In this issue of Blood, Docampo et al1 explore the role of G protein–coupled receptor (GPR)109A, a receptor for the short chain fatty acid (SCFA) butylate and the B vitamin niacin, in the pathophysiology of experimental graft-versus-host disease (GVHD).
Previous work has demonstrated that activation of GPR109A in colonic antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs), promotes anti-inflammatory responses by inducing regulatory T cell (Treg) differentiation and interleukin-10 (IL-10) production by T cells.2 In addition, GPR109A was previously shown to be essential for butyrate-mediated induction of IL-18 and tissue repair in the intestinal epithelium.2 These data suggested that GPR109A may mitigate GVHD. Docampo et al tested the role of GPR109A in murine models of GVHD and found that its absence in recipients did not alter GVHD severity or mortality. Surprisingly, however, GPR109A-deficient (GPR109A−/−) donor T cells caused dramatically less GVHD. Recipients of GPR109A−/− donor T cells had reduced GVHD histopathological scores, decreased proliferation of alloreactive T cells in target organs, and reduced accumulation of alloreactive T cells in target tissues.
Functional analyses demonstrated that GPR109A−/− T cells were more prone to apoptosis and had diminished mitochondrial oxidative phosphorylation capabilities. Hence, alloreactive GPR109A−/− T cells were metabolically dysregulated. However, antioxidant treatment with N-acetyl cysteine restored their alloreactivity and ability to drive GVHD. At steady-state, GPR109A deficiency did not alter T-cell subtypes, activation/exhaustion markers, differentiation, or Treg suppressive function, which suggests that GPR109A alters T-cell function under inflammatory conditions. Importantly, the graft-versus-tumor (GVT) and antiviral activities of GPR109A−/− donor T cells were maintained. These data collectively suggest that GPR109A in donor T cells is indispensable for expansion and metabolic homeostasis following allogeneic hematopoietic cell transplantation (allo-HCT). Thus, GPR109A in allogeneic T cells enhances their inflammatory capacity, whereas in gut epithelial cells and APCs it protects against and decreases inflammation (see figure).2,3 This study demonstrates a novel intrinsic mechanism in allogeneic T cells that is mediated by GPR109A.
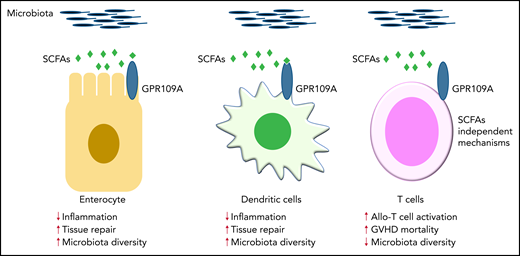
GPR109A is a receptor for the SCFA butylate. Previous work demonstrated that GPR109A is essential for butyrate-mediated induction of IL-18 in the intestinal epithelium, which facilitates repair of damaged tissue and then maintains gut homeostasis and regulates immune responses (left panel).2,3 In addition, activation of GPR109A in colonic APCs, such as DCs, promotes anti-inflammatory responses (middle panel).2,3 Docampo and colleagues have now clarified the role of GPR109A in allogeneic T cells, which enhance their inflammatory capacity in a butyrate-independent manner (right panel). Contrary to previous findings2,3 in gut epithelial cells and APCs, this study demonstrates a novel intrinsic mechanism in allogeneic T cells that is mediated by GPR109A.
GPR109A is a receptor for the SCFA butylate. Previous work demonstrated that GPR109A is essential for butyrate-mediated induction of IL-18 in the intestinal epithelium, which facilitates repair of damaged tissue and then maintains gut homeostasis and regulates immune responses (left panel).2,3 In addition, activation of GPR109A in colonic APCs, such as DCs, promotes anti-inflammatory responses (middle panel).2,3 Docampo and colleagues have now clarified the role of GPR109A in allogeneic T cells, which enhance their inflammatory capacity in a butyrate-independent manner (right panel). Contrary to previous findings2,3 in gut epithelial cells and APCs, this study demonstrates a novel intrinsic mechanism in allogeneic T cells that is mediated by GPR109A.
GVHD remains a major life-threating complication of allo-HCT. Recent advances demonstrated that the intestinal microbiome and its metabolites play critical roles in the pathogenesis of GVHD.4,5 In particular, SCFAs, such as butyrate and propionate, play a significant role in promoting and maintaining gut homeostasis and reducing GVHD in murine models.5,6 SCFAs bind to specific GPRs, such as GPR43 and GPR109A, on gut epithelial cells and immune cells, thereby regulating inflammatory immune responses and mediating tissue-protective functions.2,3 These functions include inhibiting histone deacetylase activity5 or the NLRP3 inflammasome.6 Because mice lacking GPR43 or GPR109A are more susceptible to chemically induced colitis,2 SCFA-mediated signaling pathways are thought to be indispensable for maintaining gut homeostasis. However, butyrate can also reduce GVHD in a GPR43-independent manner by maintaining enterocyte homeostasis and restoring intestinal epithelial cell (IEC) junction integrity.6 These data suggest that metabolites and their receptors have intrinsic immune-regulatory functions.
Docampo and colleagues have now clarified the role of GPR109A in allogeneic T cells. Their results suggest that GPR109A has opposing roles in donor T cells, where it increases GVHD, vs IECs, where it likely protects against GVHD. However, in T cells and IECs, GPR109A altered metabolic homeostasis, even in the absence of butyrate. Nevertheless, several outstanding questions remain. First, given GPR109A’s opposing influence on the outcome of inflammation in T cells vs APCs or IECs, what factors determine its anti- or proinflammatory immune response? Second, what upstream signaling drives GPR109A expression in alloreactive T cells? Third, what effect do niacin and butyrate have on clinical GVHD? Fourth, because conditioning and GVHD alter the gut microbiome and its metabolites, what is the role of other SCFAs in the absence of GPR109A in allogeneic T cells? Despite these remaining questions, Docompo et al’s findings may lead to new strategies for separating GVHD from GVT or antiviral activity by paving the way for research into engineered donor T cells with premodified GPR109A expression.
Conflict-of-interest disclosure: The author declares no competing financial interests.