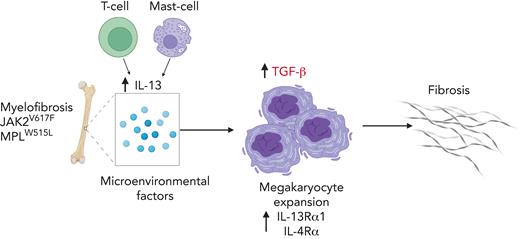
Key Points
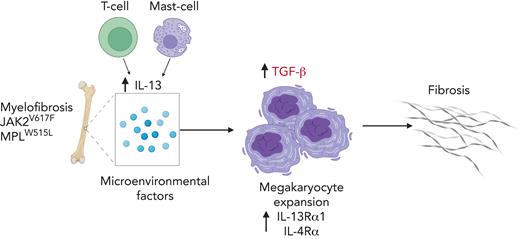
IL-13/IL-4 contributes to fibrotic progression of MF by promoting megakaryocyte growth and transforming growth factor β surface expression.
Reduction of IL-13/IL-4 signaling reduces fibrosis in mouse models.
Abstract
Myelofibrosis (MF) is a disease associated with high unmet medical needs because allogeneic stem cell transplantation is not an option for most patients, and JAK inhibitors are generally effective for only 2 to 3 years and do not delay disease progression. MF is characterized by dysplastic megakaryocytic hyperplasia and progression to fulminant disease, which is associated with progressively increasing marrow fibrosis. Despite evidence that the inflammatory milieu in MF contributes to disease progression, the specific factors that promote megakaryocyte growth are poorly understood. Here, we analyzed changes in the cytokine profiles of MF mouse models before and after the development of fibrosis, coupled with the analysis of bone marrow populations using single-cell RNA sequencing. We found high interleukin 13 (IL-13) levels in the bone marrow of MF mice. IL-13 promoted the growth of mutant megakaryocytes and induced surface expression of transforming growth factor β and collagen biosynthesis. Similarly, analysis of samples from patients with MF revealed elevated levels of IL-13 in the plasma and increased IL-13 receptor expression in marrow megakaryocytes. In vivo, IL-13 overexpression promoted disease progression, whereas reducing IL-13/IL-4 signaling reduced several features of the disease, including fibrosis. Finally, we observed an increase in the number of marrow T cells and mast cells, which are known sources of IL-13. Together, our data demonstrate that IL-13 is involved in disease progression in MF and that inhibition of the IL-13/IL-4 signaling pathway might serve as a novel therapeutic target to treat MF.
Introduction
The myeloproliferative neoplasms (MPNs) are a group of hematological disorders that affect different myeloid lineages and are characterized by the presence of specific mutations in JAK2, MPL, or CALR. Myelofibrosis (MF) is an MPN associated with poor clinical outcome. Individuals with MF have an increased risk of thrombosis and bone marrow (BM) fibrosis and a propensity to develop a form of acute myeloid leukemia termed MPN blast phase. Systemic chronic inflammation is a key feature of MF. Elevated levels of circulating proinflammatory cytokines have been associated with disease progression and clinical symptoms, and can serve as prognostic factors.1 Abnormal cytokine production is found not only in MPN-mutant cells but also in nonmalignant cells, emphasizing the role of persistent inflammation in driving the disease.2 Recent studies have highlighted the role of individual inflammatory cytokines in the fibrotic progression of MF.3-6 Although JAK inhibitors reduce the levels of certain circulating cytokines while improving clinical symptoms and reducing splenomegaly in patients with MF, the effect is relatively short lived. Prolonged treatment has a modest effect in cytokine levels with limited changes in the malignant clones or the degree of BM fibrosis.7
Atypical megakaryocytes contribute to fibrosis by secreting large amounts of profibrotic cytokines, such as transforming growth factor β (TGF-β).8 Inflammatory signaling has been shown to promote megakaryopoiesis by increasing the expression of megakaryocytic genes in megakaryocyte-biased hematopoietic stem and progenitor cells (HSPCs).9 Although several inflammatory cytokines are elevated in patients with MPN,10,11 few studies have addressed the effect of inflammatory cytokines directly on megakaryocytes. Thus, we aimed to identify the factors that promote megakaryopoiesis and subsequently result in fibrosis. We found the involvement of interleukin 13 (IL-13)/IL-4 signaling in the fibrotic progression of MF because of the increased production of TGF-β. We validated these observations by using samples from patients with MF. Targeting the IL-13/IL-4 axis leads to increased survival and reduced fibrosis in murine models of MF. Furthermore, we identified T cells and mast cells as sources of IL-13. In summary, our results highlight the effect of a previously uncharacterized signaling pathway on MF megakaryopoiesis and provide a new option for targeting the BM inflammatory milieu in MF.
Materials and methods
A list of reagents and antibodies is included in supplemental Table 1, available on the Blood website.
Mice
The animals used in this study were housed in the animal facilities of Northwestern University and St. Jude Children’s Research Hospital. All studies were performed in accordance with the Institutional Animal Care & Use Committee guidelines and approved protocols. Wild-type (WT), Jak2V617F, and Vav-Cre mice were on a C57BL/6 background, whereas Il4ra−/− mice were on a Balb/C background. Jak2V617F mice were obtained from Mullally et al,12 and other mice were obtained from the Jackson Laboratory.
Patient samples
Human CD34+ cells from patients with MF and plasma samples were obtained from the MPN research consortium. CD34+ cells from healthy controls were obtained from Fred Hutchinson Cancer Research Center. The use of these samples was approved by the St. Jude Institutional Review Board and the Mount Sinai Icahn School of Medicine.
Retrovirus production
Retroviral constructs MSCV-MPL-WT-IRES-GFP and MSCV-MPL-W515L-IRES-GFP were generously provided by Ross Levine.13 For IL-13 overexpression, we cloned murine IL-13 into a MigR1 vector (MigR1-mIL13-IRES-GFP) using NEBuilder HiFi DNA Assembly. As a control, we used a MigR1 vector expressing only green fluorescent protein (GFP). Retroviruses were produced in Plat-E Cells (Cell Biolabs) by transfection with 10 μg of plasmid using Lipofectamine 3000 (Thermo Fisher Scientific). After overnight incubation, the medium was changed with RPMI 5% containing fetal bovine serum. The virus was collected at 48h post transfection.
Cytokine analysis
Mouse plasma samples were obtained by terminal cardiac puncture in EDTA tubes. BM fluids were collected using the spin method in 200 μL of phosphate-buffered saline. The supernatant was transferred to low-protein binding tubes (Eppendorf) and stored at −80 °C. The MILLIPLEX MAP cytokine/chemokine magnetic bead panel for mice and humans was used to measure cytokine levels. Data acquisition was performed using a Luminex 200. xPONENT software was used to calculate the cytokine concentrations. Murine TGF-β levels were measured using an enzyme-linked immunosorbent assay kit (BioLegend).
Statistical analysis
The Shapiro-Wilk test was used to test the normality of the data. For the comparison of 2 groups, a 2-sample t test or Wilcoxon rank-sum test was performed depending on the normality of the data. The Kruskal-Wallis test or analysis of variance was used to compare the data among the 4 groups, depending on the normality of the data. The Jonckheere-Terpstra test was used to test for trends in cytokine values by ordered groups. The false discovery rate correction was adjusted for multiple comparisons, if relevant. Pairwise comparisons of groups were performed using Dwass, Steel, and Critchlow-Fligner multiple comparison analysis, which is based on pairwise 2-sample Wilcoxon comparisons. A generalized estimating equation method was used to compare the 2 groups and to adjust for repeated measurements per mouse. Kaplan-Meier curves were plotted to show the survival of the 2 groups, and the log-rank test was used to compare survival between the 2 groups. Analyses were performed using SAS 9.4, TS Level 1M3, R-4.1.3, and Prism 9.
Additional materials and methods are included in the supplemental Data.
Results
Disease progression is associated with increased cytokine levels particularly in the BM
To study the changes that occur in cytokines during the transition from the prefibrotic to fibrotic stage, we first profiled cytokines in the plasma and BM of the 2 MPN mouse models. The first model was generated by transplanting BM cells from Jak2V617F/Vav-Cre12 mice. Mice transplanted with BM cells expressing WT Jak2 served as controls. Jak2V617F/Vav-Cre–recipient mice presented with myeloproliferative disease characterized by elevated hematocrit and increased spleen size with decreased white blood cell (WBC) counts and platelets 6 to 8 months after transplantation (supplemental Figure 1A-B). Jak2V617F/Vav-Cre–recipient mice also showed expansion of megakaryocytes (CD41+ cells), with decreased ploidy upon disease progression (supplemental Figure 1C-D) as well as progressive BM fibrosis 6 to 8 months posttransplant (supplemental Figure 1E). For the second mouse model, we generated c-Kit+ HSPCs expressing MPLWT or MPLW515L and transplanted the cells into irradiated recipients. MPLW515L recipients developed an aggressive phenotype with shorter latency, increased WBC and platelet counts, and hepatosplenomegaly by 2 weeks posttransplant (supplemental Figure 2A-B). We also observed a progressive increase in CD41+ cells and reduced ploidy in MPLW515L-recipient mice (supplemental Figure 2C-D). Prominent BM fibrosis was apparent at 6 weeks after transplantation in MPLW515L mice (supplemental Figure 2E-F).
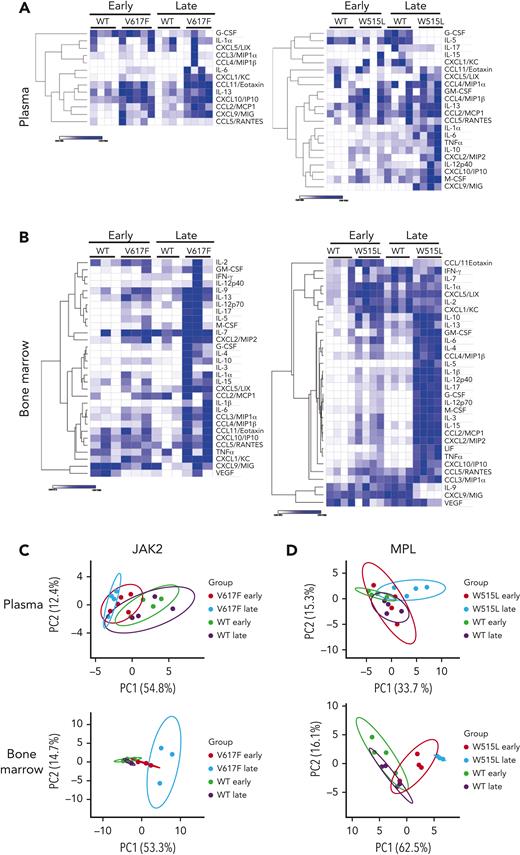
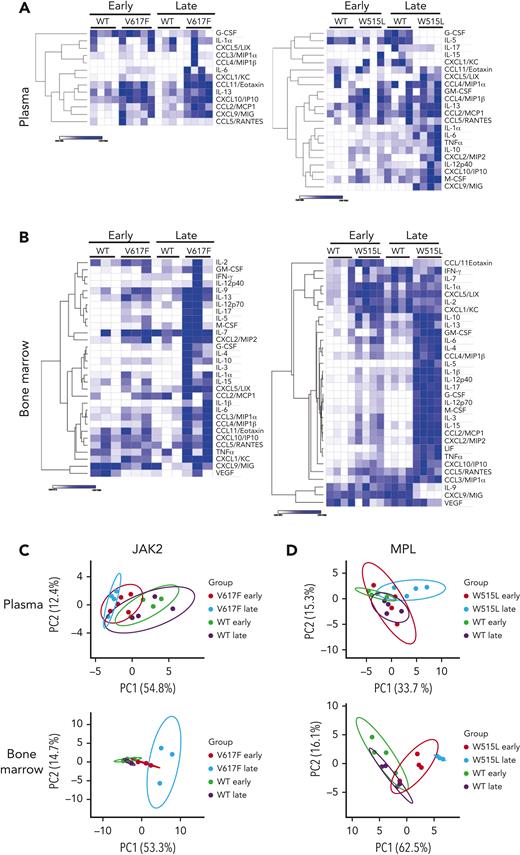
Next, we used an unbiased approach to measure the levels of 32 cytokines by Luminex 200 in the BM and plasma of both mouse models before (early time point) and after the development of fibrosis (late time point). We observed extensive cytokine changes in the BM and modest changes in the plasma (Figure 1A-B). Similar observations were made in the Gata1low mouse model of MF.14 Changes induced by the MPLW515L mutation were more profound than those induced by the Jak2V617F mutation, likely owing to the more aggressive disease course in mice (Figure 1A-B). Most cytokines were increased, with a few exceptions showing a decrease. For example, in the MPLW515L model, the levels of granulocyte colony-stimulating factor (G-CSF) and IL-5 decreased in the plasma, whereas the levels of IL-9 and CXCL9/MIG decreased in the BM at a later stage.
Numerous cytokinesare increased withdisease progression. Heat maps of cytokines detected in the plasma (A) and BM (B) of mice transplanted with Jak2WT or Jak2V617F (left) and mice transplanted with MPLWT or MPLW515L (right). N = 3 to 4 mice per group. Heat maps display the minimum and maximum values for each row independently. Principal component analysis of cytokine values detected in the plasma and BM of Jak2WT or Jak2V617F (C) or MPLWT or MPLW515L (D). G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, interferon gamma; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; PC1, principal component 1; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Numerous cytokinesare increased withdisease progression. Heat maps of cytokines detected in the plasma (A) and BM (B) of mice transplanted with Jak2WT or Jak2V617F (left) and mice transplanted with MPLWT or MPLW515L (right). N = 3 to 4 mice per group. Heat maps display the minimum and maximum values for each row independently. Principal component analysis of cytokine values detected in the plasma and BM of Jak2WT or Jak2V617F (C) or MPLWT or MPLW515L (D). G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, interferon gamma; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; PC1, principal component 1; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
To assess whether these cytokine changes correlated with the degree of fibrosis, we performed a principal component analysis. Plasma cytokines in the Jak2V617F model segregated groups based on mutation vs controls only, whereas changes in the BM further distinguished the groups based on fibrosis. In the MPLW515L model, plasma cytokine changes segregated mutant mice from the control group upon fibrosis development. In contrast, cytokines in the BM were clearly separated into groups based on their mutation and fibrosis status (Figure 1C-D). Overall, our results showed that more cytokine changes occurred in the BM than in the plasma, indicating that early changes in the BM microenvironment are not always traceable in the plasma. At the late stages of the disease, when fibrosis occurs, there are greater cytokine changes in the plasma, although not to the same degree as in the BM microenvironment.
IL-13 promotes expansion and STAT6 activation in megakaryocytes
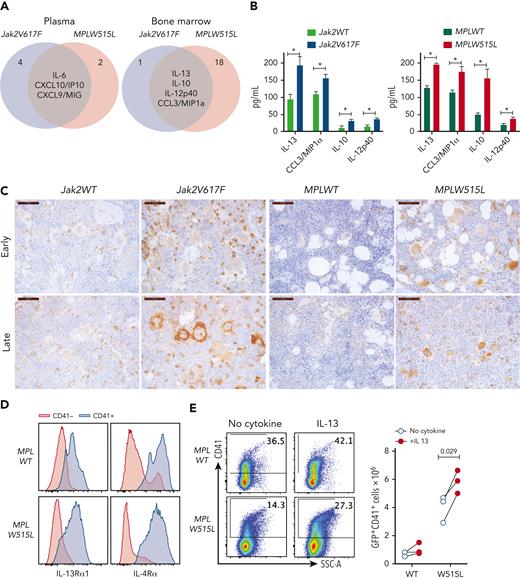
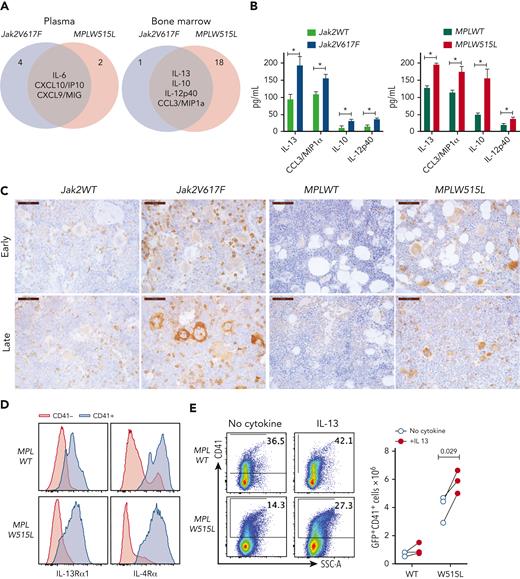
In our search for common factors that promote fibrosis, we focused on the cytokines that were upregulated in the BM of both mouse models. These cytokines included IL-13, IL-10, IL-12p40, and CCL3/macrophage inflammatory protein 1α (Figure 2A). We focused on IL-13, because its levels were the highest in the BM (Figure 2B), and because elevated IL-13 levels have been identified in patients with MF.11,15-17 Moreover, elevated levels of IL-13 persist in patients during JAK2 inhibitor treatment and among those progress to MPN blast phase.7 Notably, previous studies have implicated IL-13 involvement in fibrotic diseases of other organs, but its role in MPN has not been reported.
The IL-13 pathway is upregulated in MPN mouse models. (A) Venn diagram showing the cytokines that were upregulated in both Jak2V617F and MPLW515L mice in the plasma or BM. (B) Cytokine concentrations in the BM of Jak2V617F and MPLW515L mice and their respective controls. Data represent the mean ± standard error of the mean (SEM). N = 3 to 5. ∗P < .05, ∗∗P < .01. (C) Expression of IL-13Rα1 by immunohistochemistry in BM sections of mice from the indicated genotypes at early and late time points. (D) Expression of IL-13Rα1 and IL-4Rα in CD41+ megakaryocytes derived in vitro for 4 days after transduction of HSPCs with MPLWT or MPLW515L retroviruses. (E) In vitro cultures of HSPCs transduced with MPLWT or MPLW515L retroviruses cultured with no cytokines or in the presence of IL-13 for 4 days. Expression of CD41 was measured by flow cytometry. Total number of CD41+ cells in culture is shown (right). Each dot represents a biological replicate. Data from 3 independent experiments are shown. Paired Student t test was used for statistical analysis. MIP1a, macrophage inflammatory protein 1a; SSC-A, side scatter area.
The IL-13 pathway is upregulated in MPN mouse models. (A) Venn diagram showing the cytokines that were upregulated in both Jak2V617F and MPLW515L mice in the plasma or BM. (B) Cytokine concentrations in the BM of Jak2V617F and MPLW515L mice and their respective controls. Data represent the mean ± standard error of the mean (SEM). N = 3 to 5. ∗P < .05, ∗∗P < .01. (C) Expression of IL-13Rα1 by immunohistochemistry in BM sections of mice from the indicated genotypes at early and late time points. (D) Expression of IL-13Rα1 and IL-4Rα in CD41+ megakaryocytes derived in vitro for 4 days after transduction of HSPCs with MPLWT or MPLW515L retroviruses. (E) In vitro cultures of HSPCs transduced with MPLWT or MPLW515L retroviruses cultured with no cytokines or in the presence of IL-13 for 4 days. Expression of CD41 was measured by flow cytometry. Total number of CD41+ cells in culture is shown (right). Each dot represents a biological replicate. Data from 3 independent experiments are shown. Paired Student t test was used for statistical analysis. MIP1a, macrophage inflammatory protein 1a; SSC-A, side scatter area.
IL-13 is a signature cytokine of type 2 inflammatory responses with 2 receptors, IL-13 receptor α1 (IL-13Rα1) and IL-13Rα2. The binding of IL-13 to IL-13Rα1 leads to the formation of a heterodimer with IL-4Rα, resulting in a functional complex that activates STAT6 intracellular signaling. IL-13Rα2 has been considered a decoy receptor, although reports have shown its importance in mediating signaling by IL-13, chitinase 3–like 1, and endothelial growth factor receptor.18 Therefore, we measured the expression of IL-13Rα1 and IL-13Rα2 in BM sections from MPN mice. We found that IL-13Rα1 was highly expressed in both Jak2V617F and MPLW515L mice, whereas its expression in the respective control mice was markedly lower (Figure 2C). IL-13Rα1 expression was observed in multiple myeloid cell types, including megakaryocytes, which showed particularly intense staining. In contrast, we were unable to detect the expression of IL-13rα2 in BM. We also assayed the expression of IL-13Rα1 and IL-4Rα in megakaryocytes using flow cytometry and found that megakaryocytes express both receptors (Figure 2D).
IL-13 affects multiple cell types, including monocytes, macrophages, granulocytes, and fibroblasts. The fact that megakaryocytes express high levels of IL-13Rα1 and IL-4Rα suggests that IL-13 may also play a role in megakaryocytic lineage. Thus, we assayed the effect of IL-13 on megakaryocytes in vitro, and discovered that megakaryocytes respond to IL-13 stimulation by inducing the phosphorylation of STAT6 (supplemental Figure 3A). Moreover, in vitro cultures of HSPCs expressing MPLW515L in the presence of IL-13, but not thrombopoietin generated higher numbers of CD41+ megakaryocytes (Figure 2E). Overall, our results indicated that IL-13 levels were increased in the BM of MPN mice and induced the expansion of mutant megakaryocytes by inducing STAT6 activation.
IL-13 induces the expression of TGF-β in megakaryocytes
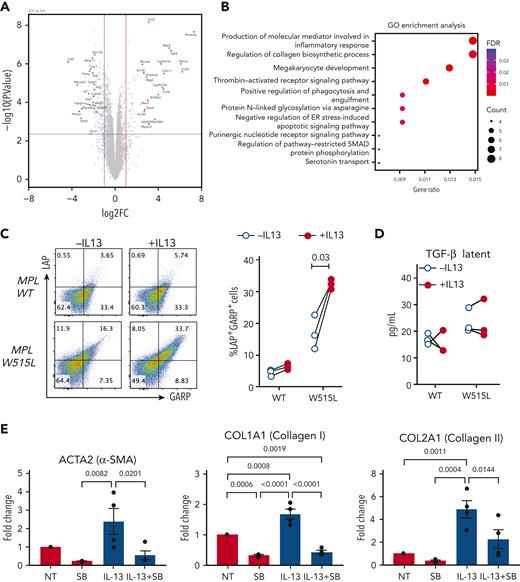
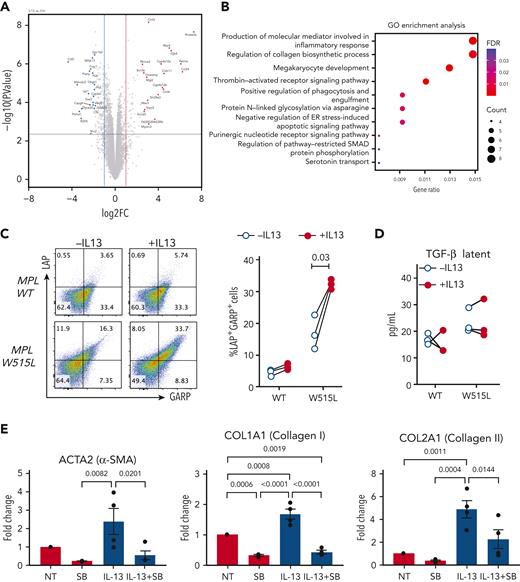
To further investigate the effect of IL-13 on megakaryocytes, we performed bulk RNA sequencing of MPLW515L cells cultured in the presence or absence of IL-13. We observed a similar number of upregulated and downregulated genes (537 and 623, respectively; false discovery rate < 0.05), with greater fold changes observed among the upregulated genes (Figure 3A). Gene ontology analysis of the upregulated genes indicated enrichment of genes involved in megakaryocyte development (Figure 3B), which is consistent with the stimulatory effect of IL-13 on megakaryocytes (Figure 2E). Additional pathways enriched in IL-13–treated cells included genes involved in the inflammatory response, collagen biosynthesis, and SMAD pathway. Previous studies in macrophages have shown the involvement of IL-13 signaling in promoting the expression of TGF-β.19,20 TGF-β is well known for its role in mediating fibrosis in MF, where it is produced by a variety of cell types including megakaryocytes.21 Collagen biosynthesis and SMAD proteins are part of the TGF-β pathway that regulate each other through an autocrine loop;22 thus, we tested the effect of IL-13 on TGF-β expression in megakaryocytes. TGF-β can be secreted in a latent form, which is then deposited in the extracellular matrix. TGF-β can also be expressed on the cell surface associated with latent associated peptide (LAP) and tethered to the cell surface by the glycoprotein-A repetitions predominant (GARP) protein, a mechanism that has been reported in regulatory T cells (Tregs), megakaryocytes, and platelets.23,24 Our results revealed an increased percentage of LAP+GARP+ double-positive cells, which were more pronounced in MPLW515L-mutant megakaryocytes than in MPLWT (Figure 3C). By contrast, we did not observe any changes in the latent TGF-β levels released upon IL-13 stimulation (Figure 3D). Next, to assess the effect of IL-13 treatment of megakaryocytes on fibrosis, we performed in vitro experiments in which we cocultured megakaryocytes isolated from the MPLW515L mouse model with leptin receptor+ (LepR+) mesenchymal stromal cells (MSCs) collected from WT mice. IL-13 treatment increased the expression of fibrosis markers, including α-SMA and collagen 1 and 2 (Figure 3E). This effect of IL-13 was abrogated when the cells were cultured with SB 431542, an inhibitor of the TGF-β receptor kinase ALK5 (Figure 3E). Importantly, IL-13 alone did not induce fibrotic gene expression in LepR+ MSCs (supplemental Figure 3B). Taken together, these results indicate that IL-13 stimulated megakaryocytes to induce fibrotic gene expression in MSCs in a TGF-β–dependent manner.
IL-13 promotes upregulation of collagen biosynthesis pathways and TGF-β expression in megakaryocytes. (A) Volcano plot of differentially expressed genes in MPLW515 cells cultured in the presence or absence of IL-13 for 4 days, as determined by bulk RNA sequencing. (B) Gene ontology enrichment analysis of the upregulated genes. (C) Expression of LAP and GARP in MPLWT and MPLW515L CD41+ megakaryocytes after 4 days of culture in the presence or absence of IL-13. Each dot represents a biological replicate. Paired Student t test was used for statistical analysis. (D) Levels of latent TGF-β in supernatants from the experiments shown in panel C. (E) Messenger RNA expression of Acta2, Col1a1, and Col2a1 in MSCs cocultured with MPLW515L megakaryocytes in the presence or absence of IL-13 and SB 431542 for 72 hours. ER, endoplasmic reticulum; FDR, false discovery rate; GO, gene ontology; MSCs, mesenchymal stromal cells; NT, no treatment; SB, SB 431542.
IL-13 promotes upregulation of collagen biosynthesis pathways and TGF-β expression in megakaryocytes. (A) Volcano plot of differentially expressed genes in MPLW515 cells cultured in the presence or absence of IL-13 for 4 days, as determined by bulk RNA sequencing. (B) Gene ontology enrichment analysis of the upregulated genes. (C) Expression of LAP and GARP in MPLWT and MPLW515L CD41+ megakaryocytes after 4 days of culture in the presence or absence of IL-13. Each dot represents a biological replicate. Paired Student t test was used for statistical analysis. (D) Levels of latent TGF-β in supernatants from the experiments shown in panel C. (E) Messenger RNA expression of Acta2, Col1a1, and Col2a1 in MSCs cocultured with MPLW515L megakaryocytes in the presence or absence of IL-13 and SB 431542 for 72 hours. ER, endoplasmic reticulum; FDR, false discovery rate; GO, gene ontology; MSCs, mesenchymal stromal cells; NT, no treatment; SB, SB 431542.
The IL-13 signaling pathway is upregulated in patients with primary MF
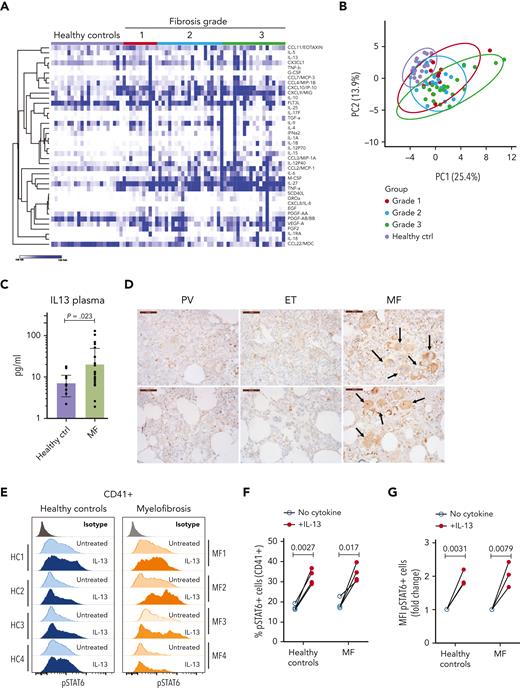
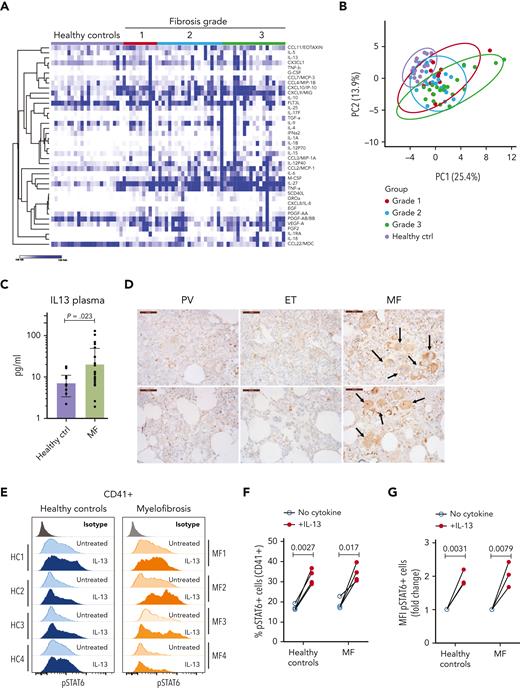
Next, we sought to identify the extent to which our observations from mice were translated into humans. We measured the cytokine levels in the plasma of healthy donors and patients with MF with different degrees of fibrosis. Our analysis showed the upregulation of several cytokines previously identified in MF (Figure 4A-B; supplemental Figure 4). We also found upregulation of other cytokines not previously described in MF, such as IL-27, IL-18, CCL22/MDC, and sCD40L.7,11,16 Principal component analysis showed that the cytokine profiles in the plasma did not segregate the groups based on the degree of fibrosis (Figure 4B). The greatest separation between the control and MF samples was observed in samples from patients with advanced disease (fibrosis grade 3). IL-13 was upregulated in samples from patients with MF, which is in agreement with previous studies (Figure 4C).11,15-17 Although IL-13 upregulation was observed in the BM of both mouse models, this increase was detected in the plasma of Jak2V617F mice but not in the plasma of MPLW515L mice (Figure 1A). Because of the relatively low incidence of MPL mutant MF cases, our study cannot rule out the possibility that there are differences in the cytokine profiles of patients with JAK2 and MPL mutations.
The IL-13 signaling pathway is activated in patients with MF. (A) Heat map of cytokines detected in the plasma of healthy controls and patients with MF (grouped by fibrosis degree). N = 11 to 20 samples per group. (B) Principal component analysis of samples shown in panel A. (C) Levels of IL-13 in plasma samples from healthy controls or patients with MF were not grouped based on fibrosis degree. P value was derived using the Mann-Whitney test with Benjamini, Krieger, and Yekutieli corrections. Data represent the average ± SEM. (D) Immunohistochemistry for IL-13Rα1 in human BM biopsies from patients with polycythemia vera, essential thrombocythemia, or MF. Scale bar, 50 μm. Black arrows indicate megakaryocytes with high IL-13Rα1 expression of the MF samples. Each image represents a different sample. (E) Representative flow cytometry plot of STAT6 phosphorylation in response to IL-13 stimulation in human megakaryocytes derived from healthy controls or patients with MF measured by flow cytometry. (F) Percentage of pSTAT6-positive cells gated on megakaryocytes CD41+. (G) Mean fluorescence intensity of pSTAT6-positive CD41+ cells. Paired Student t test was used for statistical analysis. ctrl, control; EGF, endothelial growth factor; ET, essential thrombocythemia; FGF2, fibroblast growth factor 2; HC, healthy control; MFI, mean fluorescence intensity; PDGF, platelet-derived growth factor; PV, polycythemia vera.
The IL-13 signaling pathway is activated in patients with MF. (A) Heat map of cytokines detected in the plasma of healthy controls and patients with MF (grouped by fibrosis degree). N = 11 to 20 samples per group. (B) Principal component analysis of samples shown in panel A. (C) Levels of IL-13 in plasma samples from healthy controls or patients with MF were not grouped based on fibrosis degree. P value was derived using the Mann-Whitney test with Benjamini, Krieger, and Yekutieli corrections. Data represent the average ± SEM. (D) Immunohistochemistry for IL-13Rα1 in human BM biopsies from patients with polycythemia vera, essential thrombocythemia, or MF. Scale bar, 50 μm. Black arrows indicate megakaryocytes with high IL-13Rα1 expression of the MF samples. Each image represents a different sample. (E) Representative flow cytometry plot of STAT6 phosphorylation in response to IL-13 stimulation in human megakaryocytes derived from healthy controls or patients with MF measured by flow cytometry. (F) Percentage of pSTAT6-positive cells gated on megakaryocytes CD41+. (G) Mean fluorescence intensity of pSTAT6-positive CD41+ cells. Paired Student t test was used for statistical analysis. ctrl, control; EGF, endothelial growth factor; ET, essential thrombocythemia; FGF2, fibroblast growth factor 2; HC, healthy control; MFI, mean fluorescence intensity; PDGF, platelet-derived growth factor; PV, polycythemia vera.
We then assayed the expression of IL-13Rα1 in BM biopsies from patients with MF and in samples from patients with polycythemia vera and essential thrombocythemia, which are related MPNs that typically do not present fibrosis. IL-13Rα1 staining was weak in samples from patients with polycythemia vera (PV) and essential thrombocythemia (ET). In contrast, MF samples showed a pattern reminiscent of our observations in mice; IL-13Rα1 was expressed in different cell types with a greater degree of staining in the megakaryocytic lineage (Figure 4D). We also measured the extent to which megakaryocytes from healthy controls and patients with MF responded to IL-13 stimulation by isolating CD34+ cells and differentiating them into megakaryocytes with the addition of thrombopoietin. After 7 days of culture, the cells were stimulated with IL-13 and their response was assessed by measuring the phosphorylation of STAT6. As shown in Figure 4E-G, megakaryocytes from healthy controls and patients with MF responded to IL-13 in a similar fashion, with increased pSTAT6 levels. Next, we assessed the expression of LAP and GARP in megakaryocytes after IL-13 stimulation. Our results showed that human megakaryocytes also upregulate LAP and GARP on the cell surface, although with patient-to-patient variability. We found upregulation in all controls and in 75% of MF samples (supplemental Figure 5). Overall, in patient MF samples, we observed upregulation of IL-13 in the plasma, increased expression of the IL-13 receptor in megakaryocytes, and the ability of IL-13 to increase pSTAT6 levels, showing that our observations in mice mimic those in patients.
Modulating IL-13/IL-4 signaling affects disease phenotypes in murine MPN models
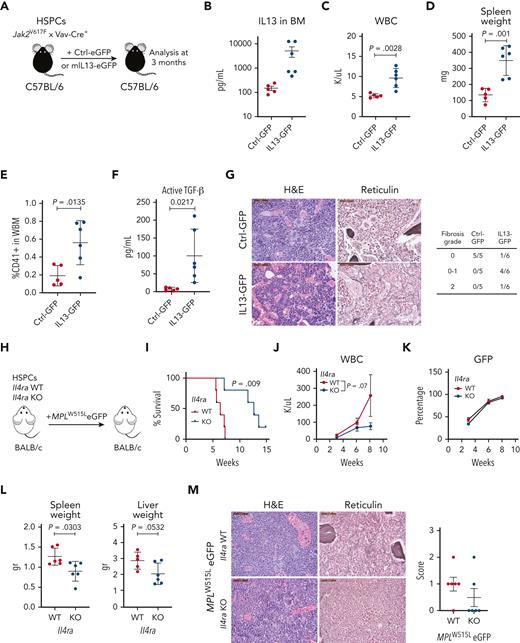
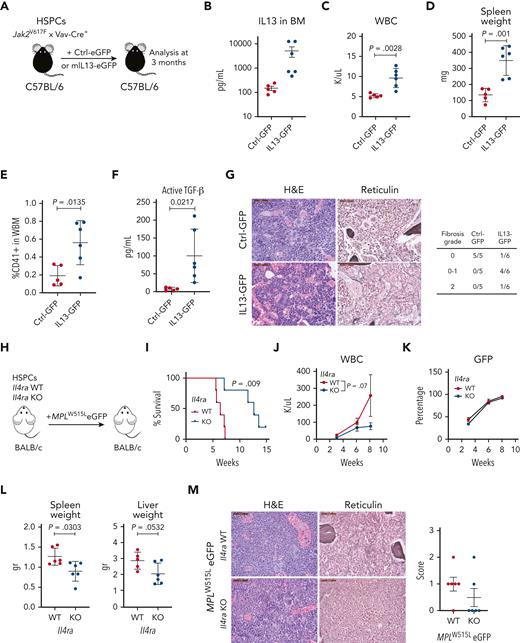
To assess the effect of modulating IL-13 signaling on the progression of MPN, we used 2 different approaches: IL-13 overexpression and IL-4Rα knockout (KO). In the first approach, we used the Jak2V617F mouse model, which develops fibrosis with a longer latency (6-8 months after transplantation). We overexpressed murine IL-13 with GFP or GFP alone in Jak2V617F/Vav-Cre HSPCs, transplanted them into irradiated recipients, and analyzed them after 3 months (Figure 5A). We confirmed that IL-13 levels were indeed elevated in vivo (Figure 5B). Overexpression of IL-13 induced an increase in WBC counts, whereas platelet counts and hemoglobin levels did not change compared with those in the control (Figure 5C; supplemental Figure 6A). Spleen weights were increased in mice receiving IL-13–expressing cells compared with those in control animals (Figure 5D). BM analysis further showed an increased percentage of CD41+ cells (Figure 5E); however, no changes were observed in CD11b+Gr1+ cells in the BM or spleen (supplemental Figure 6B). We also measured the levels of latent and active TGF-β in the BM and found increased levels of active TGF-β in mice overexpressing IL-13 (Figure 5F), whereas the levels of latent TGF-β remained unchanged (supplemental Figure 6C). Histopathological analysis of BM samples showed that Jak2V617F mice overexpressing IL-13 had dilated sinuses with intrasinusoidal hematopoiesis. In addition, 1 out of 6 mice developed grade 2 fibrosis, and 4 out of 6 mice displayed patchy grade 1 (scored 0-1) fibrosis. In contrast, mice overexpressing Ctrl-GFP showed no evidence of fibrosis (Figure 5G). In summary, these results showed that increasing IL-13 promotes features of MPN and BM fibrosis and induces TGF-β activation.
Overexpression of IL-13 promotes disease progression, whereas reduction of IL-13/IL-4 signaling ameliorates MF in vivo. (A) Schematic of experiment. (B) IL-13 levels in the BM of control (Ctrl-GFP) or IL-13 overexpressing (IL13-GFP) mice. (C) WBC counts in control and IL-13 overexpressing mice. (D) Spleen weights of indicated mice. (E) Percentage of megakaryocytes (CD41+) in the BM of control and IL-13 overexpressing mice. (F) Levels of active TGF-β in the BM of the indicated mice. (G) Hematoxylin and eosin (H&E) and reticulin staining of BM sections from Ctrl-GFP and IL13-GFP mice. Fibrosis scoring for all mice in the experiment is shown (right). (H) Schematic of the experimental setup. (I) Survival analysis of mice transplanted with Il4ra WT or KO cells. P value from the log-rank (Mantel-Cox) test is shown. WBC counts (J) and percentage of GFP+ cells (K) in peripheral blood are shown from indicated mice. (L) Spleen and liver weight of indicated mice. P value from unpaired Student t test is shown. (M) Representative images of BM sections stained with H&E and reticulin of indicated mice. Scale bar, 50 μm. Fibrosis scores are shown (right). In all graphs, the data represent the average ± SEM. eGFP, enhanced green fluorescent protein; H&E, hematoxylin and eosin; WBC, white blood cell count; WBM, whole bone marrow.
Overexpression of IL-13 promotes disease progression, whereas reduction of IL-13/IL-4 signaling ameliorates MF in vivo. (A) Schematic of experiment. (B) IL-13 levels in the BM of control (Ctrl-GFP) or IL-13 overexpressing (IL13-GFP) mice. (C) WBC counts in control and IL-13 overexpressing mice. (D) Spleen weights of indicated mice. (E) Percentage of megakaryocytes (CD41+) in the BM of control and IL-13 overexpressing mice. (F) Levels of active TGF-β in the BM of the indicated mice. (G) Hematoxylin and eosin (H&E) and reticulin staining of BM sections from Ctrl-GFP and IL13-GFP mice. Fibrosis scoring for all mice in the experiment is shown (right). (H) Schematic of the experimental setup. (I) Survival analysis of mice transplanted with Il4ra WT or KO cells. P value from the log-rank (Mantel-Cox) test is shown. WBC counts (J) and percentage of GFP+ cells (K) in peripheral blood are shown from indicated mice. (L) Spleen and liver weight of indicated mice. P value from unpaired Student t test is shown. (M) Representative images of BM sections stained with H&E and reticulin of indicated mice. Scale bar, 50 μm. Fibrosis scores are shown (right). In all graphs, the data represent the average ± SEM. eGFP, enhanced green fluorescent protein; H&E, hematoxylin and eosin; WBC, white blood cell count; WBM, whole bone marrow.
IL-13 and IL-4 mediate intracellular signaling through a shared receptor, IL-4Rα, which mediates signaling through heterodimerization with IL-13Rα1 or the common gamma chain, respectively. Notably, we observed IL-4 upregulation in the BM fluid of MPLW515L mice (Figure 1A-B; supplemental Figure 7A). Although we did not detect upregulation of IL-4 in the plasma of MPN mouse models (Figure 1A-B) and only a few human MF samples showed upregulation of IL-4 (Figure 4; supplemental Figure 4), previous studies in samples from patients with MPN have shown increased plasma levels of IL-4.25-27 Given that previous reports have shown that IL-13 and IL-4 have overlapping functions,28,29 we tested the effect of IL-4 on megakaryocytes in vitro. Our results showed that, similar to our observation with IL-13, IL-4 exhibited a tendency to promote megakaryocyte growth in vitro (supplemental Figure 7B).
Given the possibility that both cytokines contribute to fibrosis, we assessed the effect of IL-4Rα deficiency, which disrupts both cytokine functions in MPN. We transduced Il4ra+/+ (WT) or Il4ra−/− (KO) hematopoietic progenitor cells with MPLW515L and transplanted them into the irradiated recipients (Figure 5H). Notably, these mice were on a Balb/C background, which shows a more aggressive disease upon transplantation with MPLW515L than C57BL/6 mice. We found that mice receiving Il4ra-deficient cells had a longer survival time than the control mice (Figure 5I). Recipients of Il4ra-deficient MPLW515L cells showed a reduction in WBC counts, mainly owing to a reduction in neutrophils (Figure 5J; supplemental Figure 8A), but unchanged hemoglobin and platelet counts (supplemental Figure 8B). Analysis of the disease burden by measuring GFP-expressing cells showed no differences (Figure 5K; supplemental Figure 8C). Furthermore, there was a reduction in spleen and liver weights (Figure 5L), a decrease in CD11b+Gr1+ cells mainly in the spleen, and a trend toward a decrease in CD41+ megakaryocytes (supplemental Figure 8D-E). Importantly, reticulin staining of the BM showed the absence of fibrosis in most mice (4/6) in the Il4ra KO mice (Figure 5M). To assess whether the effect observed in Il4ra-deficient MPLW515L-recipient mice was not because of an intrinsic hematopoietic defect in Il4ra-deficient cells, we transplanted unmanipulated Il4ra WT and KO cells and monitored blood counts over the same time frame used for the MPLW515L-transduced cells. We found no significant differences in the engraftment of cells or downstream production of hematopoietic cells, except for a reduction in lymphocytes (supplemental Figure 8F). Taken together, our results revealed that IL-13 mediates fibrosis in MPN and that targeting IL-13/IL-4 mitigates the progression of the disease and fibrosis development in the early stages.
Mast cell and T-cell numbers increase with fibrosis
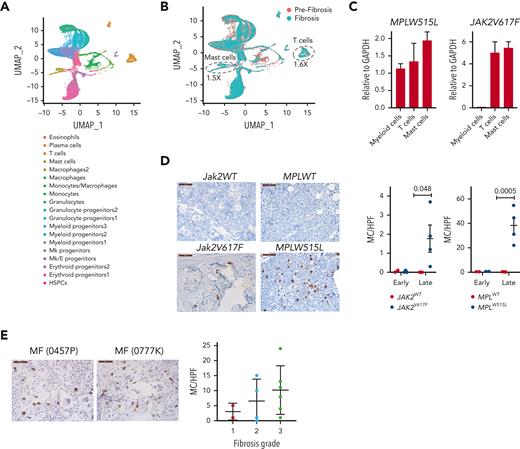
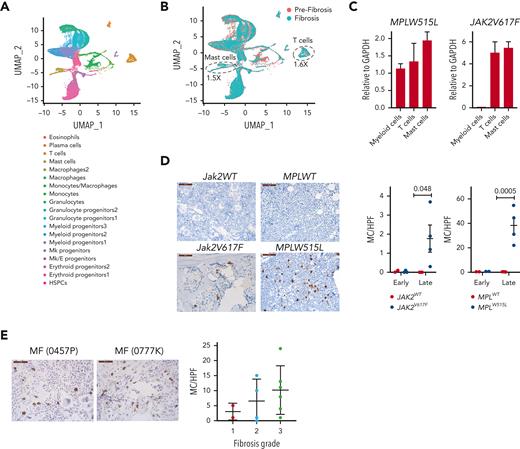
To gain further insight into the changes that occur with the progression of fibrosis, we performed single-cell RNA sequencing analysis using BM cells collected from Jak2V617F mice at 3 and 8 months posttransplant. These mice showed no fibrosis at 3 months, but showed grade 2 fibrosis at 8 months (supplemental Figure 9A). We used 2 biological replicates for each time point and performed cell hashing to identify the individual samples. A total of 16 732 and 14 080 cells were sequenced in the prefibrotic and fibrotic stages, respectively, and the data were analyzed using Seurat. Unsupervised clustering led to the identification of 19 clusters (Figure 6A). We defined the cell types in each cluster by performing differential gene expression analysis and comparing it to publicly available data sets.30-32 Selected differentially expressed genes, characteristic of each cluster, are shown in supplemental Figure 9B. We did not observe major differences among the biological replicates (supplemental Figure 9C); therefore, we pooled the samples from each time point for further analysis.
Mast cells and T cells are sources of IL-13 in MPN mouse models. (A) Uniform manifold approximation and projection (UMAP) of single-cell RNA sequencing analysis of BM cells from Jak2V617F recipient mice at 3 and 8 months after transplant (n = 2 per time point) colored by cell clusters. Data from all mice and time points are shown. (B) Uniform manifold approximation and projection of cells in prefibrotic (orange) and in fibrotic (green) stages. (C) IL-13 messenger RNA expression in sorted myeloid cells (CD11b+), T cells (CD3+), and mast cells (CD117+ FcεRIα+) from the BM of MPLW515L and Jak2V617F mice. (D) Mast cell tryptase staining in the BM of the indicated mice at early and late time points. (E) Mast cell tryptase staining of human BM biopsies from patients with MF stratified based on the fibrosis grade. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPF, high powered field; MC, mast cells; Mk, megakaryocytic.
Mast cells and T cells are sources of IL-13 in MPN mouse models. (A) Uniform manifold approximation and projection (UMAP) of single-cell RNA sequencing analysis of BM cells from Jak2V617F recipient mice at 3 and 8 months after transplant (n = 2 per time point) colored by cell clusters. Data from all mice and time points are shown. (B) Uniform manifold approximation and projection of cells in prefibrotic (orange) and in fibrotic (green) stages. (C) IL-13 messenger RNA expression in sorted myeloid cells (CD11b+), T cells (CD3+), and mast cells (CD117+ FcεRIα+) from the BM of MPLW515L and Jak2V617F mice. (D) Mast cell tryptase staining in the BM of the indicated mice at early and late time points. (E) Mast cell tryptase staining of human BM biopsies from patients with MF stratified based on the fibrosis grade. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPF, high powered field; MC, mast cells; Mk, megakaryocytic.
Gene set enrichment analysis with the hallmark gene sets revealed oxidative phosphorylation and DNA repair as dominant pathways enriched in most myeloid cell types upon fibrosis development, whereas lymphoid cells showed enrichment for interferon response genes (supplemental Figure 9D). Interestingly, megakaryocyte progenitors showed enrichment for mTOR signaling and heme metabolism. Although we did not capture mature megakaryocytes because of their large size, we performed further analysis of the megakaryocyte progenitor population to investigate whether there was enrichment for STAT6 targets. Gene set enrichment analysis with the STAT6 target gene set obtained from the ChIP Enrichment Analysis database showed enrichment of this pathway at the fibrotic stage (supplemental Figure 9E).
Next, we compared the differences in the proportion of cells between the prefibrotic and fibrotic stages. We observed an increase in T cells and mast cells in the fibrotic stage compared with those in the prefibrotic stage (Figure 6B). Given that mast cells and T cells are known to secrete IL-13, we assayed the extent to which these cells express IL-13 in the MPLW515L and Jak2V617F mouse models. To this end, we sorted T cells (CD3+) and mast cells (CD117+FcεRIα+) from the BM and measured IL-13 expression by quantitative reverse transcription polymerase chain reaction. We found that T cells and mast cells showed higher expression of IL-13 than myeloid cells (CD11b+) (Figure 6C). To further validate the cellular changes observed using single-cell RNA sequencing, we performed mast cell tryptase staining of the BM of MPLW515L and Jak2V617F mice. Mast cells have been previously implicated in MF.33 We detected significant increases in mast cells in both mouse models upon fibrosis development, with greater numbers of mast cells observed in MPLW515L mice (Figure 6D). Likewise, mast cell tryptase staining of human MF samples showed a tendency toward increased mast cells with a higher degree of fibrosis (Figure 6E).
Discussion
MPNs are characterized by a chronic state of inflammation with elevated levels of circulating cytokines. Recent evidence has highlighted how individual cytokines contribute to the progression of MF, providing important insights into the mechanisms of fibrosis development and opening novel therapeutic interventions.3,4 Although treatment with JAK inhibitors reduces the levels of certain inflammatory cytokines, several cytokines persist, including IL-13, despite therapy.7,34,35 IL-13 is an immunoregulatory cytokine that plays diverse functions in type 2 immunity, regeneration, and fibrosis. The role of IL-13 in fibrotic processes has been studied in solid organs,19,20,29 but its role in BM fibrosis has not been investigated.
We found that IL-13 induced megakaryocyte growth and increased the expression of membrane bound TGF-β as measured by the increased expression of GARP associated with LAP. Platelets, Tregs, and activated B cells express GARP on the cell surface. The function of GARP has been extensively studied in Tregs, whereas its function in megakaryocytes is unknown. Studies on platelets have shown a critical role of GARP expression in TGF-β activation. GARP deficiency in platelets drastically reduces the levels of active TGF-β.36,37 Although the mechanisms for releasing active TGF-β from the GARP-bound latent TGF-β have not been studied in megakaryocytes or platelets, studies in Tregs show that GARP binding to αvβ6 and αvβ8 integrins mediates this process.38 Interestingly, BM stromal cells from mouse and human origin express these integrins,39,40 including fibroblasts and LepR+ (or CXCL12 abundant reticular) cells, which are known to contribute to fibrosis in MF.41,42 Although further studies are necessary, the increased levels of GARP in IL-13–activated megakaryocytes suggests a mechanism to increase the local bioavailability and cell-to-cell activity of TGF-β in the stroma.
Although we focused our study on the effect of IL-13/IL-4 in megakaryocytes, other reports have shown the effect of these cytokines in other cell types. For example, IL-13/IL-4 signaling has been shown to induce alternative activation of macrophages, and upon prolonged stimulation, it mediates fibrosis, whereas its inactivation reduces fibrosis.19,43 IL-13/IL-4 stimulates collagen expression in fibroblasts,44 and enhances the proliferation of hematopoietic progenitors.45,46 Inhibition of this pathway has also been shown to reduce fibrosis in solid organs in animal models.47,48 In our mouse models, we found that modulating this signaling pathway affected not only megakaryocytes but also other myeloid cells. We observed increased numbers of neutrophils in the blood and CD11b+Gr1+ cells in the spleen upon IL-13 overexpression, whereas the opposite was observed in the Il4ra KO mice. Strategies to block IL-13/IL-4 signaling have been used in patients with asthma and atopic dermatitis, where an IL-4Rα–blocking antibody (dupilumab) and an IL-13–blocking antibody (lebrikizumab) showed clinical benefits leading to US Food and Drug Administration approval. Our results in MPN mouse models suggest that the inhibition of this pathway may be beneficial when used as an early intervention to prevent fibrosis development. In addition, because JAK inhibitors used for the treatment of patients with MF do not alter IL-13 plasma levels, but provide clinical benefits, combining a JAK inhibitor with an IL-13/IL-4–blocking strategy may provide additional benefits.
Our results showed an increase in the number of mast cells and T cells upon the development of fibrosis. The involvement of mast cells in MF has been previously reported. Mast cells in MF harbor JAK2V617F and MPLW515L mutations, express high levels of activation markers, and produce IL-13.33,49,50 Increased mast cells have also been reported in Jak2V617F mice.51 In regard to T cells, these cells are known to reside in the BM under healthy conditions,52 but less is known in MF. T cells are known to secrete IL-13, thus, we cannot exclude the possibility that the increase in IL-13 was driven by both cell subsets.
Together, our data demonstrate that IL-13/IL-4 signaling is involved in the progression of MF and suggest that inhibition of this pathway should be investigated as a therapeutic option.
Acknowledgments
The authors thank Julie Justice for assistance with immunohistochemistry, the Hartwell Center and the flow core at St. Jude Children’s Research Hospital.
This work was supported by grants from the National Institutes of Health (NIH), National Cancer Institute (NCI) (grant R35CA253096) (J.D.C.) and MPN Research Consortium (P01CA108671) (R.H., R.W., A.R.M., and J.D.C.) and in part by a grant from the NIH, NCI (grant R01CA265009) (J.C.C.). Additional support was provided by the Samuel Waxman Cancer Research Foundation and St. Jude/American Lebanese Syrian Associated Charities (J.D.C.).
The content is the sole the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
J.M.-C. is a fellow of the Leukemia and Lymphoma Society and Mark Foundation for Cancer Research.
Authorship
Contribution: J.M.-C., L.B., A.C., and C.M. designed and performed the experiments and analyzed the data; J.C.C., G.K., J.G., and S.G. analyzed the data; R.W., R.H., Y.Z., M.D., and C.R.R. provided the reagents and/or samples; J.D.C. designed the experiments and analyzed the data; and all authors wrote the manuscript.
Conflict-of-interest disclosure: J.D.C. consults for Cellarity and is a member of the scientific advisory board for Alethiomics. The remaining authors declare no competing financial interests.
Correspondence: John D. Crispino, Division of Experimental Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS341, Memphis, TN 38105; e-mail: john.crispino@stjude.org.
References
Author notes
The RNA sequencing data reported in this article have been deposited in the Gene Expression Omnibus database (accession numbers GSE205884 and GSE207691).
Data are available on request from the corresponding author, John D. Crispino (john.crispino@stjude.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.