Key Points
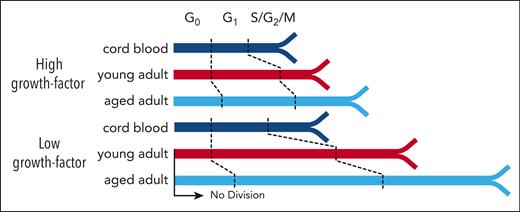
Aging HSCs display a progressive and growth-factor sensitive elongation of G1.
Increased growth factor concentrations are required to stimulate mitogenesis in aging HSCs associated with reduced AKT activation.
Abstract
Human hematopoietic stem cells (HSCs), like their counterparts in mice, comprise a functionally and molecularly heterogeneous population of cells throughout life that collectively maintain required outputs of mature blood cells under homeostatic conditions. In both species, an early developmental change in the HSC population involves a postnatal switch from a state in which most of these cells exist in a rapidly cycling state and maintain a high self-renewal potential to a state in which the majority of cells are in a quiescent state with an overall reduced self-renewal potential. However, despite the well-established growth factor dependence of HSC proliferation, whether and how this mechanism of HSC regulation might be affected by aging has remained poorly understood. To address this knowledge gap, we isolated highly HSC-enriched CD34+CD38−CD45RA−CD90+CD49f+ (CD49f+) cells from cord blood, adult bone marrow, and mobilized peripheral blood samples obtained from normal humans spanning 7 decades of age and then measured their functional and molecular responses to growth factor stimulation in vitro and their regenerative activity in vivo in mice that had undergone transplantation. Initial experiments revealed that advancing donor age was accompanied by a significant and progressively delayed proliferative response but not the altered mature cell outputs seen in normal older individuals. Importantly, subsequent dose-response analyses revealed an age-associated reduction in the growth factor–stimulated proliferation of CD49f+ cells mediated by reduced activation of AKT and altered cell cycle entry and progression. These findings identify a new intrinsic, pervasive, and progressive aging-related alteration in the biological and signaling mechanisms required to drive the proliferation of very primitive, normal human hematopoietic cells.
Introduction
Hematopoiesis is a hierarchically organized process that supports the lifelong production of many short-lived mature blood cells from a small number of cells with extensive self-sustaining ability. The stepwise changes that accompany and regulate this process at the single-cell level are now recognized to be complex and highly variable, even at a single developmental time point or from the same tissue source.1 Aging in mice and humans alike is associated with a decreased output of mature lymphoid and red blood cells, an expansion of clones bearing leukemia-associated mutations (clonal hematopoiesis of indeterminate potential), and increased incidences of hematopoietic malignancies.2-10 It is also well established that in aging mice there is a selective increase in hematopoietic stem cells (HSCs) that produce reduced outputs of mature B and T lymphocytes, despite retention of their repopulating activity and sustained outputs of other mature blood lineages in serial transplants.11-16 Documented cell-intrinsic mechanisms of aging in mice include an observed accumulation of DNA damage, metabolic changes, and epigenetic alterations affecting HSC-specific functions.4 More recent studies of cells from older humans have reported an aging-associated accumulation of cells with surface phenotypes variably enriched in functionally defined HSCs17-20 but with a 5- to 40-fold decreased frequency of cells with mouse repopulating activity within the highly HSC-enriched CD34+CD38−CD45RA–CD90+CD49f+ (CD49f+) phenotype of cells in adult as compared with cord blood (CB) sources.21-26
Label-retention studies in mice have demonstrated that their HSCs are continuously, but infrequently, recruited from a quiescent state into cycle after 4 weeks of age, before which most are rapidly cycling.27-31 A corresponding change in the cycling activity of HSCs in baboons and humans has been inferred to occur within the first 3 years of life based on the rate of telomere shortening exhibited by their short-lived neutrophil progeny.32,33 In addition, the time required for maximally stimulated HSCs from young adult mice to complete a first division in vitro was found to correlate with the longevity of their regenerative activity in vivo,34 although this correlation was not found to extend to HSCs from aged mice.35 In contrast, very little is known about how normal aging affects the properties of human HSCs for which inter-person variability is a major issue, numbers of highly-enriched phenotypes of functionally defined HSCs are much lower, and molecular heterogeneity has been revealed in single-cell analyses.21,23,24,36-40
Here, we report the results of a series of single-cell analyses of the initial kinetics of proliferation of CD49f+ cells and their differentiated cell outputs in vitro and in vivo for cells from normal donors spanning 7 decades. The results reveal a progressive age-associated delay in progressing through the cell cycle coupled with desensitization of these cells to extrinsic mitogenic growth factors (GFs).
Methods
Primary sources of human cells
Anonymized heparinized samples of human blood and bone marrow (BM) samples from donors who were hematologically normal, aged between 0 and 69 years, were obtained and used in experiments with informed consent in accordance with the University of British Columbia Research Ethics Board approved protocols.
Flow cytometry and cell sorting
CD34+ cells were isolated from fresh or thawed cells using EasySep and then suspended in Hanks balanced salt solution (STEMCELL Technologies) supplemented with 5% human serum (Millipore-Sigma) and 1.5 μg/mL antihuman CD32 antibody (clone IV.3; STEMCELL Technologies) and then stained with antibodies (supplemental Table 1, available on Blood website) for 1 to 2 hours on ice before analysis or fluorescence-activated cell sorting (FACS) on a FACSAria Fusion, FACSAria III sorter, or FACSymphony instrument (BD).
Stromal cell–containing cocultures
Individual CD49f+ cells were index sorted into wells containing mixtures of mouse stromal cells producing human GFs supplemented with additional soluble GFs as previously described.23 Cultures were maintained with weekly half-medium changes for 6 to 8 weeks as indicated after which clonal outputs were analyzed visually and when indicated for lymphocytes (B lineage and natural killer cells), neutrophils, and monocytes (NM cells; supplemental Tables 3 and 4). Clones were persistent if they remained until the end of the culture or assigned as transient if they were detectable at earlier time points but not at the end of the culture.
Xenotransplants
FACS-purified CD49f+ cells were transplanted IV (either alone or in combination with 104 green fluorescent protein–labeled [GFP+] CB cells) into sublethally irradiated 12- to 16-week-old female nonobese diabetic (NOD)-Rag1–/–-IL2Rγc–/–-W41/W41 (NRG-W41) mice41 (supplemental Tables 6 and 8). Human BM chimerism was assessed at weeks 4, 8, 12, 16, 20, and 30. Digital droplet polymerase chain reaction (ddPCR) was used to determine the extent to which the progeny of the GFP+ cells might have contributed to the outputs attributed to the nontransduced (GFP–) CD49f+ cell–derived progeny.
Single-cell tracking
Single CD49f+ cells were index sorted into Terasaki plates (Greiner) preloaded with serum-free medium (SFM) containing 10-fold dilutions of 300 ng/mL stem cell factor, 300 ng/mL FMS-like tyrosine kinase 3 ligand, and 60 ng/mL interleukin 3 (IL3) (3GF), and in 1 set of experiments also 400 nM Triciribine (t; Thermo Fisher) or dimethyl sulfoxide (d; Sigma). Cells were tracked over 7 days for survival (high refractility) and incidence of division(s).
Cell-capture and cyclic-immunofluorescence tracking
Freshly isolated CD49f+ or CD34+CD38– cells were sorted either directly into 384-well streptavidin-coated plates (Thermo Fisher) pretreated with 1 μg/mL α-CD44–biotin42 (Biolegend), or transferred after a 24- to 64-hour exposure to SFM + 100% or 1% of the 3GF cocktail and 10 μM 5-ethynyl-2´-deoxyuridine (EdU) in 96-well plates. Cells were then fixed, permeabilized, stained, and fluorescently imaged. Fluorescent antibodies used in the first round of imaging were quenched before subsequent rounds of staining and imaging.43
Phosphoflow cytometry
CD34+ cells were cultured in SFM for 3 hours either without additional GFs or with 3GF added during the last 5, 15, or 30 minutes. Cells were then immediately fixed, permeabilized, and each time point labeled with different concentrations of a Pacific Blue succinimidyl ester dye (Thermo Fisher), after which cell were combined, stained, and analyzed on a FACSymphony.
Data analysis
Data analysis and statistical tests were performed in R using publicly available packages unless otherwise specified. Statistical tests and significance levels are indicated for each data set.
Results
Human CD49f+ cells from CB and adult BM sources display similar clonogenic frequencies in vitro but with an age-associated delay in the kinetics of their outputs
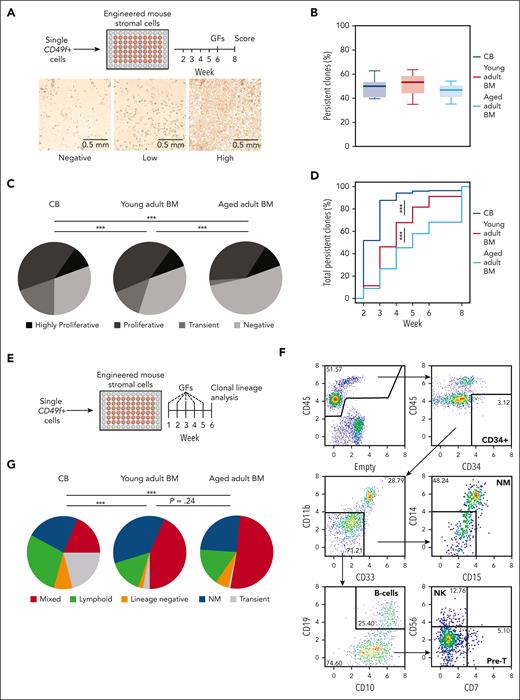
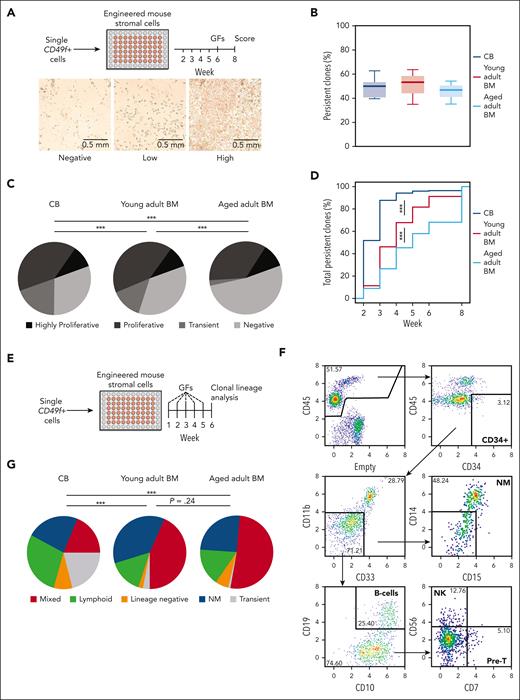
Cell outputs of single CD49f+ cells from CB and adult BM were first assessed over an 8-week period in stromal cocultures that support differentiated NM outputs exclusively (Figure 1A; supplemental Figure 1A). The results showed that ∼50% of the CD49f+ cells from all sources were persistent and produced clones of ≥50 refractile cells detectable at the end of 8 weeks (Figure 1B; pairwise t tests P > .05). However, the CD49f+ cells from the older donors produced fewer early-appearing but ultimately transient clones (pairwise Fisher exact test, P < .001; Figure 1C; supplemental Table 2) and their persistent clones became initially detectable at later time points (Figure 1D; pairwise Komolgerov-Smirnov tests, all P < .0001). Index sorting data confirmed higher levels of CD33, CD34, and CD90 expression on the most proliferative CD49f+ CB cells23 (supplemental Figure 1B). However, this was not maintained on CD49f+ cells from adult sources. Altogether, these findings suggest an age-associated change in mechanism(s) regulating the kinetics of CD49f+ cell–output responses to mitogenic stimuli that support their proliferation and NM outputs.
Sustained long-term and multilineage output potential of CD49f+cells from birth to late adulthood in clonal in vitro assays. (A) Design of the stromal cell coculture system used to measure the 8-week clonal kinetics of NM cell production from individual human CD49f+ cells obtained from differently aged donors. (B) Proportion of individually assessed CD49f+ cells that showed persistent outputs to week 8 based on analyses of 898 cells from 5 CB samples, 538 BM cells from 3 young adult donors, and 539 BM cells from 3 samples of aged adult donors. (C) Distribution of clonal outputs from each donor age group (clone categories: H, highly proliferative; P, proliferative; T, transient; N, negative; ∗∗∗P < .001; Fisher exact test). (D) Cumulative distribution of the time at which each persistent clone first appeared (∗∗∗P < .001; Komoglerov-Smirnov test). (E) Design of the stromal coculture system used to measure the 6-week multilineage (lymphoid and/or NM) cell output potential of individual CD49f+ cells obtained from differently aged donors. (F) Representative gating strategy of indicated lineages present in the 6-week harvests of the multilineage cocultures described in panel E. (G) Average distribution of the 6-week clone types obtained from the different sources of CD49f+ cells tested, based on analyses of 239 cells from 2 CB samples, 240 cells from 2 young adult BM samples, and 240 cells from 2 aged adult BM samples; ∗∗∗P < .001; Fisher exact test.
Sustained long-term and multilineage output potential of CD49f+cells from birth to late adulthood in clonal in vitro assays. (A) Design of the stromal cell coculture system used to measure the 8-week clonal kinetics of NM cell production from individual human CD49f+ cells obtained from differently aged donors. (B) Proportion of individually assessed CD49f+ cells that showed persistent outputs to week 8 based on analyses of 898 cells from 5 CB samples, 538 BM cells from 3 young adult donors, and 539 BM cells from 3 samples of aged adult donors. (C) Distribution of clonal outputs from each donor age group (clone categories: H, highly proliferative; P, proliferative; T, transient; N, negative; ∗∗∗P < .001; Fisher exact test). (D) Cumulative distribution of the time at which each persistent clone first appeared (∗∗∗P < .001; Komoglerov-Smirnov test). (E) Design of the stromal coculture system used to measure the 6-week multilineage (lymphoid and/or NM) cell output potential of individual CD49f+ cells obtained from differently aged donors. (F) Representative gating strategy of indicated lineages present in the 6-week harvests of the multilineage cocultures described in panel E. (G) Average distribution of the 6-week clone types obtained from the different sources of CD49f+ cells tested, based on analyses of 239 cells from 2 CB samples, 240 cells from 2 young adult BM samples, and 240 cells from 2 aged adult BM samples; ∗∗∗P < .001; Fisher exact test.
We then asked if and how aging might affect the timing and cell outputs from single CD49f+ cells in a 6-week stromal coculture system (Figure 1E; supplemental Table 4) that efficiently supports NM and B-lymphoid differentiation from CB CD49f+ cells in ratios similar to the clonal outputs of this population in immunodeficient mice that had undergone transplantation.23 In this second in vitro assay, a high frequency of the CD49f+ cells from all age groups produced persistent clones (28%-55%, supplemental Table 5). However, the frequency of transient clones produced from the oldest donor–derived CD49f+ cells compared with that from CB cells was again markedly reduced (21% vs 1%, Figure 1F-G; supplemental Figure 2A-C; pairwise Fisher exact tests, P < 5 × 10–8). Moreover, persistent clones from the oldest donors of CD49f+ cells also appeared later than those from CB (supplemental Figure 2D; pairwise Komolgerov-Smirnov tests, P = 6.9 × 10–6), also suggesting an age-associated blunting of the mitogenic responsiveness of aging human CD49f+ cells.
Adult BM but not CB CD49f+ cells display enhanced repopulating activity when cotransplanted with CD34+ CB cells
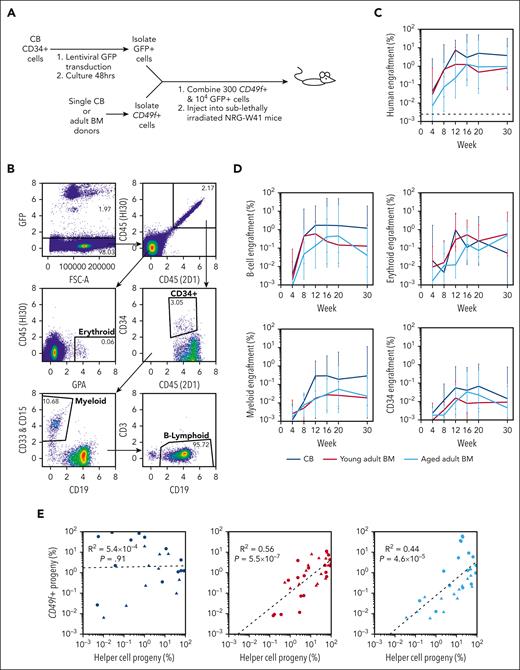
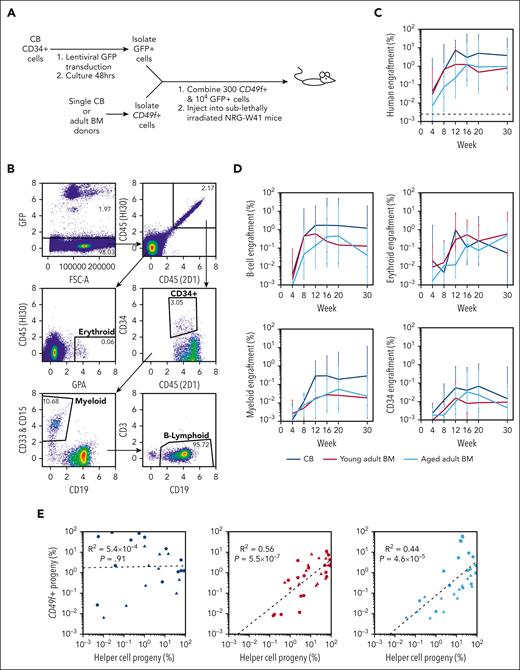
Preliminary transplants of CD49f+ cells from normal CB and adult BM into sublethally irradiated immunodeficient mice (supplemental Table 6) confirmed previously reported lower levels of repopulation by adult sources.22,26 However, the finding of their similar clonogenic frequencies in supportive in vitro systems (Figure 1) suggested that the conventional xenotransplant assay might provide a suboptimal stimulatory environment for eliciting the intrinsic output potential of adult human CD49f+ cells. To test this possibility, we cotransplanted 104 FACS-purified GFP-labeled CB CD34+ cells together with each test inoculum of 300 nontransduced CD49f+ cells to allow distinction of their respective progeny (Figure 2A; supplemental Tables 7 and 8). The contributions of the tested CD49f+ cells to the total detectable human, and separated B-lymphoid, NM, and erythroid lineages, and the CD34+ cell compartment were highly variable and no significant age-associated differences in the ratio of their progeny were detected, although only 2 donors per age group were tested (Figure 2B-D; pairwise t tests P > .05). T cells were not detected in the BM of any of these mice within the first 20 weeks after transplant, although low levels of human T cells were detected in some mice at week 30 after transplant, when the experiments were terminated. The low frequency of emergent GFP− human cells in samples from mice that had received only GFP-transduced cells, and ddPCR analyses of FACS-purified human GFP+ and GFP– cells from mice transplanted with both nontransduced CD49f+ cells and GFP-transduced CB CD34+ cells indicated that the outputs attributed to the CD49f+ cells contained negligible contamination from the cotransplanted GFP+ CB cells (supplemental Figure 2B-F).
Similar in vivo repopulating activity of neonatal and adult CD49f+cells when cotransplanted with CD34+CB “helper” cells. (A) Design of a cotransplantation strategy to compare the regenerative potential of CD49f+ cells isolated from donors of different ages. (B) Flow-cytometric analysis of the cells in a representative BM aspirate obtained from a mouse that had undergone cotransplantation 12 weeks earlier with CB CD49f+ cells and GFP-labeled CD34+ CB cells. (C-D) Percentage of human CD49f+ cell (GFP−) (C), and NM, B-lymphoid, erythroid, and CD34+ progeny (D) from input CB, young adult BM, or aged adult BM measured in the total number of viable cells present in the BM aspirate obtained at each time point assessed. Values indicate the geometric mean of pooled values from all mice in each group assessed in 2 independent experiments (2-3 mice per donor per experiment). Bars indicate the geometric means ± 1 standard error of the mean (SEM). (E) Linear regression analyses of CD49f+-progeny (GFP−) and GFP+ chimerism levels for CB (left), young adult BM (center), and aged adult BM (right). Points indicate paired results within each individual mouse at each different time point assessed after transplant. Symbols indicate mice that had undergone transplantation with different donors of the CD49f+ cells tested.
Similar in vivo repopulating activity of neonatal and adult CD49f+cells when cotransplanted with CD34+CB “helper” cells. (A) Design of a cotransplantation strategy to compare the regenerative potential of CD49f+ cells isolated from donors of different ages. (B) Flow-cytometric analysis of the cells in a representative BM aspirate obtained from a mouse that had undergone cotransplantation 12 weeks earlier with CB CD49f+ cells and GFP-labeled CD34+ CB cells. (C-D) Percentage of human CD49f+ cell (GFP−) (C), and NM, B-lymphoid, erythroid, and CD34+ progeny (D) from input CB, young adult BM, or aged adult BM measured in the total number of viable cells present in the BM aspirate obtained at each time point assessed. Values indicate the geometric mean of pooled values from all mice in each group assessed in 2 independent experiments (2-3 mice per donor per experiment). Bars indicate the geometric means ± 1 standard error of the mean (SEM). (E) Linear regression analyses of CD49f+-progeny (GFP−) and GFP+ chimerism levels for CB (left), young adult BM (center), and aged adult BM (right). Points indicate paired results within each individual mouse at each different time point assessed after transplant. Symbols indicate mice that had undergone transplantation with different donors of the CD49f+ cells tested.
We then asked whether the cotransplanted GFP+ CB cells had differentially influenced the outputs of the CD49f+ cells from any of the donor sources tested (Figure 2E). Despite the limited donors compared, analyses showed a significant positive correlation exclusively between the outputs attributable to the input CD49f+ cells from the young and aged adult donors and the progeny of the cotransplanted GFP+ CB cells but not for the CD49f+ CB cells.
CD49f+ cells display a progressive age-related delay in their GF-stimulated division kinetics in vitro
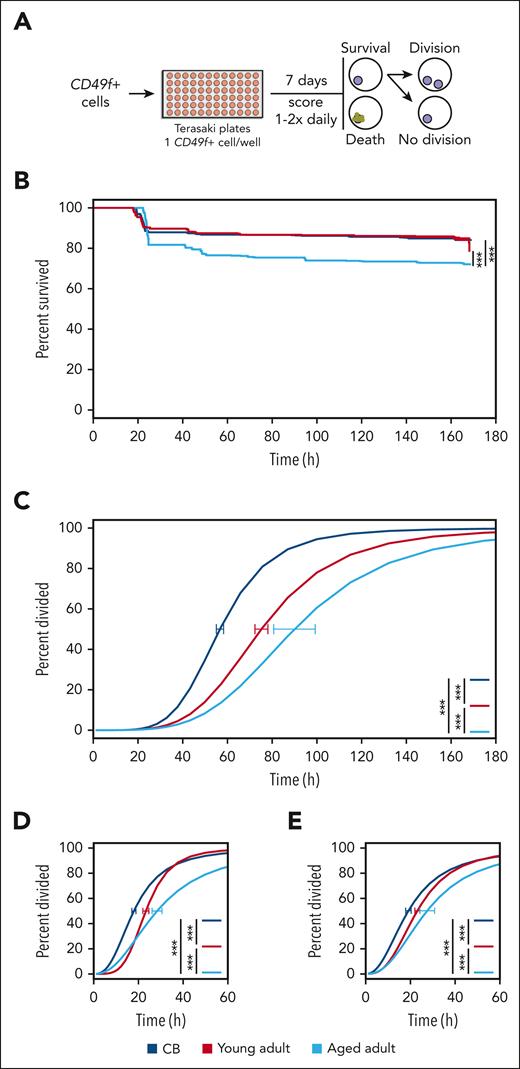
Next, we asked whether the delayed clone development in vitro exhibited by CD49f+ cells from adult donors (Figure 1D) would be evident in their initial mitogenic responses. Accordingly, we tracked the behaviors of single CD49f+ cells for 7 days in SFM + 3GF (Figure 3A; supplemental Table 9). All sources of these cells showed high levels of survival, with those from CB and the young adults being slightly higher than those from the aged adults (85% vs 72%, log-rank test P < .001; Figure 3B; supplemental Figure 4A). However, the time preceding the first division of the viably maintained cells demonstrated a highly significant and progressive increase with increasing donor age (increasing from 57 to 90 hours; Figure 3C; supplemental Figure 4B; all pairwise Holm-corrected, P < 1 × 10–23). Notably, this age-associated delay in the initial mitogenic response of isolated CD49f+ cells was not restricted to the first division but was followed by similarly lengthened times to complete a second and third division (Figure 3D-E). Least absolute shrinkage and selection operator (LASSO) regression models from the initial index sorted data showed that only CD38 and cell-size (forward-scatter area) values were consistently negatively associated with the first division timing across all examined groups (supplemental Figure 4C). Taken together, these findings are consistent with the expectation of an early postnatal alteration in the cycling dynamics of HSCs27,32,33,44,45 and suggest progressive aging-associated alterations to the regulation of their cell cycle kinetics.
GF-stimulated CD49f+cells display a progressive age-related delay in vitro. (A) Design of the culture system used to compare the survival and cell division kinetics of CD49f+ cells isolated from differently aged donors over 7 days of culture. (B) Kaplan-Meier curves of CD49f+ cell survival in the cultures described in panel A (log-rank test, ∗∗∗P < .001). (C) Weighted dose-response curves describing the completion of a first division of the different sources of CD49f+ tested. Curves reflect only CD49f+ cells that remained viable past a first division, or until the end of the assay without dividing. Error bars are indicated at the median point of each curve (CB = 57 hours, young adult = 75 hours, aged adult = 90 hours) and indicate the range defined by ± 1 SEM of this estimate (∗∗∗P < .001). Time required for clones to complete a second (D) or third (E) division after completing their previous division (∗∗∗P < .001).
GF-stimulated CD49f+cells display a progressive age-related delay in vitro. (A) Design of the culture system used to compare the survival and cell division kinetics of CD49f+ cells isolated from differently aged donors over 7 days of culture. (B) Kaplan-Meier curves of CD49f+ cell survival in the cultures described in panel A (log-rank test, ∗∗∗P < .001). (C) Weighted dose-response curves describing the completion of a first division of the different sources of CD49f+ tested. Curves reflect only CD49f+ cells that remained viable past a first division, or until the end of the assay without dividing. Error bars are indicated at the median point of each curve (CB = 57 hours, young adult = 75 hours, aged adult = 90 hours) and indicate the range defined by ± 1 SEM of this estimate (∗∗∗P < .001). Time required for clones to complete a second (D) or third (E) division after completing their previous division (∗∗∗P < .001).
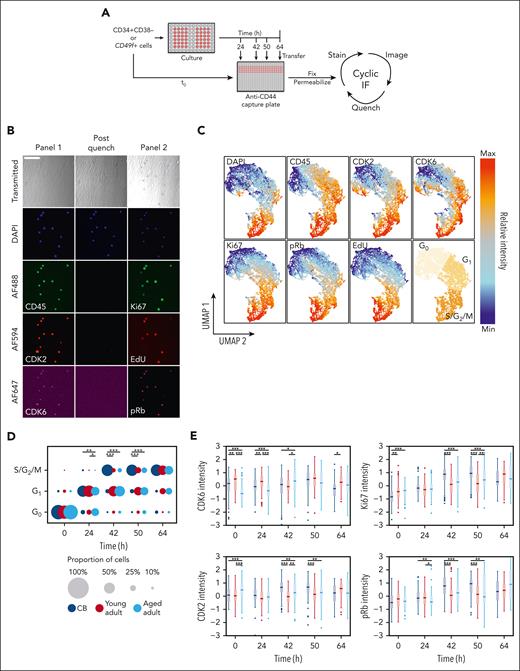
CD49f+ cells display a progressive age-related elongation of their G1 phase
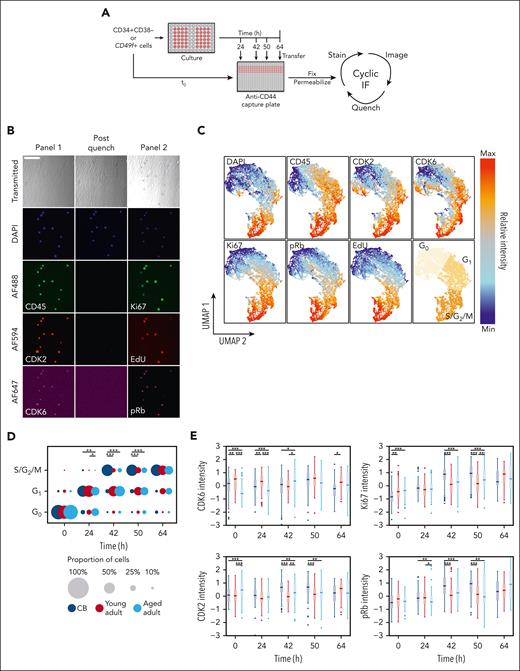
To examine which cell cycle phase(s) in adult CD49f+ cells were affected, we used a cell-capture cyclic immunofluorescence approach42,43 that allowed time-dependent changes in 7 features to be quantified over several measurements on the same individual cells (ie, their DNA content and levels of CD45, CDK2, CDK6, Ki67, pRb, and EdU). This assessment was performed on a combined total of >10 000 cells from 3 samples each of CB, young adult BM, and aged adult BM (supplemental Table 10) cultured in SFM supplemented with high (100%) or low (1%) concentrations of 3GF (Figure 4A-B). This method retained >80% of the initially analyzed cells after 2 additional rounds of imaging and analysis (supplemental Figure 5A-C). Visualization of the cell cycle progression kinetics of the tracked cells was then achieved by reducing the fluorescence data to a 2D format using the UMAP algorithm46,47 and separating the data into 3 regions using K-means clustering representative of different phases of the cell cycle (Figure 4C).
Paired cell cycle state and molecular analysis of individually tracked primitive human hematopoietic cells reveal an age-associated prolongation of G1in the adult cells. (A) Experimental design to enable the in vitro serial immunofluorescence tracking of changes in the properties of individual cells isolated from donors of different ages over time. (B) Representative fluorescence images obtained from the experimental design described in panel A; scale bar = 100 μm. (C) UMAP distribution of different phenotypic properties of individually assessed CD49f+ and CD34+CD38– cells from CB and young or aged adult BM cultured for 0, 24, 42, 50, or 64 hours and analyzed as described in panel A. Assignment of regions of the UMAP distribution to the different phases of the cell cycle by K-means clustering (bottom-right). (D) Proportion of CD49f+ cells in different phases of the cell cycle after different times in culture. The size of each circle is scaled to show the proportion of total cells in each cell cycle phase at the culture time point when the assessment was made (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests). (E) Scaled intensity (Z scores) of CDK6, CDK2, Ki67, and pRb protein levels measured in the CD49f+ cells analyzed at each time point shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests).
Paired cell cycle state and molecular analysis of individually tracked primitive human hematopoietic cells reveal an age-associated prolongation of G1in the adult cells. (A) Experimental design to enable the in vitro serial immunofluorescence tracking of changes in the properties of individual cells isolated from donors of different ages over time. (B) Representative fluorescence images obtained from the experimental design described in panel A; scale bar = 100 μm. (C) UMAP distribution of different phenotypic properties of individually assessed CD49f+ and CD34+CD38– cells from CB and young or aged adult BM cultured for 0, 24, 42, 50, or 64 hours and analyzed as described in panel A. Assignment of regions of the UMAP distribution to the different phases of the cell cycle by K-means clustering (bottom-right). (D) Proportion of CD49f+ cells in different phases of the cell cycle after different times in culture. The size of each circle is scaled to show the proportion of total cells in each cell cycle phase at the culture time point when the assessment was made (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests). (E) Scaled intensity (Z scores) of CDK6, CDK2, Ki67, and pRb protein levels measured in the CD49f+ cells analyzed at each time point shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests).
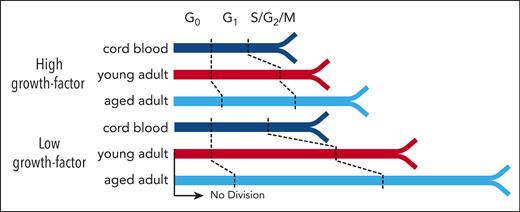
The proportion of cells in each of these regions was then determined at each time point for each culture condition analyzed to compare the cell cycle progression of CD49f+ and CD34+CD38− cells from all 3 sources (Figure 4D; supplemental Figure 5D; supplemental Table 11). As expected from their initial quiescent state,48,49 the majority of the freshly isolated CD49f+ and CD34+CD38– cells (supplemental Figure 6A) were in the G0 region of the UMAP distribution. However, within 24 hours of exposure to the 100% 3GF cocktail, ∼70% of all the CB and young adult BM CD49f+ cells but only 50% of aged adult BM cells had transitioned into G1. After 42 hours, the majority of CB CD49f+ cells had progressed into S/G2/M phase. In contrast, most of the cells obtained from young or aged adults were still in G1 (Holm-corrected Wilcox tests, P = 1.7 × 10–9 and P = 2.9 × 10–10, respectively) in which they remained for at least another 8 hours (Holm-corrected Wilcox tests, P = 5.7 × 10–6 and P = 7.3 × 10–7; Figure 4D). Baseline levels of CDK6 were markedly lower in the aged adult BM cells (Holm-corrected Wilcox tests P < 1.4 × 10–13) and remained lower for 24 hours. Levels of Ki67, pRb, and CDK2 (a regulator of the G1 phase to S phase transition), were all lower in the adult CD49f+ cells after 42 to 50 hours (Figure 4E). However, after 64 hours in culture, most of the adult CD49f+ cells had transitioned into S/G2/M, indicative of a total prolongation of their first cell cycle transit time of ∼14 to 22 hours, in agreement with our independent direct visualization experiments (Figure 3C).
Taken together, these results indicate that the prolonged first division timing in young adult CD49f+ cells compared with CB CD49f+ cells can be explained primarily by an increased duration of G1 rather than an increased time required to exit G0. However, CD49f+ cells from aged donors remained in G0 and G1 for prolonged periods of time. Interestingly, similar altered cell cycle progression kinetics were exhibited by the CD34+CD38– cells from the same donors (supplemental Figure 6), suggesting the aging-related prolonged cell cycle transit features persist into the more differentiated cells present in the CD34+CD38– subset.
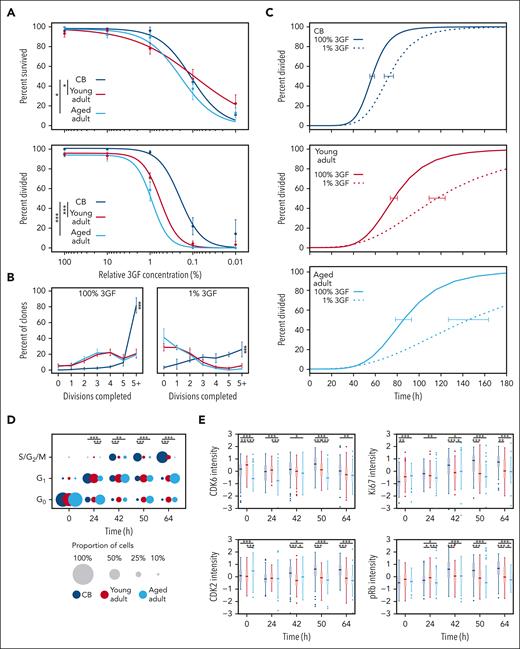
Exposure to reduced GF concentrations exaggerates the age-related elongation of G1 in CD49f+ cells
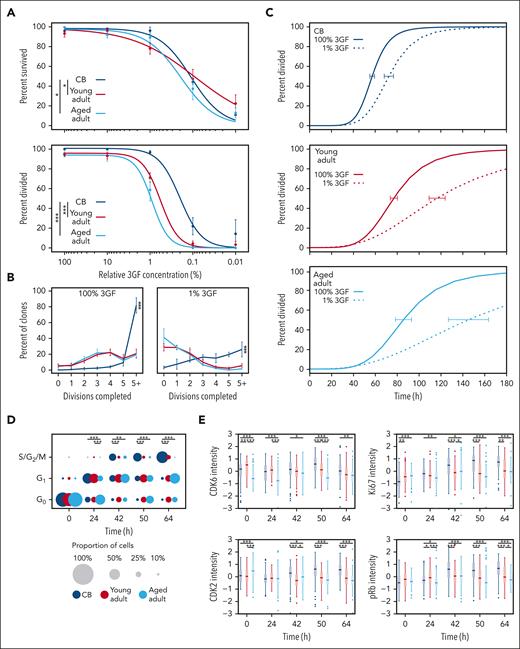
Given that progression into, and through, G1 relies on mitogenic stimulation,50-52 we examined how reduced GF stimulation impacts CD49f+ cell proliferation. This showed that CD49f+ cell survival was maintained at high levels (>75% of maximum) for all cell sources, even at the low concentration of 1% 3GF cocktail, but then steeply decreased thereafter (Figure 5A top panel; supplemental Figure 7A). In contrast, the 3GF concentration required to stimulate CD49f+ cells to divide at least once within 7 days was 3 to 5 fold higher for adult cells than for CB cells (CB vs young adult, P = 1.7 × 10–5; and CB vs aged adult, P = 1.5 × 10–17; Figure 5B bottom panel), although the difference between young and aged adult CD49f+ cells was not significant (P = .13). The clone sizes measured after 7 days were heterogeneous for all sources of CD49f+ cells, but the CB-derived clones were consistently larger than those derived from the adults (Holm-corrected pairwise Kruskal-Wallis tests, P < 1 × 10–17; Figure 5B). Furthermore, the CD49f+ cells that were able to divide in response to 1% 3GF demonstrated progressive age-related delays in their timing to complete a first division compared with that exhibited in response to 100% 3GF (ie, delayed by 1.3 fold in CB CD49f+ cells extending to 1.5 fold and 1.7 fold for the young and aged CD49f+ cells, respectively; Figure 5C; supplemental Figure 7B).
CD49f+cells show an aging-related increase in their 3GF concentration requirement to stimulate their division. (A) Weighted 3GF dose-response curves for CD49f+ cell survival (top) and proliferation (bottom) monitored over a 7-day period in vitro as described in Figure 3A. Proliferation curves are normalized to cells that survived either past a first division or for 7 days without dividing. Points indicate the mean ± 1 SEM of the survival and proliferation responses of each group of cell types assessed (∗P < .05; ∗∗∗P < .001). The 50% effective dose concentrations were interpolated from the fitted curves (survival: CB = 0.11%, young adult = 0.09%, aged adult = 0.19%; proliferation: CB = 0.2%, young adult = 0.6%, aged adult = 0.9%). (B) Percent of clones that completed the indicated number of divisions by day 7 at 100% 3GF (left) or 1% 3GF (right). Lines connect the observed mean ± 1 SEM of the proportion of cells from each donor source that had completed the indicated number of divisions within 7 days. (∗∗∗P < .001; pairwise Kruskal-Wallis tests). (C) Kinetics of 3GF-stimulated timing of a first division of CD49f+ cells from different sources exposed to 100% (solid lines) or 1% (dotted lines) 3GF cocktail (CB: 100% and 1% 3GF, 57 hours and 72 hours, respectively; young adult: 100% and 1% 3GF, 77 hours and 116 hours, respectively; aged adult: 100% and 1% 3GF, 85 hours and 145 hours, respectively). Error bars drawn at the median value of each curve indicate the range defined by ± 1 SEM of this estimate for each sample type. (D) Proportion of CD49f+ cells cultured in 1% 3GF in different phases of the cell cycle assessed at different times. The size of each circle represents the proportion of total cells in each phase at the time shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests). (E) Scaled intensity (Z scores) of CDK6, CDK2, Ki67, and pRb protein levels found in the CD49f+ cells analyzed (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests).
CD49f+cells show an aging-related increase in their 3GF concentration requirement to stimulate their division. (A) Weighted 3GF dose-response curves for CD49f+ cell survival (top) and proliferation (bottom) monitored over a 7-day period in vitro as described in Figure 3A. Proliferation curves are normalized to cells that survived either past a first division or for 7 days without dividing. Points indicate the mean ± 1 SEM of the survival and proliferation responses of each group of cell types assessed (∗P < .05; ∗∗∗P < .001). The 50% effective dose concentrations were interpolated from the fitted curves (survival: CB = 0.11%, young adult = 0.09%, aged adult = 0.19%; proliferation: CB = 0.2%, young adult = 0.6%, aged adult = 0.9%). (B) Percent of clones that completed the indicated number of divisions by day 7 at 100% 3GF (left) or 1% 3GF (right). Lines connect the observed mean ± 1 SEM of the proportion of cells from each donor source that had completed the indicated number of divisions within 7 days. (∗∗∗P < .001; pairwise Kruskal-Wallis tests). (C) Kinetics of 3GF-stimulated timing of a first division of CD49f+ cells from different sources exposed to 100% (solid lines) or 1% (dotted lines) 3GF cocktail (CB: 100% and 1% 3GF, 57 hours and 72 hours, respectively; young adult: 100% and 1% 3GF, 77 hours and 116 hours, respectively; aged adult: 100% and 1% 3GF, 85 hours and 145 hours, respectively). Error bars drawn at the median value of each curve indicate the range defined by ± 1 SEM of this estimate for each sample type. (D) Proportion of CD49f+ cells cultured in 1% 3GF in different phases of the cell cycle assessed at different times. The size of each circle represents the proportion of total cells in each phase at the time shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests). (E) Scaled intensity (Z scores) of CDK6, CDK2, Ki67, and pRb protein levels found in the CD49f+ cells analyzed (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Holm-corrected Wilcox tests).
To determine the specific phases of the cell cycle affected by culture in 1% 3GF, the proportions of cells from all 3 sources of CD49f+ cells present in the different regions of the 2D UMAP distribution were examined (Figure 4C) and compared (Figure 5D; supplemental Table 12). This showed that the majority of young adult CD49f+ cells (like their CB counterparts) cultured in 1% 3GF entered G1 within 24 hours but, unlike the CB CD49f+ cells, had not transitioned into S/G2/M even after 64 hours. Under the same conditions, the aged adult CD49f+ cells also showed a delayed entry into, as well as out of, G1. The extended G1 in the adult cells was reflected by reduced levels of CDK2, pRb, and Ki67, between 42 and 64 hours (Figure 5E). In addition, after 64 hours, approximately one-third of the young and aged adult CD49f+ cells continued to remain in G0 compared with only approximately one-tenth of the CB CD49f+ cells (Figure 5D), the majority of which would be predicted to remain undivided for at least 7 days (Figure 5A, bottom panel). Similar trends were again seen in the coexamined CD34+CD38– subset but more of the adult cells transitioned into S/G2/M within 64 hours (supplemental Figure 7C-D). Taken together, these findings indicate that a reduced 3GF stimulus, which can maintain the viability of a range of CB and adult primitive human hematopoietic cells, is differentially sufficient to induce their exit from G0 and passage through G1 in an age- and state-specific fashion.
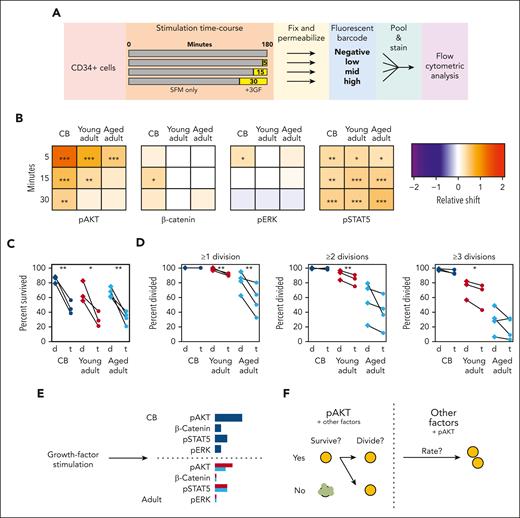
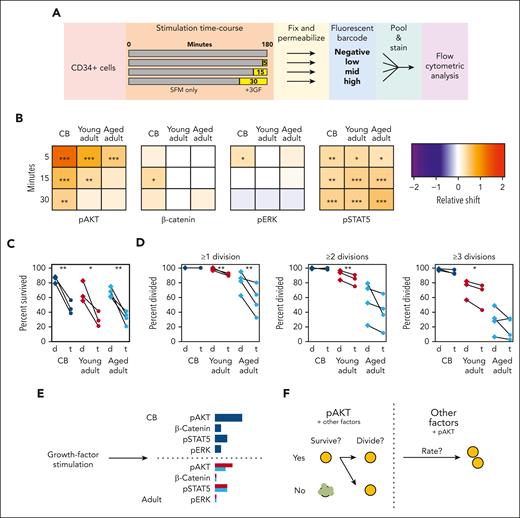
Adult CD49f+ cells display selectively reduced activation of AKT by 3GF
To explore the mechanistic basis of these aging-associated GF-mediated effects on cell cycle progression, we examined several signaling responses previously shown to be activated by a similar GF cocktail (Figure 6A).38 The relative median shift of specific signaling pathway intermediates at each stimulation time point was compared with that of the corresponding unstimulated fraction contained within the same analyzed sample (supplemental Figure 8A-B). CD49f+ cells from all sources displayed robust activation of AKT and STAT5 from 3GF stimulation (Figure 6B; supplemental Figure 8C-D; bootstrapped P values < .05). However, the activation of the AKT response in the CD49f+ cells was consistently reduced and shorter in the adult cells, with a continued decline between the young and aged adult cells (similar AKT responses were also seen in the CD34+CD38–CD45RA– fraction; supplemental Figure 8E). Activation of ERK1/2 and β-catenin was also observed but only in CB cells within the times assessed.
AKT signaling drives the 3GF-activated survival and division of primitive human hematopoietic cells. (A) Experimental design used to compare the 3GF-stimulated time course of signaling protein activation assessed by flow cytometric analysis. (B) Median relative shift in the intensity of the indicated marker from the corresponding unstimulated sample in CD49f+ cells exposed to 3GF for different times. Asterisks indicate significant differences from the unstimulated samples (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; bootstrapped probabilities). Paired survival (C) and proliferation (D) effects of dimethyl sulfoxide (d)- or triciribine (t)-mediated inhibition of AKT activation. (E) Model of CD49f+ pathway activation after short-term 3GF stimulation. The length of the colored bars indicates the relative strength of activation of the indicated marker. CB responses are shown in navy, young adult in red, and aged adult in teal. (F) A model of the role of AKT signaling and other factors in regulating different stages of primitive human hematopoietic cell survival and proliferation.
AKT signaling drives the 3GF-activated survival and division of primitive human hematopoietic cells. (A) Experimental design used to compare the 3GF-stimulated time course of signaling protein activation assessed by flow cytometric analysis. (B) Median relative shift in the intensity of the indicated marker from the corresponding unstimulated sample in CD49f+ cells exposed to 3GF for different times. Asterisks indicate significant differences from the unstimulated samples (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; bootstrapped probabilities). Paired survival (C) and proliferation (D) effects of dimethyl sulfoxide (d)- or triciribine (t)-mediated inhibition of AKT activation. (E) Model of CD49f+ pathway activation after short-term 3GF stimulation. The length of the colored bars indicates the relative strength of activation of the indicated marker. CB responses are shown in navy, young adult in red, and aged adult in teal. (F) A model of the role of AKT signaling and other factors in regulating different stages of primitive human hematopoietic cell survival and proliferation.
To further test the biological importance of this differentially reduced activation of AKT in primitive cells from donors of increasing age, we compared the effect of Triciribine (an AKT inhibitor) on CB and adult CD49f+ cells cultured in SFM + 10% 3GF (supplemental Table 15). This showed strong but relatively equal reductions in the survival of all 3 sources (Figure 6C, paired t tests, P < .05). However, only the surviving adult CD49f+ cells showed a decreased ability to divide (Figure 6D, paired t tests, P < .05). However, the time taken to complete a first division was only marginally extended (1.1-fold, P < 1.0 × 10–4, CB and young adult; 1.2-fold, P = 4.6 × 10–3, aged adult) in all groups with subsequent division cycles also minimally extended (supplemental Figure 9). Taken together, these results point to a selectively pronounced aging effect on the control of AKT signaling in GF-activated human HSCs that differentially affects their proliferative responses.
Discussion
This study presents new evidence of a negative effect of aging on the intrinsic mitogenic response properties of the CD49f+ subset of CD34+ human hematopoietic cells, a phenotype shown to highly enrich for cells with long-term in vivo repopulating activity.21,23-25 A key result was the progressively increased donor age–associated delay in the ability of these initially quiescent cells to enter and advance through the cell cycle under a wide range of stimulatory conditions. These conditions included several in vitro systems with and without stromal cells that allowed the detection of only mature NM cells or also erythroid and B-lineage outputs from single input cells. Evidence of a reduced GF responsiveness of the CD49f+ cells from both young and aged adult human donors was also suggested by the selective dependence of their 30-week repopulating activity on a coinjected transplant of CD34+ CB cells.
Interestingly, in both the clonal in vitro assays and bulk in vivo assays able to detect both B-lymphoid and NM potential, it was surprising to detect no evidence of the relative reduction in B-lymphoid output reported in previous studies on aging mice11-13,35,53 and humans.18,20 This discrepancy could have several explanations. First, in this study, the cells analyzed were obtained from only 2 samples per age group, with the adult samples originating from BM harvests rather than the CB comparator. Second, the cell outputs examined in the mice that had received transplantation were assessed under more supportive conditions found to be selectively beneficial for adult CD49f+ cells achieved using a cotransplant of CD34+ CB cells. In this regard, it is interesting to note that long-term repopulating mouse cells that exhibit only a single mature lineage (platelets) long term in vivo, can be stimulated to express a multilineage output rapidly in vitro when appropriately stimulated.54 Similarly, lymphoid outputs in vivo have been shown to be influenced by extrinsic factors.55,56 Future efforts to examine the variability of CD49f+ cells from different donors of the same age groups and different lineage output assay conditions should help to discriminate the relevance of these different factors.
The age-related delays in CD49f+ cell progeny output in the stromal coculture experiments described here were accompanied by a highly apparent delayed timing of the first and subsequent divisions of the initially quiescent CD49f+ cells. Taken together, these results suggest an intrinsically determined delay in their GF response mechanism that is established during the aging process and sustained through several division cycles, even as the cells begin to differentiate. Use of a cell-capture and multiplexed molecular phenotyping strategy42,43 revealed this age-related delay to be because of prolonged G1 in both young and aged adult cells and a preceding G0 exit delay observed in only the aged adult cells. The increased baseline levels of CDK6 in the young adult CD49f+ cells suggest differential priming of these cells to exit G0 in response to a mitogen-induced program, because this protein has been demonstrated to affect the kinetics of G0/G1 transition in human HSCs.48 However, baseline CDK6 levels were lower in the aged adult cells than in either the CB or young adult cells, likely contributing to their delayed exit from G0. CDK2 levels also remained lower for at least 50 hours in adult CD49f+ cells, suggesting that the G1 delay occurs before CDK2 activation that typically follows phosphorylation of Rb by CDK4/6 with cyclin D and inhibition of CDK inhibitors, including p21, p27, and p15.50,51,57
This delayed proliferation phenotype was selectively exaggerated under a reduced, nonsaturating GF stimulus, with many adult cells remaining in G1 for at least 26 hours and an increased proportion failing to exit G0, resulting in approximately one-third of adult cells remaining viable but undivided for 7 days. These findings suggest a rheostat mode of GF control of both the G0-to-G1 and G1-to-S phase transitions and an aging-associated increase in the stimulation-dependent signaling events required to promote these transitions. In agreement with this model, we found that AKT activation was progressively reduced in both adult CD49f+ cells and CD34+CD38–CD45RA– cells compared with their CB counterparts after a strong mitogenic stimulus (Figure 6E). Similarly, inhibition of AKT activity equally reduced survival across the different sources but only reduced the ability of adult CD49f+ cells to complete ≥1 divisions with overall lesser impacts on the rate at which divisions were completed. These findings suggest that AKT signaling responses control 2 key activities. The first is whether or not the cell will survive, and the second is whether the cell will proceed into cycle or remain quiescent. The relatively small impact on division timing observed in cells with selectively inhibited AKT suggests that AKT signaling contributes to, but is not solely responsible for, the overall extended proliferation kinetics observed between the different age groups, with the differential activity of other factors then providing a larger impact (Figure 6F). Recent studies have also demonstrated the impact of ERK signaling dynamics and metabolic activation on the immediate progeny of GF-stimulated HSCs, suggestive of a connected mechanism of regulation.58-60
Importantly, these findings do not preclude additional delays at other stages of cell cycle progression control, including other points within G1. Indeed, the finding of decreased minichromosome maintenance helicase levels in aged mouse HSCs compared with those of younger adult mice, as well as the increased replication stress experienced by these cells during S phase, suggest that multiple points of cell cycle progression may be affected.35 A recent report of altered Cdc42 activity as a potential contributor to age-related proliferative delays in primitive human hematopoietic cells adds further weight to this possibility.17
Taken together, the demonstration here of a profound and progressive postnatal decline in GF sensitivity of very primitive normal human hematopoietic cells attributed, at least in part, to altered AKT signaling could be relevant to many aspects of human hematopoiesis. These include not only potential effects of aging on mature blood cell outputs,2-5,7,8 differential induction of long-term repopulating activity under conditions that promote regenerative responses vs established homeostasis, and the ability of clonal hematopoiesis of indeterminate potential clones to gain ascendancy with increasing age.6
Acknowledgments
The authors thank M. Hale, G. Edin, D. Wilkinson, and the staff of the British Columbia Cancer Agency Stem Cell Assay Laboratory for technical assistance, including the acquisition and some of the initial processing of CB, mPB, and BM samples.
This work was supported by subawards to C.J.E. from the Terry Fox Foundation Program Project grant #1074 and the Canadian Cancer Society grant #705047. C.A.H. held a CIHR Frederick Banting and Charles Best Doctoral Scholarship and a UBC Graduate Fellowship.
Authorship
Contribution: C.A.H. and C.J.E. designed the experiments and wrote the manuscript; S.W.W. performed the GFP and Y-chromosome ddPCRs and assisted with other experiments; F.W. assisted with the multilineage coculture experiments; M.E.M. assisted with the AKT inhibition experiments; C.A.H. performed experiments, all data analysis, and figure preparation; and all authors read and approved this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Connie J. Eaves, British Columbia Cancer Research Institute, Terry Fox Laboratory 675 W. 10th Ave, Vancouver, British Columbia V5Z 1L3 Canada; e-mail: ceaves@bccrc.ca.
References
Author notes
Data are available on request from the corresponding author, Connie J. Eaves (ceaves@bccrc.ca).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement”in accordance with 18 USC section 1734.