Abstract
Background: Investigations into targeting apoptosis in acute myeloid leukemia (AML) have resulted in breakthrough therapies utilizing Bcl-2 inhibition by venetoclax; however, acquired resistance poses major clinical challenges. Non-apoptotic regulated cell death (RCD) pathways have recently drawn attention especially in solid tumors, but studies targeting these pathways including ferroptosis in leukemia are limited. While ferroptosis is well-defined by iron- and lipid peroxidation-dependency, accumulating evidence suggests that there are also context-specific mechanisms. Since leukemia cells exhibit increased oxidative stress with particular dependency of cell survival on mitochondria, we hypothesized that AML exhibits a unique vulnerability to ferroptosis involving mitochondria. We here study targeting ferroptosis pathway in AML and its mitochondrial (mito-) regulation to develop mechanism-based combination with mito-targeting agents.
Results: We first analyzed DepMap datasets to determine the essentiality of ferroptosis-regulating genes in cancer cell lines. Among major anti-ferroptosis genes, GPX4 exhibits high essentiality prominently in leukemia cells, suggesting its potential as a therapeutic target. AML patient survival data from TCGA revealed that high GPX4 expression is associated with shorter survival, indicating prognostic impact. GPX4 is the most downstream molecule to block ferroptosis by reducing lipid hydroperoxide. Although GPX4 is essential for mouse embryonic development, acquired depletion is reported to have no significant effect on hematopoietic stem cells, suggesting tolerability of GPX4-targeted therapies for normal hematopoiesis.
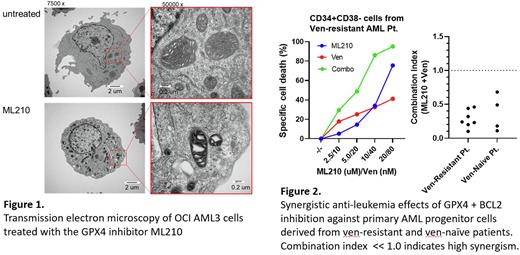
We demonstrate that GPX4 inhibition with doxycycline-inducible shRNA or the specific inhibitor ML210 induces ferroptosis in leukemia cells, evidenced by iron-dependent lipid peroxidation and blockade of cell death by the iron chelator deferoxamine. Of note, the classical ferroptosis inducers (xCT inhibitors) sulfasalazine and sorafenib did not induce ferroptosis in AML cells, suggesting a specific relevance of GPX4 in AML ferroptosis. Consistent with solid tumor cells, AML cells show early (6h post-treatment) morphological alterations in mitochondria characterized by shrinkage and increased membrane density upon GPX4 inhibition (Figure 1). Importantly, and in contrast to most solid tumor studies, mitochondria-targeted antioxidants MitoQ and MitoTEMPO completely blocked ferroptosis, suggesting that AML ferroptosis is regulated predominantly by mitochondrial rather than cytoplasmic redox metabolism. To further determine the role of mitochondria in ferroptosis, we utilized HL60 rho0 cells that exhibit abrogated mito-respiration due to lack of mito-DNA. Surprisingly, HL60 rho0 cells were nearly 10 times more sensitive to ML210 than HL60 parental cells, in clear contrast to sarcoma studies demonstrating similar or lower sensitivity to ferroptosis of mito-DNA deficient cells. Consistently, degradation of the respiratory complex with hyperactivation of mito-protease ClpP by imipridone ONC212 sensitized cells to GPX4 inhibition in HL60 parental cells but not in rho0 cells, further supporting the role of mito-respiration in the protection of cells against ferroptosis. As venetoclax (Ven) is one of the most widely used mitochondria-targeting agents in AML that also inhibits mito-respiration, we tested the combination of GPX4 inhibitor ML210 and Ven. This combinatorial treatment exerts synergistic anti-leukemia effects in AML cell lines and primary cells from AML patients including those with Ven resistance (Figure 2). Unexpectedly, and as a novel mechanism of GPX4 inhibition-mediated ferroptosis, we found that ML210 induces BAX/BAK-independent cytochrome C release from mitochondria, which is blocked by MitoQ. Combination with Ven further enhanced cytochrome C release, as one of potential mechanisms of the observed synergistic cell death.
Conclusion: Our data suggest therapeutic potential of ferroptosis induction in AML by targeting GPX4 with the mechanistic involvement of mito-respiration. The combination of GPX4 and BCL-2 inhibition is synergistic and capable of overcoming Ven resistance. Studies are in progress to elucidate the molecular link between mito-respiration/redox and cytochrome C release and to investigate ferroptosis induction in vivo.
Disclosures
Carter:PinotBio: Research Funding; Syndax: Research Funding; Revolution Medicines: Research Funding. Andreeff:Breast Cancer Research Foundation: Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Senti Bio: Consultancy, Research Funding; AstraZeneca: Research Funding; Kintor Pharmaceutical: Research Funding; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Oxford Biomedical UK: Research Funding; Pinot Bio: Research Funding; Brooklyn ITX: Research Funding; Syndax: Consultancy, Research Funding; Glycomimetics: Consultancy; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Current holder of stock options in a privately-held company; Chimerix: Current holder of stock options in a privately-held company; Reata: Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.