In this issue of Blood, Gu et al dissect the different role of phosphotidylinositol 3-kinase (PI3K) isoforms in acute myeloid leukemia (AML) and identify a critical function of PI3Kγ in regulation of the pentose phosphate pathway in leukemia stem cells (LSCs).1 LSCs are generally accepted as the reservoir for AML relapse, and curative therapy in AML may require eradication of the LSC compartment. Although this is sometimes achievable using intensive chemotherapy with or without an allogeneic stem cell transplant, most patients with AML relapse and succumb to their disease. Selective targeting of LSCs remains elusive as many stem cell–directed therapies also impair normal hematopoietic stem cell (HSC) function.
Activation of class I PI3Ks and their downstream pathways (eg, AKT, mammalian target of rapamycin complex 1) is frequently observed in primary human AML specimens.2-4 Of the class I PI3K isoforms, α and β are ubiquitously expressed, whereas δ and γ are only expressed in leukocytes. However, the specific isoform dependencies of PI3K for LSCs are unknown.
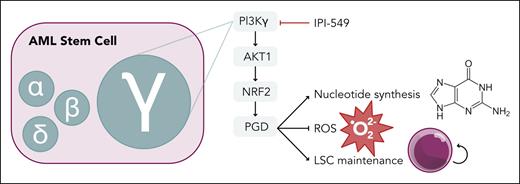
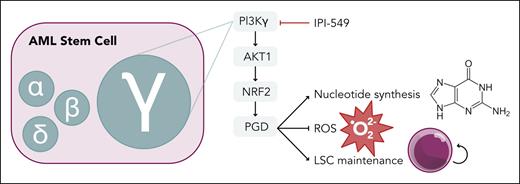
Gu et al thoroughly dissect the contribution of each class I PI3K isoform in the histone lysine N-methyltransferase 2A (KMT2A, referred to as MLL)-rearranged, Mll-Af9, mouse model of AML. They found high expression of the gene encoding PI3Kγ, Pik3cg, in the LSC compartment compared with normal HSCs. Genetic ablation of Pik3cg led to improved survival in primary and secondary murine transplants, demonstrating a critical role of PI3Kγ in disease initiation and LSC maintenance (see figure). This was confirmed in an alternative mouse model using the Aml1-Eto9a driver, AML cell lines, and multiple patient-derived xenograft models. More important, transplantation experiments with nonleukemic marrow from Pik3cg knockout mice showed normal repopulating ability. This indicates a unique function of PI3Kγ in LSCs and not normal HSCs, providing a therapeutic window for PI3Kγ targeting in AML. In contrast, genetic inhibition of the α, β, and δ isoforms did not have an impact on Mll-Af9 leukemic burden or survival. A recent abstract suggested a role of PI3Kα in LSC function using the same Mll-Af9 model with Pi3kca-null cells.5 This discrepancy may be due to the degree of Pi3kca ablation (knockdown vs knockout). Thus, the precise role of PI3Kα in LSCs needs further clarification.
AML stem cell dependency on PI3Kγ isoform through an AKT1-NRF2-PGD pathway. PGD supports nucleotide synthesis in the pentose phosphate pathway and suppresses ROS production to promote LSC maintenance. Professional illustration by Somersault18:24.
AML stem cell dependency on PI3Kγ isoform through an AKT1-NRF2-PGD pathway. PGD supports nucleotide synthesis in the pentose phosphate pathway and suppresses ROS production to promote LSC maintenance. Professional illustration by Somersault18:24.
Mechanistically, loss of Pik3cg led to downregulation of genes involved in the pentose phosphate pathway (PPP). Reduced expression of phosphogluconate dehydrogenase (PGD) in Pi3kcg-null LSCs led to decreased PPP metabolites, decreased nucleotide synthesis, decreased reduced NAD phosphate, and accumulation of reactive oxygen species (ROS). Elevated ROS levels are a hallmark of the loss of LSC potential.6 More important, exogenous expression of downstream components of the PI3Kγ pathway (Akt1, nuclear factor erythroid 2–related factor 2, or Pgd) was sufficient to counteract these metabolic derangements and restore the leukemic potential of Pi3kcg-null Mll-Af9 cells. Anti-leukemic effects were seen with the selective PI3Kγ inhibitor, IPI-549, in Mll-Af9 leukemia and primary human AML specimens in vitro and in vivo. Limited mutational data were available for the primary AML specimens, but the effect of IPI-549 was not restricted to MLL-rearranged leukemia. IPI-549 significantly improved survival for the aggressive leukemia models used in this study; however, the difference in survival was on the order of days. Analysis of leukemic cells harvested after 6 weeks of treatment demonstrated restoration of PGD expression as an adaptive response to PI3Kγ inhibition, supporting the proposed mechanistic role of PGD in LSC function. Further studies into suppressing this adaptive response will be needed to enhance the translational potential of PI3Kγ targeting.
Several PI3K inhibitors recently had their US Food and Drug Administration approval withdrawn because of their unfavorable risk/benefit profile in phase 3 randomized studies for follicular lymphoma and small lymphocytic lymphoma. Idelalisib (PI3Kδ selective) and duvelisib (PI3Kδ/γ selective) remain on the market for patients with chronic lymphocytic leukemia who have progressed on multiple lines of therapy. Although this raises concerns over clinical development of PI3Kγ inhibitors, the withdrawn agents were largely PI3Kδ targeted with presumed on-target immune-related toxicities and infectious complications. Isoform specificity will be essential for future PI3K inhibitors to improve the therapeutic window. IPI-549 (eganelisib), the PI3Kγ selective inhibitor used in this study, had an acceptable toxicity profile in phase 1 and 2 studies in solid malignancies and would be readily translatable for AML.7,8 To date, therapeutic targeting of PI3K as monotherapy in AML has not yielded much clinical success, which may be due to signaling redundancy, as demonstrated in this study. This cause of failure has been a common theme for molecularly targeted therapies. Preclinical testing of IPI-549 in combination with standard AML treatments is needed. Unfortunately, the company developing this agent filed for bankruptcy in October 2023, leaving the fate of IPI-549 in question. Nevertheless, the metabolic vulnerabilities of LSCs are becoming increasingly appreciated (as previously reviewed9), and we can now add PI3Kγ to the growing list of potential therapeutic targets for AML.
Conflict-of-interest disclosure: The author declares no competing financial interests.