stem cell level and that downstream monocytes undergo transcriptomic rewiring and acquire survival mechanisms that induce therapy resistance and further accelerate disease progression.
In conclusion, our results elucidate the differentiation hierarchies and transcriptional programs associated with CMML's initiation and its progression after HMA therapy. Our data suggest that therapies targeting downstream effectors of NF-kB-mediated survival signaling could overcome treatment failure.
Wei: Daiichi Sanko: Research Funding. Kantarjian: AbbVie: Honoraria, Research Funding; Immunogen: Research Funding; KAHR Medical Ltd: Honoraria; Jazz: Research Funding; Ipsen Pharmaceuticals: Honoraria; Astellas Health: Honoraria; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astra Zeneca: Honoraria; Ascentage: Research Funding; Aptitude Health: Honoraria; Daiichi-Sankyo: Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria.