Abstract
Background: Dasatinib is a second generation BCR::ABL1 tyrosine kinase inhibitor approved at the dose of 100 mg daily for the frontline therapy of patients with chronic myeloid leukemia in chronic phase (CML-CP). Low-dose dasatinib (50mg daily) has been previously reported to be effective and better tolerated compared to the standard dose. Here, we report updated data with longer follow-up of patients with newly diagnosed CML-CP treated with dasatinib 50 mg daily.
Method: Patients with newly diagnosed CML-CP who were treated with low-dose dasatinib (50 mg daily) were included. Response criteria were standards defined in previous protocols. The overall survival (OS) was calculated from the start date of the therapy to the date of death from any cause at any time or date of last follow-up; the event-free survival (EFS) to the date of any of the events while on study as defined in the IRIS study; failure-free survival (FFS) was calculated from the start date of therapy to the dates of treatment discontinuation for any reason except of treatment-free remission; transformation-free survival (TFS), to the date of transformation to accelerated or blast phases during study. Patients on low-dose dasatinib who had suboptimal response by ELN 2013 criteria had an option to increase the dose to 100 mg/day as well as decreasing the dose to 20 mg/day in case of significant toxicity.
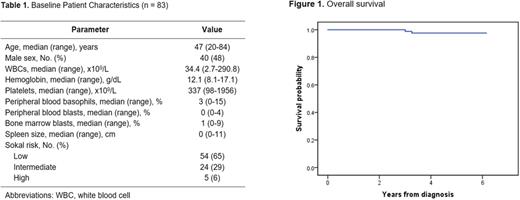
Results: Eighty-three patients were included, with a median age of 47 years (range, 20-84); 48% were males (Table 1). By Sokal risk score, most of the patients had low-risk (65%) or intermediate-risk (29%) disease. Eighty-one patients were evaluable for response. At 3 months, 78 (96%) patients achieved BCR::ABL1/ABL1 ≤10%, and 62 (77%) patients achieved BCR::ABL1/ABL1 ≤1%. The 12-month major molecular response (MMR) rate was 81%. The cumulative incidence of molecular response (MR)4, MR4.5, and complete molecular response (CMR) rates within 1 year were 63%, 53%, and 46%, respectively.
After a median follow-up of 5 years, the 5-year FFS, EFS, TFS and OS rates were 88%, 97%, 100% and 98%, respectively. Treatment was well-tolerated overall; the rates of pleural effusion of any Grade and of Grade 3-4 were 5% and 3%, respectively. Due to safety concerns, 5 (6%) patients had treatment interruption within 12 months of starting therapy and for a median of 10 days (range, 7-22 days). At 12 months, 2 patients had a dose reduction to 20 mg and 40 mg, respectively. The mean daily dose administered was 49 mg.
Conclusions: With longer follow-up, dasatinib 50 mg daily continues to be effective and safe in newly diagnosed CML-CP and therefore should be considered as standard of care.
Disclosures
Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria. Issa:Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding; Novartis, Kura Oncology, Nuprobe: Consultancy. Takahashi:Ostuka Pharmaceuticals: Honoraria; Agios: Consultancy; GSK: Consultancy; Celgene/BMS: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy; Mission Bio: Honoraria; Illumina: Honoraria. Burger:BeiGene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AstraZeneca: Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Bose:Telios: Research Funding; Pharma Essentia: Honoraria; AbbVie: Consultancy; Sierra Oncology (now GSK): Consultancy; Promedior: Research Funding; Karyopharm: Consultancy; Astellas: Research Funding; Disc Medicine: Research Funding; Novartis: Honoraria; BMS: Consultancy, Research Funding; NS Pharma: Research Funding; Pfizer: Research Funding; Ionis: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; Kartos: Research Funding; Cogent: Honoraria, Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; Incyte: Honoraria, Research Funding. Garcia-Manero:Genentech: Honoraria, Research Funding; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Acceleron Pharma: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Astex: Consultancy, Honoraria, Research Funding. Jabbour:Amgen: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Kantarjian:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.