TO THE EDITOR:
Venetoclax (VEN) in combination with low-dose cytarabine (LDAC) is FDA–approved for the treatment of unfit patients with newly diagnosed AML ineligible for intensive chemotherapy, based on a response rate of 54% (complete remission with or without blood count recovery [CR/CRi]) in the original phase Ib/II study.1 The VIALE-C phase 3 study (ClinicalTrials.gov Identifier: NCT03069352), compared VEN vs placebo (PBO) in combination with LDAC in 211 patients with untreated AML ineligible for intensive chemotherapy.2,3 The primary overall survival (OS) endpoint was event-driven and did not show a significant benefit in favor of VEN + LDAC after a median follow-up time of 12 months.2 This initial analysis was associated with substantial early censoring of patients with <6 months follow-up. In a subsequent post hoc analysis with median follow-up of 17.5 months (range 0.1-23.5), median OS was significantly longer in patients receiving VEN + LDAC (8.4 vs 4.1 months; HR = 0.70; 95% CI, 0.50-0.99; P = .04). Rates of CR/CRi were higher for patients receiving VEN + LDAC (48.3%), compared with PBO + LDAC (13.2%). In the present study, a final analysis with 2-years additional follow-up was undertaken to determine if the survival benefit of VEN +LDAC was sustained. In addition, clinical and molecular correlates of survival among patients receiving VEN + LDAC were assessed. These analyses demonstrated that survival outcome was influenced by prior exposure to hypomethylating agents, clinical response, cytogenetic risk, and molecular genotype, with best outcomes observed for patients with NPM1 mutation. This longer-term final analysis confirmed the survival improvement of VEN + LDAC in patients unfit for intensive chemotherapy.
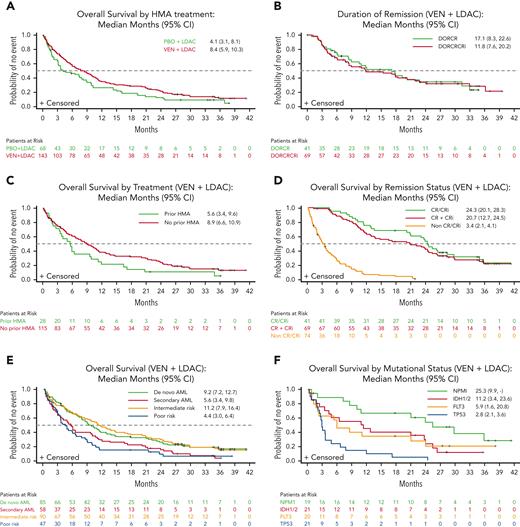
At the last follow-up on 15 February 2021 (median follow-up of 34.7 months, range 0.1-41.3), 83.9% (n = 120, VEN + LDAC) and 89.7% (n = 61, PBO + LDAC) of patients had died with 7% of patients (n =10) still receiving VEN + LDAC. No patient was receiving LDAC + PBO at this follow-up. A total of 2 patients on VEN + LDAC were lost to follow-up, and 5 withdrew consent (VEN + LDAC 3 [2.1%] and PBO + LDAC 2 [2.9%]). With an additional 2 years of follow-up from the last analysis,3 improvement in median OS with VEN + LDAC vs PBO + LDAC was unchanged (8.4 vs 4.1 months) (Figure 1A, supplemental Table 1, available on the Blood website). Two-year OS was 21.5% for patients in the VEN + LDAC arm and 12.4% for patients receiving PBO + LDAC (number needed to treat = 11). No new adverse event signal was noted (supplemental Table 2). We then investigated the correlates of outcome in the VEN + LDAC treated arm.
Survival outcomes and response in patients treated with VEN compared withPBO. (A) Kaplan-Meier OS curves of all patients. Number of patients at risk for each time is shown below and separated by treatment arms. (B) Kaplan-Meier duration of response curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by response. (C) Kaplan-Meier OS by prior HMA treatment curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by prior HMA treatment. (D) Kaplan-Meier OS by remission status curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by response. (E) Kaplan-Meier OS by AML type curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by AML type. (F) Kaplan-Meier OS by mutational status curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by mutational status. AML, acute myeloid leukemia; CR, complete remission; CRi, complete remission with incomplete blood count recovery; DOR, duration of remission; HMA, hypomethylating agent; LDAC, low dose cytarabine; PBO, placebo; VEN, venetoclax.
Survival outcomes and response in patients treated with VEN compared withPBO. (A) Kaplan-Meier OS curves of all patients. Number of patients at risk for each time is shown below and separated by treatment arms. (B) Kaplan-Meier duration of response curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by response. (C) Kaplan-Meier OS by prior HMA treatment curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by prior HMA treatment. (D) Kaplan-Meier OS by remission status curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by response. (E) Kaplan-Meier OS by AML type curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by AML type. (F) Kaplan-Meier OS by mutational status curves in patients treated with VEN + LDAC. Number of patients at risk for each time is shown below and separated by mutational status. AML, acute myeloid leukemia; CR, complete remission; CRi, complete remission with incomplete blood count recovery; DOR, duration of remission; HMA, hypomethylating agent; LDAC, low dose cytarabine; PBO, placebo; VEN, venetoclax.
Clinical response rates were similar to the previously published 6-month follow-up (supplemental Table 1), with 28.7% and 19.6% achieving CR and CRi, respectively. At 2 years, clinical response was sustained in 34.3% and 31.6% of patients initially achieving CR or CR/CRi, respectively, in the VEN + LDAC arm, indicating a similar duration of response for both response categories (Figure 1B). In contrast to the VIALE-A study, patients enrolled in the VIALE-C trial included 20% patients with a history of HMA treatment. Patients who did not receive prior HMA treatment had a longer OS (8.9 months [95% CI, 6.6-10.9]) than those who received prior HMA therapy (5.6 months [95% CI, 3.4-9.6]) (Figure 1C). Among the 29% of patients achieving CR, median OS was 24.3 months (95% CI, 20.1-28.3) (Figure 1D). Of the 48% patients achieving CR/CRi, median OS was 20.7 months (95% CI, 12.7-24.5), compared with 3.4 months (95% CI, 2.1-4.1) for those not achieving remission (Figure 1D). OS for patients with de novo AML was 9.2 months (95% CI, 7.2-12.7), compared with 5.6 months (95% CI, 3.4-9.8) in patients with secondary AML (Figure 1E). Patients categorized according to NCCN classification as intermediate risk had a longer OS of 11.2 months (95% CI, 7.9-16.4) compared with 4.4 months (95% CI, 3.0-6.4) in patients at poor risk (Figure 1E). We further analyzed OS according to the presence of somatic mutations in patients treated with VEN + LDAC. Median OS was 25.3 (95% CI, 9.9-not reached), 11.2 (95% CI, 3.4-23.6), 5.9 (95% CI, 1.6-20.8), and 2.8 (95% CI, 2.1-3.6) months in patients with NPM1, IDH1/2, FLT3, and TP53 mutations, respectively (Figure 1F). These outcomes should be interpreted with caution, because of the limited size of these subgroups.
Although a mOS difference was apparent between patients with NPM1 or IDH1/2 mutations at baseline, this may be attributed to co-occurrence of other poor prognostic factors in the IDH1/2 mutated cohort. Patients with IDH1/2 mutations were represented in secondary AML (38%), poor cytogenetic risk per NCCN 2016 classification (24%), and received prior treatment with HMA (19%) more frequently when compared with patients with NPM1 mutation (16%, 16%, and 5%, respectively) (supplemental Table 3).
In conclusion, among patients with newly diagnosed AML ineligible for intensive chemotherapy, longer-term follow-up confirmed that patients receiving VEN + LDAC had longer mOS than patients receiving PBO + LDAC. CR/CRi responses in the VEN + LDAC were durable, with 31.6% remaining in remission for >2 years. Notably, for patients with NPM1 mutation treated with VEN + LDAC, OS at 24 months was ∼50%. In contrast, outcomes for patients with TP53 mutation remained poor. This 2-year follow-up analysis confirms long-term benefit for patients treated with VEN + LDAC, with no new safety findings.
Acknowledgments
AbbVie and the authors thank all the trial investigators and patients who participated in this clinical trial. Medical writing support was provided by Susan Olalekan of AbbVie, Inc. Editorial support was provided by Angela T. Hadsell of AbbVie, Inc.
Authorship
Contribution: All authors contributed to provision, collection, and assembly of data; A.H.W., Q.J., Y.S., B.C., and W.M. contributed in data analysis and interpretation; all authors contributed thereafter in manuscript writing, critical revision, and approved the final version for submission. AbbVie, Inc contributed to the study conception and design.
Conflict-of-interest disclosure: AbbVie sponsored the study (NCT03069352), contributed to its design, collection, analysis, and interpretation of the data, and participated in the writing, review, and approval of the final version of the manuscript. All authors had access to relevant data. No honoraria or payments were made for authorship. VEN (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech. A.H.W. is a consultant for AbbVie, Amgen, Astellas Pharma, Celgene, Janssen, MacroGenics, Novartis, Roche, and Servier; received research funding from AbbVie, Celgene, Novartis, and Servier; is an employee of Walter and Eliza Hall Institute of Medical Research, which receives royalties related to VEN; and is entitled to a fraction of these payments. P.P. received grant/research support from AbbVie, Genesis, Novartis, and Roche; honoraria from AbbVie, Genesis, Gilead, Janssen, Novartis, and Roche. P.M. received grant/research support from Astellas Pharma, Celgene, Daiichi Sankyo, Janssen, Karyopharm Therapeutics, Novartis, Pfizer, and Teva; is a speaker/plays an advisory role for AbbVie, Celgene, Daiichi Sankyo, Incyte, Janssen, Karyopharm Therapeutics, Novartis, Pfizer, Teva, and Tolero; is a consultant for Agios, Astellas Pharma, Celgene, Daiichi Sankyo, Oryzon, and Tolero. K.L. received grant/research support from AbbVie, Novartis, Roche, Sandoz, and Takeda and personal fees from AbbVie, Astellas Pharma, BeiGene, Celgene, iQone Healthcare Switzerland, Janssen, Novartis, and Sandoz. V.I. is an investigator in AbbVie–sponsored clinical trials. I.K. is an investigator in AbbVie–sponsored clinical trials. J.N. is a consultant/plays an advisory role for Amgen, Novartis, Pfizer, Roche, and Takeda; receives travel expenses from Amgen and Janssen. R.C. is on the speakers bureau for Bristol Myer Squibb and AbbVie and the advisory board for ADC therapeutics and BeiGene. W.F. has a membership on an entity’s board of directors or advisory committee for AbbVie, Amgen, ARIAD/Incyte, Celgene, Jazz Pharmaceuticals, MorphoSys AG, Novartis, Stemline, Clinigen, and Pfizer; received patents and royalties from Amgen and support for meeting attendance from Amgen, Daiichi Sankyo, Gilead, Jazz Pharmaceuticals, and Servier; received research funding from Amgen and Pfizer. M.P. is a speaker/plays an advisory role for AbbVie, Amgen, Astellas Pharma, Genesis, Janssen, Novartis, AstraZeneca, Bristol Meyers Squibb, Gilead, Sobie and Pfizer. J.B. is a consultant for AbbVie, Servier, Amgen, Astellas Pharma, BMS, Jazz Pharmaceuticals, Novartis, and Pfizer; receives travel support from Amgen and Novartis. S.B.T. is a consultant for AbbVie and an investigator in AbbVie–sponsored clinical trials. J.Z.H., A.A., and A.M. are investigators in AbbVie–sponsored clinical trials. V.M. received conference attendance support from AbbVie, Celgene, Janssen, Novartis, and Takeda; is a consultant for AbbVie, Celgene, Novartis, and Janssen. T.Y. receives research support/honoraria from, and advisory role for AbbVie, Astellas Pharma, Gilead, Janssen, Nippon Shinyaku, Otsuka, Pfizer, Solasia, SymBio, and Takeda. J.W. plays an advisory role for AbbVie; research support from Celgene. B.C., Y.S., Q.J. and W.M. are employees of AbbVie and may hold stock or stock options. C.D.D. receives research support from AbbVie/Genentech, Agios, BMS/Celgene, Calithera, Cleave, Daiichi Sankyo, Immune-Onc, and Loxo; is a consultant/advisory board member for AbbVie, Agios, Aprea, BMS/Celgene, Immune-Onc, Kura, Novartis, Takeda, and Notable Labs.
Correspondence: Andrew H. Wei, The Peter MacCallum Cancer Centre and Royal Melbourne Hospital, 305 Grattan St, Melbourne, VIC 3000, Australia; e-mail: andrew.wei@petermac.org.
References
Author notes
Data sharing statement: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
The online version of this article contains a data supplement.