Key Points
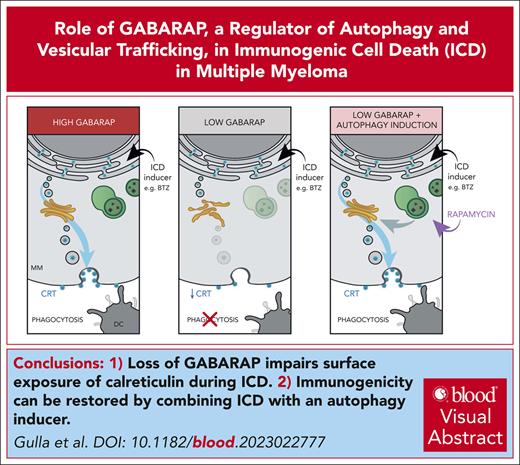
Loss of GABARAP abrogates the surface exposure of CRT in dying cancer cells, thus reducing anti-MM immune response after bortezomib.
Immunogenicity can be restored by combining bortezomib with an autophagy inducer, providing the framework for their clinical translation.
Visual Abstract
Immunogenic cell death (ICD) is a form of cell death by which cancer treatments can induce a clinically relevant antitumor immune response in a broad range of cancers. In multiple myeloma (MM), the proteasome inhibitor bortezomib is an ICD inducer and creates durable therapeutic responses in patients. However, eventual relapse and resistance to bortezomib appear inevitable. Here, by integrating patient transcriptomic data with an analysis of calreticulin (CRT) protein interactors, we found that GABA type A receptor–associated protein (GABARAP) is a key player whose loss prevented tumor cell death from being perceived as immunogenic after bortezomib treatment. GABARAP is located on chromosome 17p, which is commonly deleted in patients with high risk MM. GABARAP deletion impaired the exposure of the eat-me signal CRT on the surface of dying MM cells in vitro and in vivo, thus reducing tumor cell phagocytosis by dendritic cells and the subsequent antitumor T-cell response. Low GABARAP was independently associated with shorter survival in patients with MM and reduced tumor immune infiltration. Mechanistically, we found that GABARAP deletion blocked ICD signaling by decreasing autophagy and altering Golgi apparatus morphology, with consequent defects in the downstream vesicular transport of CRT. Conversely, upregulating autophagy using rapamycin restored Golgi morphology, CRT exposure, and ICD signaling in GABARAPKO cells undergoing bortezomib treatment. Therefore, coupling an ICD inducer, such as bortezomib, with an autophagy inducer, such as rapamycin, may improve patient outcomes in MM, in which low GABARAP in the form of del(17p) is common and leads to worse outcomes.
Introduction
Immunogenic cell death (ICD) is a form of cell death that triggers the release of damage-associated molecular patterns and other signals that activate the immune system.1,2 ICD is a critical mechanism by which cancer treatments, such as chemotherapy, radiation therapy, and targeted therapy, can induce an antitumor immune response and promote the elimination of cancer cells.2,3 In fact, ICD is important for treatment efficacy in multiple cancers, including breast,4-7 colon,8,9 and lung10-12 cancers and hematologic neoplasms.13-16
In general, during ICD, the dying tumor cell will emit specific prophagocytic signals, including exposing the endoplasmic reticulum (ER) protein calreticulin (CRT) on the cell surface.2,3,17-19 Exposure of this “eat-me” signal promotes the phagocytosis of tumor cells by antigen-presenting cells, such as dendritic cells (DCs) and macrophages,17,18,20 which process and present the tumor antigens to T cells,20,21 thus initiating an adaptive antitumor immune response.2,3,22,23 However, cancer cells can exploit several pathways to subvert the induction of ICD,3,24,25 and the exact mechanisms they use and how to combat those mechanisms remain open questions.
Multiple myeloma (MM) is an incurable malignancy of the plasma cells that accounts for ∼10% of hematologic cancers.26 It is characterized by dysfunction of the immune system, particularly of anti-MM immunity, over the course of the disease.27-29 As such, immunogenic chemotherapy stands out as an ideal therapeutic opportunity to restore endogenous T-cell competence in MM. In fact, the clinical success of the standard-of-care drug bortezomib (BTZ) significantly relies on its ability to kill MM cells in an immunogenic fashion, thus rendering them beacons to the immune system.30-34 BTZ stimulates the exposure of CRT on the dying cell surface, which stimulates an antitumor response.30 Yet, patients with MM inevitably become resistant to BTZ and relapse. We believe this suggests that tumor cells may develop resistance not only to the process of cell death but more precisely to its immunogenic consequences. Therefore, we integrated transcriptomic and proteomic data to identify genes that affect the exposure of CRT, thus potentially causing resistance to immunogenic chemotherapy. We found that losing GABA type A receptor–associated protein (GABARAP), a well-known regulator of autophagy and vesicular trafficking,35,36 2 processes that are important for CRT exposure and ICD,19,37,38 is a novel mechanism of tumor escape from phagocytosis that contributes to resistance and poor clinical outcomes. Our findings suggest that clinical response can be restored using autophagy inducers.
Methods
Cell lines and drugs
Cell lines were grown at 37°C at 5% CO2. Detailed information on cell lines and drugs are included in supplemental Methods, available on the Blood website.
PBMCs
Healthy donor peripheral blood mononuclear cells (PBMCs) were obtained after written informed consent, approved by the institutional review board of the Dana-Farber Cancer Institute. PBMCs were separated by Ficoll-Hypaque method (Lonza Group Ltd).
Fluorescence protein detection and immunofluorescence analysis of protein colocalization and Golgi area were performed.
Detailed information about the protocol and list of antibodies are included in supplemental Methods.
CRISPR/Cas9 gene knockout and stable gene expression
Coimmunoprecipitation, immunoblotting, and proteomic analysis
Coimmunoprecipitation was performed using the Pierce Co-Immunoprecipitation Kit (ThermoFisher Scientific; catalog no. 26149). Detailed information on the procedures, list of antibodies, and proteomic analysis can be found in supplemental Methods.
Proximity labeling assay
AMO1, H929, and U266 cell lines were transduced with CRT-3xHA-TurboID or 3xHA-TurboID doxycycline-inducible expressing vector as previously described. The complementary DNA sequence coding for Turbo-ID-3xHA40 or the CRT (NM_004343)-3xHA-TurboID sequence was synthesized and cloned into the pLVX-Tet-One-Puro inducible expression system41 from Azenta (Azenta US, Inc). Detailed information on the assay can be found in supplemental Methods.
Analysis of apoptosis and adenosine triphosphate release
Detailed information on these procedures is included in supplemental Methods.
Generation of monocyte-derived DCs and phagocytosis assay
Generation of monocyte-derived DCs and phagocytosis assay were performed as previously described.30 Detailed information on these procedures can be found in supplemental Methods.
T-cell cytotoxicity assay
T-cell cytotoxicity assay was performed as previously described.30 A detailed description of the procedure is included in supplemental Methods.
Transmission electron microscopy (TEM) and immunohistochemistry analysis
A detailed description of sample preparation and analysis is included in supplemental Methods.
In vivo studies
Six-week-old female immunocompetent C57BL/KaLwRijHsd (Envigo) mice were housed in the animal facility at the Dana-Farber Cancer Institute (DFCI). All experiments were performed after approval by the Animal Ethics Committee of the DFCI and using institutional guidelines. Detailed information can be found in supplemental Methods.
RNAseq data of patients with MM
We used RNA sequencing (RNAseq) from a previously published data set of patients with newly diagnosed clinically annotated MM from the Intergroupe Francophone du Myélome (IFM)/DFCI 2009 clinical trial.42 After quality control, all RNAseq data were quantified with Salmon. Raw counts and transcript per million values were summed to gene levels using tximport, and DESeq2 was used for all differential gene expression analyses. All figures were created with R and ggplot2. Survival analysis was performed using the survival package in R, and the log-rank test was used to compare groups.
Analysis of RNAseq and single-cell RNAseq data sets
Analysis of publicly available RNAseq and single-cell RNAseq data sets is detailed in supplemental Methods. Single-cell data from patients with normal bone marrow (n = 15), monoclonal gammopathy of undetermined significance (MGUS; n = 19), smoldering MM (n = 10), newly diagnosed MM (n = 17), and relapsed/refractory MM (pretherapy; n = 19) were retrieved from GSE145977, GSE124310,43 GSE161801,44 and GSE16327845 data sets.
Statistical analysis
Statistical significance of differences was determined using the Student t test (unless otherwise specified for comparison of >2 groups), with the minimal level of significance specified as P value <.05. Kaplan-Meier survival curves were compared by the log-rank test. All statistical analyses were performed using GraphPad software (http://www.graphpad.com).
Approval of your Institutional Review Board or Animal Care and Use Committee have been obtained for the studies.
Results
GABARAP is a clinically relevant binding partner of CRT
We first identified CRT’s binding partners by performing mass spectrometry analysis on CRT-bound proteins in AMO1 MM cells. This analysis was performed before and after treatment with BTZ (supplemental Table 1), which induces ICD and CRT exposure in this specific cell line.30 To find proteins that potentially drive CRT exposure, we focused on the proteins enriched after BTZ treatment. Within these proteins, gene ontology analysis found an enrichment in proteins involved in Golgi transport vesicles and membrane protein complexes, consistent with the vesicular transport of CRT to the plasma membrane (false discovery rate [FDR] < 1%; supplemental Figure 1A; supplemental Table 2). To focus on the clinically relevant interactors, we integrated these results with the transcriptomic analysis of patients with MM. We interrogated RNAseq data from newly diagnosed, uniformly treated, and clinically annotated patients with MM (IFM/DFCI 2009; NCT01191060)42 for a list of genes differentially expressed among patients with MM with longer survival (>5 years) vs poor survival (<1.5 years) after BTZ-based treatment (P < .01) (supplemental Figure 1B). By combining these 2 analyses, we found that GABARAP and carnitine palmitoyl transferase 1A (CPT1A) were both binding partners of CRT during the ICD process and had lower expression in patients with worse clinical outcome (Figure 1A).
GABARAP is a clinically relevant binding partner of CRT. (A) Schematic representation of the analysis combining proteomic and transcriptomic data. (B-C) Prognostic relevance (overall survival [OS] [B] or progression-free survival [PFS] [C]) of low GABARAP level estimated in patients enrolled in the IFM/DFCI. P value was calculated with a log-rank test. (D-E) Same analysis as in panels B and C but excluding patients from the IFM/DFCI carrying 17p deletion. P value was calculated with a log-rank test. (F-G) Immunoblot of GABARAP, CRT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on total protein lysates or proteins bound to CRT or Immunoglobulin G isotype control in AMO1 cells untreated or treated with BTZ (5 nM; 10 hours) (F) or CFZ (10 nM, 16 hours) (G). (H) Representative confocal images of coimmunofluorescence of intracellular staining of GABARAP (green) and CRT (red) in AMO1 WT cells untreated or treated with BTZ (5 nM; 10 hours). DAPI (4′,6-diamidino-2-phenylindole) was used to stain nuclei. An enlargement of the squared area shows colocalization with yellow fluorescence due to colocalizing signals; scale bars, 25 μm; enlargement scale bar, 10 μm. (I) Immunoblot of GABARAP, CRT, streptavidin, and GAPDH on total protein lysates and biotin pull-down proteins before and after doxycycline treatment (1 μg/mL; 24 hours) in AMO1, H929, and U266 CRT-3xHA-TurboID cells.
GABARAP is a clinically relevant binding partner of CRT. (A) Schematic representation of the analysis combining proteomic and transcriptomic data. (B-C) Prognostic relevance (overall survival [OS] [B] or progression-free survival [PFS] [C]) of low GABARAP level estimated in patients enrolled in the IFM/DFCI. P value was calculated with a log-rank test. (D-E) Same analysis as in panels B and C but excluding patients from the IFM/DFCI carrying 17p deletion. P value was calculated with a log-rank test. (F-G) Immunoblot of GABARAP, CRT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on total protein lysates or proteins bound to CRT or Immunoglobulin G isotype control in AMO1 cells untreated or treated with BTZ (5 nM; 10 hours) (F) or CFZ (10 nM, 16 hours) (G). (H) Representative confocal images of coimmunofluorescence of intracellular staining of GABARAP (green) and CRT (red) in AMO1 WT cells untreated or treated with BTZ (5 nM; 10 hours). DAPI (4′,6-diamidino-2-phenylindole) was used to stain nuclei. An enlargement of the squared area shows colocalization with yellow fluorescence due to colocalizing signals; scale bars, 25 μm; enlargement scale bar, 10 μm. (I) Immunoblot of GABARAP, CRT, streptavidin, and GAPDH on total protein lysates and biotin pull-down proteins before and after doxycycline treatment (1 μg/mL; 24 hours) in AMO1, H929, and U266 CRT-3xHA-TurboID cells.
For confirmation, we tested the association of GABARAP and CPT1A to clinical outcome in the IFM/DFCI data set and 2 additional independent data sets (GSE9782 and GSE4581).46 We found that low expression of GABARAP, but not of CPT1A, correlated with inferior clinical outcome in patients with MM (Figure 1B-C; supplemental Figure 1C-D). Furthermore, the GABARAP gene locus is on chr17p13.1, a chromosomal region whose deletion is a high-risk marker in patients with MM.47 Indeed, although GABARAP is broadly downregulated in patients with MM compared with healthy individuals (supplemental Figure 1E), its expression among subgroups of patients with MM is significantly lower in those carrying del(17p) (supplemental Figure 1F). However, the prognostic significance of GABARAP levels was still maintained even after excluding patients with MM with del(17p) from the analysis, thus suggesting its independent role as a risk predictor (Figure 1D-E). By interrogating The Cancer Genome Atlas database48-51 we also found that low levels of GABARAP were associated with poor clinical outcome in other cancers, including brain lower-grade glioma, kidney renal papillary cell carcinoma, mesothelioma, pancreatic adenocarcinoma, and uterine corpus endometrial carcinoma (supplemental Figure 1G).
To molecularly validate the CRT-GABARAP interaction, we immunoprecipitated CRT in cells treated or untreated with BTZ. This experiment confirmed a GABARAP-CRT protein interaction and its increase upon BTZ treatment (Figure 1F). Interaction with another microtubule-associated protein 1A/1B-light chain 3 (LC3) protein, LC3B, previously reported to interact with CRT,52 was not observed (supplemental Figure 1H). Treatment with another proteasome inhibitor, carfilzomib, which is also an ICD inducer,53,54 confirmed GABARAP-CRT but not LC3B interaction (Figure 1G; supplemental Figure 1I). Induction of ER stress by tunicamycin treatment (8 hours) did not produce a CRT-GABARAP interaction, whereas it confirmed the CRT-LC3B interaction (supplemental Figure 1J). These findings were confirmed by confocal microscopy, by which we found that BTZ treatment triggered the colocalization of GABARAP and CRT (Figure 1H).
To validate these findings in living cells, we used the ultrafast TurboID–based proximity labeling assay40 (supplemental Figure 1K). We generated a C-terminally fused CRT-3xHA-TurboID doxycycline-inducible Tet-On lentiviral construct (supplemental Figure 1L). The fusion of 3xHA-TurboID at the C-terminal of CRT mimics a translocation signal by altering the recognition interface of the KDEL sequence, which is an ER retention signal, as shown in the 3-dimensional protein structure predicted using AlphaFold and ChimeraX55-57 (supplemental Figure 1L). This way, we generated an artificial system in which CRT translocation was induced by doxycycline, independently of BTZ and ER stress. Validation of the CRT-3xHA-TurboID system and subsequent CRT exposure was performed in 3 MM cell lines: AMO1, H929, and U266 (supplemental Figure 1M-N). We used this approach to validate GABARAP as an interactor of CRT during the translocation process. As such, AMO1, H929, or U266 CRT-3XHA-TurboID cells were induced or uninduced with doxycycline for 24 hours in the presence of biotin, and western blot analysis of the streptavidin pull-down proteins confirmed the binding of GABARAP with CRT during the exposure on the surface in all cell lines (Figure 1I). These results identify GABARAP as a clinically relevant binding partner of CRT and provide the basis for further investigating whether GABARAP levels may interfere with CRT exposure and induction of the ICD process in MM cells.
Loss of GABARAP abrogates CRT exposure during ICD
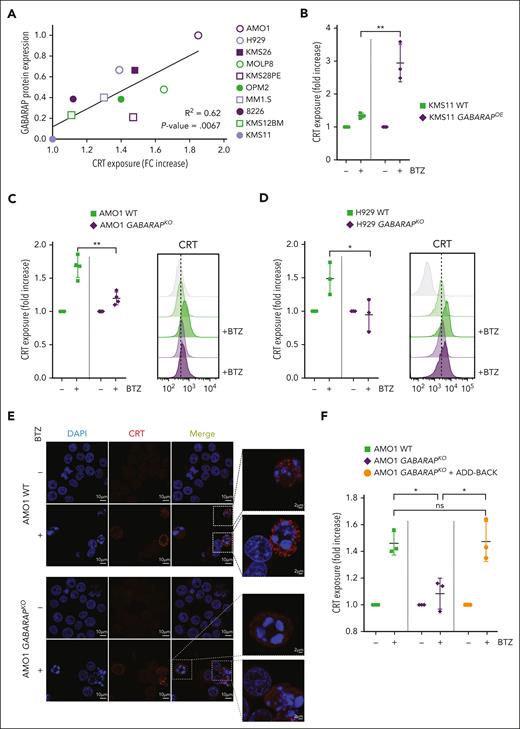
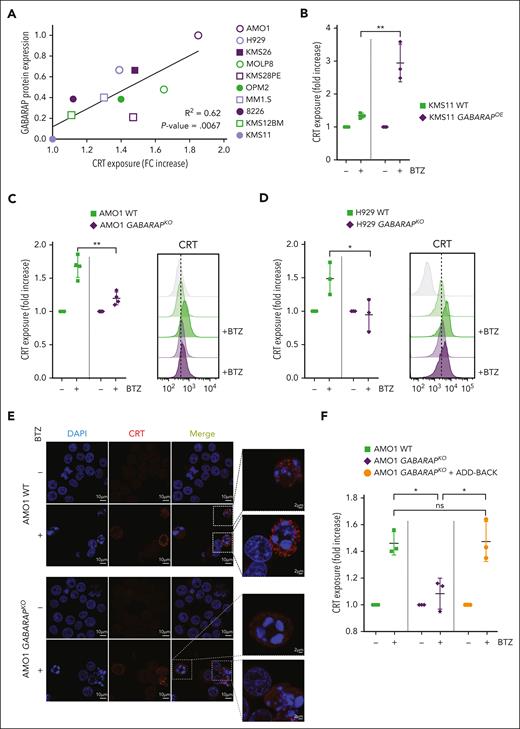
We next explored the role of GABARAP in the cell surface exposure of CRT. We treated a panel of 10 MM cell lines with varying concentrations of BTZ to obtain a similar degree of cell death among cell lines. We found a strong positive linear correlation (R2 = 0.62) between the endogenous expression level of GABARAP protein (supplemental Figure 2A) and the exposure of CRT on the cell surface induced during BTZ-mediated cell death (Figure 2A). To further confirm the above findings, we used KMS11 cells, which exhibit undetectable levels of GABARAP and show an absence of CRT exposure after BTZ. Overexpression of GABARAP in these cells restored CRT translocation to the cell surface during BTZ treatment (Figure 2B; supplemental Figure 2B). Conversely, the KO of GABARAP in 2 ICD-sensitive and GABARAPhigh cell lines, AMO1 and H929 (supplemental Figure 2C-D), abrogated CRT exposure after BTZ treatment, as assessed by flow cytometry (Figure 2C-D) and fluorescent microscopy of nonpermeabilized cells (Figure 2E). Add-back experiments using GABARAPOE in the KO clones restored CRT exposure after BTZ, confirming the on-target effect of GABARAP loss (Figure 2F; supplemental Figure 2E). Importantly, no significant changes in drug-induced cytotoxicity were detected (supplemental Figure 2F), indicating that this pathway purely affected the immunogenicity of the cell death.
Loss of GABARAP abrogates CRT exposure during ICD. (A) Correlation between CRT exposure and GABARAP protein expression in a panel of 10 MM cell lines. The surface exposure of CRT was determined by flow cytometry on viable cells after 16 hours of treatment of different cell lines, according to their BTZ sensitivity. Fold change of CRT increase was correlated with abundance of GABARAP protein (as shown in supplemental Figure 2A). (B) Analysis of surface CRT exposure in KMS11 WT and GABARAPOE after treatment with BTZ (7.5 nM; 16 hours) by flow cytometry of viable cells. (C-D) Effect of BTZ treatment (16 hours) on the exposure of surface CRT in AMO1 (5 nM) (C) and H929 (2.5 nM) cells (D) both with WT and GABARAPKO as assessed by flow cytometry of viable cells (left). Representative overlay histogram (right) of surface CRT fluorescence (MFI) in AMO1 (C) and H929 (D). (E) Representative images of immunofluorescence staining of surface CRT (red) in nonpermeabilized AMO1 WT and GABARAPKO before and after treatment with BTZ. DAPI was used to stain nuclei; scale bars, 10 μm. Enlargement pictures of the squared area show CRT exposure on dying cells only in WT condition; scale bars, 2 μm. (F) Analysis of surface CRT exposure in AMO1 WT, GABARAPKO, and GABARAPKO in which GABARAP was re-expressed (GABARAPKO + add-back) after treatment with BTZ (5 nM; 16 hours) by flow cytometry of viable cells. For panels B-D,F, ∗P < .05; ∗∗P < .01. ns, not significant (unpaired Student t test).
Loss of GABARAP abrogates CRT exposure during ICD. (A) Correlation between CRT exposure and GABARAP protein expression in a panel of 10 MM cell lines. The surface exposure of CRT was determined by flow cytometry on viable cells after 16 hours of treatment of different cell lines, according to their BTZ sensitivity. Fold change of CRT increase was correlated with abundance of GABARAP protein (as shown in supplemental Figure 2A). (B) Analysis of surface CRT exposure in KMS11 WT and GABARAPOE after treatment with BTZ (7.5 nM; 16 hours) by flow cytometry of viable cells. (C-D) Effect of BTZ treatment (16 hours) on the exposure of surface CRT in AMO1 (5 nM) (C) and H929 (2.5 nM) cells (D) both with WT and GABARAPKO as assessed by flow cytometry of viable cells (left). Representative overlay histogram (right) of surface CRT fluorescence (MFI) in AMO1 (C) and H929 (D). (E) Representative images of immunofluorescence staining of surface CRT (red) in nonpermeabilized AMO1 WT and GABARAPKO before and after treatment with BTZ. DAPI was used to stain nuclei; scale bars, 10 μm. Enlargement pictures of the squared area show CRT exposure on dying cells only in WT condition; scale bars, 2 μm. (F) Analysis of surface CRT exposure in AMO1 WT, GABARAPKO, and GABARAPKO in which GABARAP was re-expressed (GABARAPKO + add-back) after treatment with BTZ (5 nM; 16 hours) by flow cytometry of viable cells. For panels B-D,F, ∗P < .05; ∗∗P < .01. ns, not significant (unpaired Student t test).
To widen these observations to other tumor contexts and different ICD inducers, we also tested the outcome of GABARAP loss in A549 lung cancer cells. We generated A549 GABARAPKO cells (supplemental Figure 2G) and treated them with crizotinib, a drug previously described as an ICD inducer in this tumor context.11 Interestingly, GABARAP loss decreased CRT exposure after crizotinib treatment (supplemental Figure 2H). Altogether, these findings support the role of GABARAP in mediating CRT exposure during the induction of ICD.
Loss of GABARAP impairs ICD-induced phagocytosis and antitumor T-cell activation
Given that surface CRT is an “eat-me” signal, we tested whether GABARAP KO reduced tumor phagocytosis by DCs. Indeed, GABARAP loss impaired DC-mediated phagocytosis of human AMO1, H929, and U266 and murine 5TGM1 myeloma cells (Figure 3A; supplemental Figure 3A-C). Cotreatment of GABARAPKO cells with BTZ and recombinant CRT protein, which binds directly to the surface of tumor cells, restored DC-mediated phagocytosis, confirming that GABARAP loss impairs phagocytosis via inhibition of CRT translocation (Figure 3B). Similarly, overexpression of GABARAP in KMS11 GABARAPlow cells increased cell phagocytosis after treatment with BTZ (Figure 3C).
Loss of GABARAP impairs ICD-induced phagocytosis and antitumor T-cell activation. (A) For phagocytosis assay, MM cells and DCs were prestained with different dyes (either far-red or carboxyfluorescein diacetate succinimidyl ester [CFSE]). Dye-stained AMO1, H929, and 5TGM1 cells either WT or GABARAPKO were left untreated or treated with BTZ (5, 2.5, and 7.5 nM, respectively) for 16 hours. Then, they were cocultured with dye-stained DCs. Analysis was performed after 4 hours. Shown in the graph is the fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells, as assessed by flow cytometry. (B) Phagocytosis assay of BTZ-treated (5 nM; 16 hours) or -untreated stained AMO1 WT, GABARAPKO, and GABARAPKO with the addition of exogenous recombinant CRT (rCRT) cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. Representative overlay histograms confirm the exposure of surface CRT in the different conditions (right), as assessed by flow cytometry. (C) Phagocytosis assay of BTZ-treated (7.5 nM; 16 hours) or -untreated stained KMS11 WT or GABARAPOE cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. (D) BTZ-treated (16 hours) or -untreated U266 either WT or GABARAPKO cells were cocultured with HLA-matched DCs and T cells from the same healthy donors. After 5 days, T cells were negatively selected from all 4 coculture conditions (α, WT untreated; β, WT treated with BTZ, δ, GABARAPKO untreated; and γ, GABARAPKO treated with BTZ) and then cultured for 24 hours with new U266 cells at 1:5 target:effector (T:E) ratio, followed by 7-AAD staining and quantification of MM cell lysis by flow cytometry. Shown in the graph is the fold change increase of MM cell lysis induced by the T cells retrieved from the treated conditions versus the untreated ones. For panels A-D, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). GFP, green fluorescent protein.
Loss of GABARAP impairs ICD-induced phagocytosis and antitumor T-cell activation. (A) For phagocytosis assay, MM cells and DCs were prestained with different dyes (either far-red or carboxyfluorescein diacetate succinimidyl ester [CFSE]). Dye-stained AMO1, H929, and 5TGM1 cells either WT or GABARAPKO were left untreated or treated with BTZ (5, 2.5, and 7.5 nM, respectively) for 16 hours. Then, they were cocultured with dye-stained DCs. Analysis was performed after 4 hours. Shown in the graph is the fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells, as assessed by flow cytometry. (B) Phagocytosis assay of BTZ-treated (5 nM; 16 hours) or -untreated stained AMO1 WT, GABARAPKO, and GABARAPKO with the addition of exogenous recombinant CRT (rCRT) cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. Representative overlay histograms confirm the exposure of surface CRT in the different conditions (right), as assessed by flow cytometry. (C) Phagocytosis assay of BTZ-treated (7.5 nM; 16 hours) or -untreated stained KMS11 WT or GABARAPOE cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. (D) BTZ-treated (16 hours) or -untreated U266 either WT or GABARAPKO cells were cocultured with HLA-matched DCs and T cells from the same healthy donors. After 5 days, T cells were negatively selected from all 4 coculture conditions (α, WT untreated; β, WT treated with BTZ, δ, GABARAPKO untreated; and γ, GABARAPKO treated with BTZ) and then cultured for 24 hours with new U266 cells at 1:5 target:effector (T:E) ratio, followed by 7-AAD staining and quantification of MM cell lysis by flow cytometry. Shown in the graph is the fold change increase of MM cell lysis induced by the T cells retrieved from the treated conditions versus the untreated ones. For panels A-D, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). GFP, green fluorescent protein.
DC phagocytosis promotes T-cell priming and tumor cell recognition, so we next tested whether GABARAP loss in tumor cells impaired downstream T-cell activation. We incubated wild-type (WT) or GABARAPKO HLA.A2.1+ U266 cells, in the presence or absence of BTZ, with donor-matched DCs and T cells, in a system previously described to induce T-cell activation.30 After 5 days of culture, T cells isolated from cocultures with U266 GABARAPKO cells lost the ability to recognize and lyse MM cells (Figure 3D). Overall, these data support the role of GABARAP as a modulator of the antitumor response after BTZ immunogenic chemotherapy.
Loss of GABARAP impairs autophagy induction and alters Golgi morphology
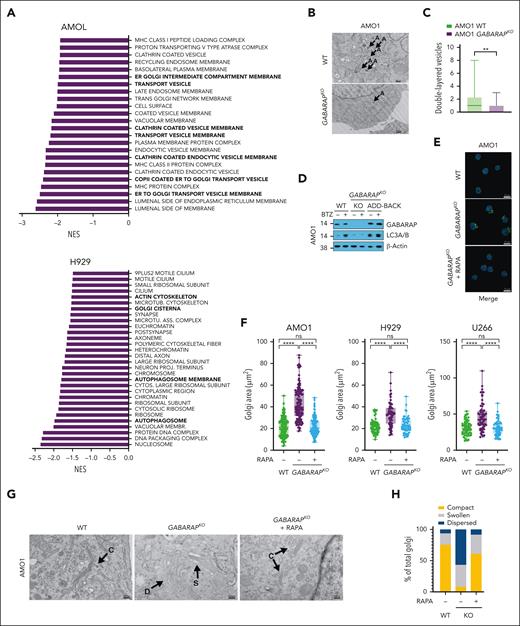
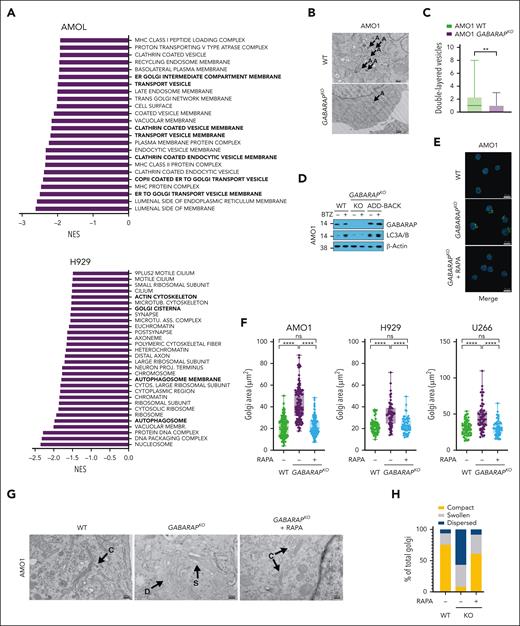
To molecularly characterize MM cells exhibiting GABARAP loss, we conducted a comprehensive proteomic analysis comparing GABARAP WT and knockout (KO) in AMO1 and H929 cells. We found that GABARAP KO altered the expression of 209 proteins in AMO1 cells (126 downregulated and 83 upregulated) and of 102 proteins in H929 cells (51 downregulated and 51 upregulated; supplemental Tables 3 and 4). Gene set enrichment analysis found a negative enrichment (FDR <1% in AMO1 and FDR <25% in H929) in pathways linked to vesicular transport, autophagosome, ER-to-Golgi trafficking, and Golgi composition (Figure 4A; supplemental Tables 5 and 6). Given GABARAP’s known role in vesicular transport and autophagy,35,36 we postulated that, in the absence of GABARAP, MM cells exhibiting lower basal autophagy might undergo biological adaptation within organelles crucial for maintaining their proteostasis, including the Golgi apparatus.
Loss of GABARAP impairs autophagy induction and alters Golgi morphology. (A) AMO1 and H929 WT and GABARAPKO were subjected to proteomic analysis by multiplexed proteomics with mass spectrometry. Shown in panel A is the gene set enrichment analysis (GSEA) gene ontology cellular components (GOCC) that were significantly negatively enriched after GABARAP KO. (FDR <1% for AMO1 and FDR <25% for H929). (B-C) Analysis of autophagy in AMO1 WT and GABARAPKO cells by TEM. (B) Representative TEM images depicting Golgi morphology (A = double-layered vesicles); scale bars, 500 nm. (C) Histograms showing the number of double-layered vesicles as determined in a total of 30 images for AMO1 WT and 30 images for AMO1 GABARAPKO cells. (D) AMO1 WT, GABARAPKO, and GABARAPKO in which GABARAP was re-expressed (GABARAPKO + add-back) were left untreated or treated with BTZ (5 nM; 16 hours). Immunoblot of GABARAP and LC3A/B is shown. β-Actin was used as a loading control. (E) Representative confocal images of Golgi apparatus stained with GM-130 antibody (green) in AMO1 WT, GABARAPKO, and GABARAPKO treated with rapamycin (50 nM; 24 hours). DAPI was used to label nuclei. This merged figure is also reported as supplemental Figure 4L together with the ones of the single channels; scale bars, 20 μm. (F) Box plot showing the Golgi area (μm2) in the different conditions as determined in a total of 119 cells per condition for AMO1, 60 cells per condition for H929, and 60 cells per condition for U266. (G) Representative TEM images depicting Golgi morphology in AMO1 WT, GABARAPKO, and GABARAPKO treated with rapamycin (50 nM; 24 hours) (C = compact; D = dispersed; and S = swollen); scale bars, 500 nm. (H) Histogram showing the percentage of compact, swollen, and dispersed Golgi in each condition. Specifically, 61 Golgi were visible in 29 TEM images taken in AMO1 WT; 37 Golgi in 30 TEM images taken in AMO1 GABARAPKO; and 47 Golgi in 29 TEM images taken in AMO1 GABARAPKO treated with rapamycin. For panel C, ∗∗P < .01 based on the unpaired Student t test; for panel F, ∗∗∗∗P < .0001 Kruskal-Wallis test. RAPA, rapamycin.
Loss of GABARAP impairs autophagy induction and alters Golgi morphology. (A) AMO1 and H929 WT and GABARAPKO were subjected to proteomic analysis by multiplexed proteomics with mass spectrometry. Shown in panel A is the gene set enrichment analysis (GSEA) gene ontology cellular components (GOCC) that were significantly negatively enriched after GABARAP KO. (FDR <1% for AMO1 and FDR <25% for H929). (B-C) Analysis of autophagy in AMO1 WT and GABARAPKO cells by TEM. (B) Representative TEM images depicting Golgi morphology (A = double-layered vesicles); scale bars, 500 nm. (C) Histograms showing the number of double-layered vesicles as determined in a total of 30 images for AMO1 WT and 30 images for AMO1 GABARAPKO cells. (D) AMO1 WT, GABARAPKO, and GABARAPKO in which GABARAP was re-expressed (GABARAPKO + add-back) were left untreated or treated with BTZ (5 nM; 16 hours). Immunoblot of GABARAP and LC3A/B is shown. β-Actin was used as a loading control. (E) Representative confocal images of Golgi apparatus stained with GM-130 antibody (green) in AMO1 WT, GABARAPKO, and GABARAPKO treated with rapamycin (50 nM; 24 hours). DAPI was used to label nuclei. This merged figure is also reported as supplemental Figure 4L together with the ones of the single channels; scale bars, 20 μm. (F) Box plot showing the Golgi area (μm2) in the different conditions as determined in a total of 119 cells per condition for AMO1, 60 cells per condition for H929, and 60 cells per condition for U266. (G) Representative TEM images depicting Golgi morphology in AMO1 WT, GABARAPKO, and GABARAPKO treated with rapamycin (50 nM; 24 hours) (C = compact; D = dispersed; and S = swollen); scale bars, 500 nm. (H) Histogram showing the percentage of compact, swollen, and dispersed Golgi in each condition. Specifically, 61 Golgi were visible in 29 TEM images taken in AMO1 WT; 37 Golgi in 30 TEM images taken in AMO1 GABARAPKO; and 47 Golgi in 29 TEM images taken in AMO1 GABARAPKO treated with rapamycin. For panel C, ∗∗P < .01 based on the unpaired Student t test; for panel F, ∗∗∗∗P < .0001 Kruskal-Wallis test. RAPA, rapamycin.
To test this hypothesis, we first confirmed the observed changes at proteomic levels by western blot analysis of several proteins involved in the autophagy machinery (LC3B, ATG4B, GABARAPL2, and ATG3) and Golgi trafficking and morphology (PAQR11, GODZ, GOSR1, and SORL1) in both AMO1 and H929 WT or GABARAPKO cells (supplemental Figure 4A). To further confirm the outcome of GABARAP KO on autophagy, we performed TEM to compare the number of double-layered or multilayered vesicles in GABARAPKO cells (n = 30 images) or WT cells (n = 30 images), which showed significantly fewer vesicles in the absence of GABARAP (Figure 4B-C). Furthermore, confocal microscopy analysis of the cis-Golgi matrix protein, GM130, showed an increased area of the Golgi apparatus in GABARAPKO cells (supplemental Figure 4B). TEM similarly depicted a more compact or a more dispersed appearance of the apparatus stacks in AMO1 WT and GABARAPKO, respectively (supplemental Figure 4C). Protein trafficking of surface proteins, such as CD138 and MHC-I, as well as paraprotein secretion, was not significantly altered in GABARAPKO conditions (supplemental Figure 4D-E), suggesting an adaptation of MM cells to this condition and a specific impairment of protein relocation (such as CRT) triggered by specific stimuli (such as ICD).
We then explored the molecular events induced by BTZ treatment in GABARAP WT and KO cells. Western blot analysis of LC3B confirmed that GABARAPKO impaired BTZ-induced autophagy in AMO1 and 5TGM1 cells (Figure 4D; supplemental Figure 4F). This effect was restored after GABARAP add-back (Figure 4D). Consistently, we found that impairment of autophagy induction after BTZ in AMO1 GABARAPKO cells was also associated with decreased release of adenosine triphosphate, another autophagy-related immunogenic damage–associated molecular pattern, during ICD38 (supplemental Figure 4G). Similarly, BTZ did not induce adenosine triphosphate release in GABARAP-low KMS11 cells, and this release was efficiently restored after GABARAP overexpression (supplemental Figure 4H). Although we did not observe higher BTZ cytotoxicity at the concentration used (supplemental Figure 2F), nor a difference in poly-ubiquitinated protein levels (supplemental Figure 4I), the induction of ER stress after drug treatment was slightly higher after GABARAP loss, consistent with lower autophagy induction (supplemental Figure 4I).
We further confirmed that the impairment of CRT exposure is dependent on compromised ER-Golgi trafficking and vesicular exocytosis of CRT and not on processes that happen before.19 Specifically, CRT exposure starts with the induction of ER stress. Two drugs that increase ER stress, tautomycin and salubrinal, did not affect CRT exposure when combined with BTZ in GABARAPKO cells (supplemental Figure 4J). In addition, subapoptotic cleavage of caspase 8, the following step required for CRT exposure, did not differ between WT and GABARAPKO cells (supplemental Figure 4K). Taken together, these data show that GABARAP loss compromised the vesicular trafficking of CRT by altering autophagy and Golgi morphology.
Because autophagy and Golgi homeostasis are intricately linked,58 we tested whether increasing autophagy by treating AMO1, H929, and U266 GABARAPKO cells with the mammalian target of rapamycin (mTOR) inhibitor rapamycin59 could restore Golgi morphology and CRT trafficking. We found, by confocal microscopy analysis of the GM130 protein, that rapamycin reverted Golgi morphology to resemble that of WT cells by decreasing Golgi area and increasing the compactness of the apparatus stacks in all 3 cell lines (Figure 4E-F; supplemental Figure 4L). TEM analysis performed in AMO1 cells further confirmed the effect of rapamycin on autophagy induction and formation of double-layered vesicles in GABARAPKO cells (Figure 4G), which was correlated with a decrease in the dispersion of Golgi morphology, with a higher frequency of cells with a more compact Golgi (Figure 4H).
Treatment with autophagy inducer restores CRT translocation after BTZ and in vivo drug efficacy
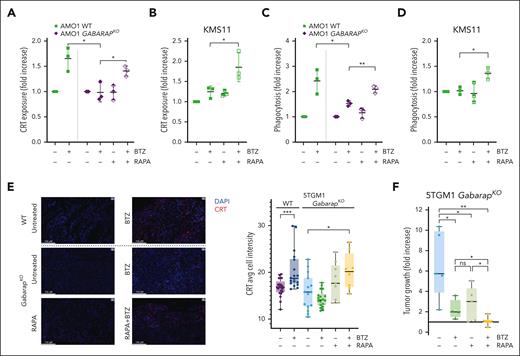
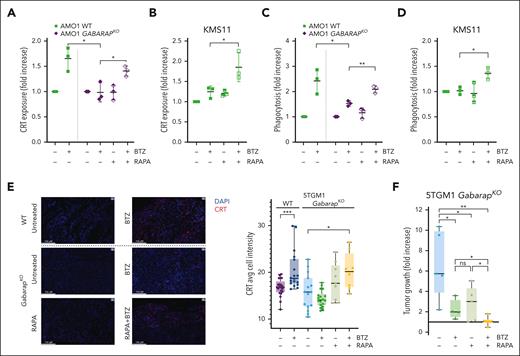
We then tested whether a clinically active autophagy inducer, rapamycin, in combination with BTZ would restore CRT translocation and DC-mediated phagocytosis of MM cells. We found that the combination efficiently restored CRT exposure in GABARAPKO AMO1 cells (Figure 5A) and in GABARAPlow KMS11 cells (Figure 5B). Consistently, combined treatment increased phagocytosis by DCs of AMO1 cells (GABARAPKO) and GABARAPlow KMS11 cells (Figure 5C-D).
Treatment with autophagy inducer restores CRT translocation after BTZ and in vivo drug efficacy. (A) Flow cytometry analysis of CRT exposure of AMO1 WT or GABARAPKO untreated or treated with BTZ (4 nM; 16 hours), rapamycin (100 nM; 24 hours) or a combination of both drugs. Fold increase as compared with untreated cells is shown. (B) Fold increase of CRT levels on surface of KMS11 cells untreated or treated with BTZ (6 nM; 16 hours), rapamycin (500 nM; 24 hours) or a combination of both drugs. (C) Phagocytosis assay of AMO1 WT or GABARAPKO untreated or pretreated with BTZ (4 nM; 16 hours), rapamycin (100 nM; 24 hours), or a combination of both drugs cocultured with far-red DCs for 4 hours. Shown is the fold increase of the percentage of double-positive DCs in treated conditions compared with untreated cells. (D) Phagocytosis assay of CFSE-stained KMS11 untreated or pretreated with BTZ (6 nM; 16 hours), rapamycin (500 nM; 24 hours), or a combination of both drugs cocultured with far-red DCs for 4 hours. Shown is the fold increase of the percentage of double-positive DCs in treated conditions compared with untreated cells. (E) 5TGM1 WT or GabarapKO were subcutaneously injected in immunocompetent C57BL/KaLwRijHsd mice. When tumors became palpable, mice bearing WT tumors were randomized to receive either BTZ (1 mg/kg) or phosphate-buffered saline (PBS); whereas mice bearing GabarapKO tumors were randomized to receive: PBS, BTZ (1 mg/kg), rapamycin (4 mg/kg), or a combination of both drugs. Tumors were retrieved 48 hours after BTZ treatment or in the combination group, 48 hours after BTZ and 24 hours after rapamycin. CRT expression was detected by immunofluorescence. Representative images of tumors retrieved from the different groups (left) stained with CRT antibody (red). DAPI was used to label nuclei (blue); scale bars, 100 μm (63× magnification). Average of cell intensity of CRT signal is shown (right), as analyzed by the Halo software. The numbers of observations reported are as follow: WT - BTZ (21 sections from 7 tumors); WT+BTZ (18 sections from 6 tumors); GabarapKO – BTZ (15 sections from 5 tumors); GabarapKO + BTZ (18 sections from 6 tumors); GabarapKO + RAPA (9 sections from 3 tumors); and GabarapKO + RAPA + BTZ (8 sections from 2 tumors); the signal from each section is represented as a dot in the graph. (F) Fold increase of tumor growth from day 1 (start of treatment) of subcutaneous 5TGM1 GabarapKO xenografts in C57BL/KaLwRijHsd mice treated with PBS (n = 5), BTZ (1 mg/kg twice per week for 2 weeks; n = 4), rapamycin (4 mg/kg per day for 5 days; n = 5), or a combination of both drugs (n = 6) ± standard error of the mean (SEM) for each group is reported. For panels A-F, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). RAPA, rapamycin.
Treatment with autophagy inducer restores CRT translocation after BTZ and in vivo drug efficacy. (A) Flow cytometry analysis of CRT exposure of AMO1 WT or GABARAPKO untreated or treated with BTZ (4 nM; 16 hours), rapamycin (100 nM; 24 hours) or a combination of both drugs. Fold increase as compared with untreated cells is shown. (B) Fold increase of CRT levels on surface of KMS11 cells untreated or treated with BTZ (6 nM; 16 hours), rapamycin (500 nM; 24 hours) or a combination of both drugs. (C) Phagocytosis assay of AMO1 WT or GABARAPKO untreated or pretreated with BTZ (4 nM; 16 hours), rapamycin (100 nM; 24 hours), or a combination of both drugs cocultured with far-red DCs for 4 hours. Shown is the fold increase of the percentage of double-positive DCs in treated conditions compared with untreated cells. (D) Phagocytosis assay of CFSE-stained KMS11 untreated or pretreated with BTZ (6 nM; 16 hours), rapamycin (500 nM; 24 hours), or a combination of both drugs cocultured with far-red DCs for 4 hours. Shown is the fold increase of the percentage of double-positive DCs in treated conditions compared with untreated cells. (E) 5TGM1 WT or GabarapKO were subcutaneously injected in immunocompetent C57BL/KaLwRijHsd mice. When tumors became palpable, mice bearing WT tumors were randomized to receive either BTZ (1 mg/kg) or phosphate-buffered saline (PBS); whereas mice bearing GabarapKO tumors were randomized to receive: PBS, BTZ (1 mg/kg), rapamycin (4 mg/kg), or a combination of both drugs. Tumors were retrieved 48 hours after BTZ treatment or in the combination group, 48 hours after BTZ and 24 hours after rapamycin. CRT expression was detected by immunofluorescence. Representative images of tumors retrieved from the different groups (left) stained with CRT antibody (red). DAPI was used to label nuclei (blue); scale bars, 100 μm (63× magnification). Average of cell intensity of CRT signal is shown (right), as analyzed by the Halo software. The numbers of observations reported are as follow: WT - BTZ (21 sections from 7 tumors); WT+BTZ (18 sections from 6 tumors); GabarapKO – BTZ (15 sections from 5 tumors); GabarapKO + BTZ (18 sections from 6 tumors); GabarapKO + RAPA (9 sections from 3 tumors); and GabarapKO + RAPA + BTZ (8 sections from 2 tumors); the signal from each section is represented as a dot in the graph. (F) Fold increase of tumor growth from day 1 (start of treatment) of subcutaneous 5TGM1 GabarapKO xenografts in C57BL/KaLwRijHsd mice treated with PBS (n = 5), BTZ (1 mg/kg twice per week for 2 weeks; n = 4), rapamycin (4 mg/kg per day for 5 days; n = 5), or a combination of both drugs (n = 6) ± standard error of the mean (SEM) for each group is reported. For panels A-F, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). RAPA, rapamycin.
To confirm our in vitro observations, we performed 2 different in vivo studies using immunocompetent C57BL/KaLwRijHsd mice carrying tumors of murine 5TGM1 cells. In the first one, we aimed to assess the exposure of CRT on tumors retrieved after BTZ treatment (1 mg/kg; 48 hours). Immunofluorescence staining of CRT protein confirmed that BTZ treatment significantly induced CRT exposure only in WT but not in GabarapKO tumors (Figure 5E; supplemental Figure 5A). However, the signal from CRT+ cells in GabarapKO tumors significantly increased after combining BTZ with rapamycin (4 mg/kg; 24 hours) (Figure 5E; supplemental Figure 5A). Consistently, although we confirmed that BTZ induces a significant regression for WT tumors as previously observed30 (supplemental Figure 5B), we found that drug efficacy was significantly lower in mice carrying GabarapKO tumors (Figure 5F). However, combination with rapamycin significantly increased BTZ efficacy in vivo with no sign of overt toxicity (Figure 5F).
Tumor intrinsic GABARAP correlates with tumor immune infiltration in patients with MM
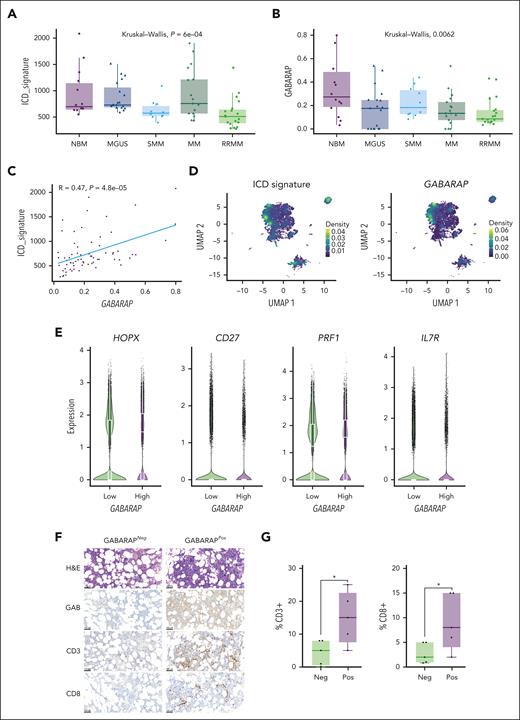
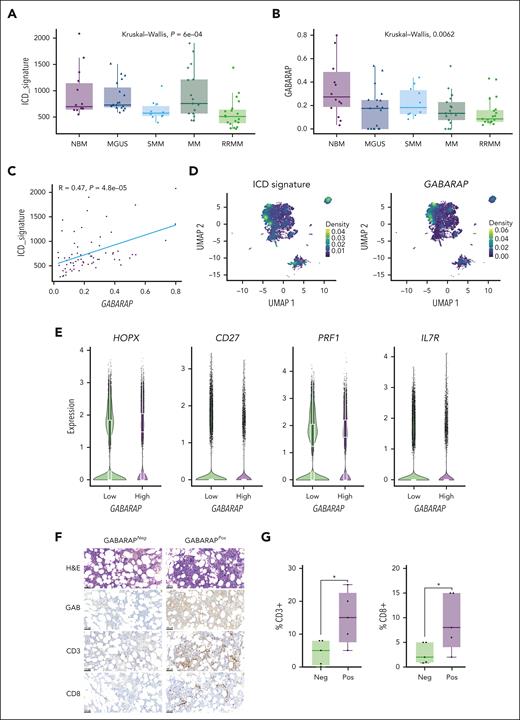
To evaluate the clinical significance of intratumor GABARAP in the context of anti-MM immunity, we analyzed published data sets of single-cell RNAseq43-45 for a total of 80 samples including normal bone marrow (n = 15), MGUS (n = 19), smoldering MM (n = 10), MM (n = 17), and relapsed/refractory MM (n = 19). First, we focused the analysis on MM cells identified according to the expression of their main markers (SDC1, CD38, TNFRSF17, GPRC5D, FCRL5, and CD19; supplemental Figure 6A) and assessed their expression of the ICD gene signature.30 We found that the expression of the ICD signature in malignant plasma cells progressively decreased during the disease course, consistent with a refractory state in which cells become less responsive to immunogenic stimuli (Figure 6A). Intratumor GABARAP expression similarly decreased over MM disease evolution (although heterogeneous expression was observed in the MGUS patient subgroup; Figure 6B) and was significantly correlated with ICD signature expression (Figure 6C). At the single-cell level, the ICD signature was still downregulated in tumor cells over the disease course (supplemental Figure 6B) and clustered similarly with GABARAP expression (Figure 6D). Concordantly, the expression of GABARAP and the ICD signature was correlated at the single-cell level (supplemental Figure 6C); thus, pointing at the likelihood of a similar outcome on poor tumor immunogenicity.
Tumor intrinsic GABARAP correlates with tumor immune infiltration in patients with MM. (A-B) Analysis of ICD signature30 (A) and GABARAP (B) expression on data aggregated per a total of 80 patients across MM disease stages (NBM, n = 15; MGUS, n = 19; SMM, n = 10 ; MM, n = 17; RRMM, n = 19).39-41 (C) Linear regression of GABARAP with ICD signature expression in the same patient cohort. (D) Uniform manifold approximation and projection (UMAP) plots of single-cell transcriptomic of 80 patients with MM showing the density of ICD signature (left) and GABARAP (right) expression on MM plasma cells. (E) Quantification of the expression of selected markers in CD8+ T cells significantly differentially expressed between patients with MM with low vs high intratumoral GABARAP expression (median as dichotomizing value). (F) Representative images of hematoxylin and eosin (H&E) and immunohistochemistry (IHC) analysis of GABARAP expression in plasma cells, and CD3 and CD8 staining of T cells from bone marrow biopsies from patients with MM; scale bars, 100 μm. (G) Statistical analysis of the percentage of CD3+ or CD8+ T cells in 10 patients with negative (neg; n = 5) or positive (pos; n = 5) staining for intratumoral GABARAP. For panels A-B, P values were calculated using the Kruskal-Wallis test. For panel G, ∗P < .05, unpaired Student t test. NBM, normal bone marrow; RRMM, relapsed/refractory MM; SMM, smoldering MM.
Tumor intrinsic GABARAP correlates with tumor immune infiltration in patients with MM. (A-B) Analysis of ICD signature30 (A) and GABARAP (B) expression on data aggregated per a total of 80 patients across MM disease stages (NBM, n = 15; MGUS, n = 19; SMM, n = 10 ; MM, n = 17; RRMM, n = 19).39-41 (C) Linear regression of GABARAP with ICD signature expression in the same patient cohort. (D) Uniform manifold approximation and projection (UMAP) plots of single-cell transcriptomic of 80 patients with MM showing the density of ICD signature (left) and GABARAP (right) expression on MM plasma cells. (E) Quantification of the expression of selected markers in CD8+ T cells significantly differentially expressed between patients with MM with low vs high intratumoral GABARAP expression (median as dichotomizing value). (F) Representative images of hematoxylin and eosin (H&E) and immunohistochemistry (IHC) analysis of GABARAP expression in plasma cells, and CD3 and CD8 staining of T cells from bone marrow biopsies from patients with MM; scale bars, 100 μm. (G) Statistical analysis of the percentage of CD3+ or CD8+ T cells in 10 patients with negative (neg; n = 5) or positive (pos; n = 5) staining for intratumoral GABARAP. For panels A-B, P values were calculated using the Kruskal-Wallis test. For panel G, ∗P < .05, unpaired Student t test. NBM, normal bone marrow; RRMM, relapsed/refractory MM; SMM, smoldering MM.
Next, we sought to assess how the immune microenvironment, and specifically the T-cell compartment, is modulated in the context of differential intratumoral GABARAP expression. We first identified the immune cell clusters using known markers (supplemental Figure 6D-F) and singled out CD8+ T cells for analysis. We found 29 genes differentially expressed between CD8+ T cells from patients with “high” vs “low” intratumor GABARAP expression (according to the median as the dichotomizing value) (supplemental Figure 6G). We found higher PRF1 and HOPX and lower CD27 and CD127 expression in CD8+ T cells from patients with high GABARAP expression, indicating a more mature, effector phenotype and higher antigen stimulation mediated by CD4+ T cells (Figure 6E). Moreover, the T cells of patients with GABARAPhigh tumors also showed higher expression of the NeoTCR8 signature, which identifies neoantigen-reactive T cells across metastatic human cancers60 (supplemental Figure 6H). Furthermore, immunohistochemical analysis of bone marrow specimens from 10 patients with MM found that infiltration of CD3+ and CD8+ T cells was significantly higher in patients with MM and GABARAPhigh tumors (Figure 6F-G). Altogether, these results suggest that tumor intrinsic GABARAP levels are associated with markers of ICD and of higher T-cell activity, implying that GABARAP may be a determinant of both spontaneous and ICD-mediated antitumor immunity.
Discussion
We have previously reported that BTZ promotes tumor phagocytosis and antitumor adaptive immunity through ICD, thus resulting in a clinical benefit for patients with MM.30,33 However, this dependence on ICD suggests an innovative hypothesis whereby resistance to BTZ may be derived not only from resistance to cell death and defective host immunity but also from a cell’s death not being immunogenic enough to trigger antitumor immunity. Here, we identified GABARAP as an intrinsic regulator of CRT externalization and tumor immunogenicity.
Importantly, the exposure of CRT after immunogenic chemotherapy has been correlated with the clinical outcome of several cancers,61-65 and mechanisms that interfere with this pathway contribute to poor clinical outcome and response to immune therapies.24,65 Notably, we found that GABARAP expression is correlated with the clinical outcome of various cancer types in which ICD induction has been found to be beneficial for patient outcome: ICD signature predicts prognosis in lower-grade glioma66,67 and endometrial cancer68; ICD induction is emerging as a promising therapeutic opportunity in mesothelioma69; and immunogenic chemotherapies and radiation therapies appear to reactivate the immune system in pancreatic cancer.70,71
The gene locus of GABARAP is on chromosome 17p, which is frequently deleted in high-risk MM47 as well as other cancer types.72-74 In the case of MM, no specific mechanisms of BTZ resistance have been ascribed to 17p deletion; however, BTZ treatment in these patients cannot overcome the adverse impact of del(17p) on outcome.75 Therefore, we propose that GABARAP deletion is a form of primary resistance to BTZ, because BTZ will be less effective in these patients due to an associated lack of spontaneous and ICD-mediated antitumor immunity. Although we did not investigate the clinical and biological consequences of GABARAP loss in other tumor types, nor the correlation of GABARAP with the status of del(17p), the prevalence of this deletion in many cancers,72-74 among other chromosome copy number variations,76 prompts future investigation into the role of GABARAP in patients carrying this abnormality in a broad range of cancers.
GABARAP is a well-known regulator of autophagy and vesicular trafficking.35,77 It also interacts with the GM130 protein,36 and so, GABARAP loss has been previously reported to also alter Golgi morphology.78 A fragmented Golgi can be observed in a variety of cancers58 and is associated with tumor proliferation and invasion, drug resistance, and reprogramming of the tumor microenvironment.79,80 As such, although we posit that CRT and GABARAP interact when induction of ER stress is followed by CRT exposure, our data also pinpoint a strong impairment of autophagy in tumor cells with GABARAP loss, which is the cause of a disrupted Golgi trafficking, which, in turn, renders the translocation of CRT to the cell surface unattainable. Therefore, our study establishes that GABARAP null cells cannot expose CRT because of the autophagy and Golgi dysfunction but leaves open the question about the contribution of the GABARAP-CRT interaction in the process; and about the nature of this interaction, whether direct or indirect, as previously reported81,82; and about how other proteins, such as GM130, play a role. Moreover, further studies are necessary to elucidate the mechanisms through which cancer cells downregulate GABARAP and whether components of the tumor microenvironment may influence its expression. In addition, our study uncovered significant correlations between GABARAP loss, autophagy, protein trafficking, and immunogenicity, which warrants further investigation to understand the intricate interplay between these processes in plasma cell biology.
Importantly, our research demonstrated that inducing autophagy alongside immunogenic chemotherapy restored CRT exposure in GABARAPlow conditions and converted a non-ICD into an immunogenic one. Although immunotherapy is an ideal strategy for addressing immunosuppression in cancer, including MM,28 we believe that restoring the tumor intrinsic immunogenicity of GABARAP-low cells first is essential for effective tumor clearance. Our findings provide the rationale for a combination treatment using an ICD inducer, such as BTZ, and an autophagy inducer, such as rapamycin, in patients with cancer with low GABARAP levels, such as those carrying del(17p), to restore antitumor immune recognition and long-term disease control. Additional studies are required to assess the effect of the drug combination on immune effectors and regulators and are necessary to translate this combination into the clinical setting.
Acknowledgments
The authors gratefully acknowledge the members of their laboratories for technical advice and critical discussions. The authors thank Christina Usher of the Dana-Farber Cancer Institute for editing the manuscript and for insightful comments. The results shown here are in whole or part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga).
This work is supported by National Institutes of Health/National Cancer Institute grants SPORE-P50CA100707 and P01CA155258 (N.C.M. and K.C.A.); VA Healthcare System (grant 5I01BX001584; N.C.M.); the Paula and Roger Riney Foundation grant (N.C.M. and K.C.A.); and the Sheldon and Miriam Adelson Medical Research Foundation (K.C.A.). A.G. is a fellow of The Leukemia & Lymphoma Society and a Scholar of the American Society of Hematology; she received support from the International Myeloma Society; she is supported by an individual Start-UP grant from the Italian Association for Cancer Research (project number 27750); a Fondazione Piemontese per la Ricerca sul Cancro “5xmille” 2019 Ministry of Health project (IDEE) and a FPRC “5xmille” 2021 Ministry of Health project (EMAGEN-FaBer). E.M. is supported by a Special Fellow grant from The Leukemia & Lymphoma Society, by a scholar award from the American Society of Hematology, by an individual Start-UP grant from the Italian Association for Cancer Research (project number 29106), and by a FPRC “5xmille” 2021 Ministry of Health project (EMAGEN-LongMynd). C.C. and D.V. are supported by National Cancer Institute, National Insitutes of Health grant (5R25CA174650). K.C.A. is an American Cancer Society Clinical Research Professor. A.G, E.M., F.B., M.T., A.S. and A.B. are supported by the Italian Ministry of Health, Ricerca Corrente 2024.
Authorship
Contribution: A.G. and K.C.A. conceived and designed the research studies; A.G., E.M., and K.C.A. wrote the manuscript; M.T., M.K.S., and C.B. performed in silico analysis of transcriptomic data; A.G., M.J., M.T., and S.C. generated DCs, performed T-cell experiments and flow cytometry analysis; P.F. performed microscopy experiments; M.J. and P.F. performed coimmunoprecipitation experiments; S.T. generated MM cells expressing Cas9; E.M., M.J., S.C., P.F., D.V., F.B., C.C., R.P., G.B., M.F., K.W., K.K., J.L., P.G.R., D.C., T.H., and N.C.M. contributed to the design, execution, and interpretation of key experiments; V.K.F., D.M, P.F., A.G., and E.M. performed the in vivo study; J.P. and R.D.C. performed the immunohistochemistry staining of patient samples; A.B. performed the analysis of the immunohistochemistry staining; and A.S. supervised the immunohistochemistry analysis.
Conflict-of-interest disclosure: N.C.M. serves on advisory boards of and as consultant to Takeda, Bristol Myers Squibb, Celgene, Janssen, Amgen, AbbVie, Oncopep, Karyopharm, Adaptive Biotechnology, and Novartis; and holds equity ownership in Oncopep. K.C.A. is a consultant for Janssen, Pfizer, and AstraZeneca; and serves as a board member with equity ownership in Oncopep, C4Therapeutics, Starton, NextRNA, Window, and Dynamic Cell Therapies. A.G. and K.C.A filed a provisional patent on the role of GABARAP as modulator of ICD. M.K.S. is a consultant for AbbVie and serves as advisor for Neuberg Center for Genomic Medicine. G.B. is a consultant for Prothena. D.C. has equity ownership in C4 Therapeutics outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Annamaria Gulla, Candiolo Cancer Institute, FPO-IRCCS, Strada Provinciale 142, km. 3.95, 10060 Candiolo, Italy; email: annamaria.gulla@ircc.it; and Kenneth C. Anderson, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: kenneth_anderson@dfci.harvard.edu.
References
Author notes
E.M., M.J., and M.T. have contributed equally.
Data are available on request, according to Blood policy, from the corresponding authors, Annamaria Gulla (annamaria.gulla@ircc.it) and Kenneth C. Anderson (kenneth_anderson@dfci.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![GABARAP is a clinically relevant binding partner of CRT. (A) Schematic representation of the analysis combining proteomic and transcriptomic data. (B-C) Prognostic relevance (overall survival [OS] [B] or progression-free survival [PFS] [C]) of low GABARAP level estimated in patients enrolled in the IFM/DFCI. P value was calculated with a log-rank test. (D-E) Same analysis as in panels B and C but excluding patients from the IFM/DFCI carrying 17p deletion. P value was calculated with a log-rank test. (F-G) Immunoblot of GABARAP, CRT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on total protein lysates or proteins bound to CRT or Immunoglobulin G isotype control in AMO1 cells untreated or treated with BTZ (5 nM; 10 hours) (F) or CFZ (10 nM, 16 hours) (G). (H) Representative confocal images of coimmunofluorescence of intracellular staining of GABARAP (green) and CRT (red) in AMO1 WT cells untreated or treated with BTZ (5 nM; 10 hours). DAPI (4′,6-diamidino-2-phenylindole) was used to stain nuclei. An enlargement of the squared area shows colocalization with yellow fluorescence due to colocalizing signals; scale bars, 25 μm; enlargement scale bar, 10 μm. (I) Immunoblot of GABARAP, CRT, streptavidin, and GAPDH on total protein lysates and biotin pull-down proteins before and after doxycycline treatment (1 μg/mL; 24 hours) in AMO1, H929, and U266 CRT-3xHA-TurboID cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/25/10.1182_blood.2023022777/2/m_blood_bld-2023-022777-gr1.jpeg?Expires=1765170734&Signature=D9aWtk2reBZDJ8oFbykfoxSfKCDf8GM-aATNG7xhtay0NLlIIksgoCHN3NFJ6CDIUvwSe0ymoTR~bOxaVGVD10gI5FcNmCxfqQ~-6HQWY3bhmXUKFvw2qy5dZtGB6uTTAzLX4Wxzr-hB-UBCB8hmxKum6gjpYAQ7kXNiV1WHSA6KJWvLZh1UON~3AWpGSHbisgl2ykRiOTuLfEnejK0bN~NM1hA~UWm0yEliqFlMdl2Nfd2L1vjcxffgpeJzhips1Zet6l1QvU3Pt3wy2zczA39C63j1akuSdkkmkAkaUSSWTzZqXyBA6UBLJdb27S~Qb7OHYRL30Jt7KF62859xEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Loss of GABARAP impairs ICD-induced phagocytosis and antitumor T-cell activation. (A) For phagocytosis assay, MM cells and DCs were prestained with different dyes (either far-red or carboxyfluorescein diacetate succinimidyl ester [CFSE]). Dye-stained AMO1, H929, and 5TGM1 cells either WT or GABARAPKO were left untreated or treated with BTZ (5, 2.5, and 7.5 nM, respectively) for 16 hours. Then, they were cocultured with dye-stained DCs. Analysis was performed after 4 hours. Shown in the graph is the fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells, as assessed by flow cytometry. (B) Phagocytosis assay of BTZ-treated (5 nM; 16 hours) or -untreated stained AMO1 WT, GABARAPKO, and GABARAPKO with the addition of exogenous recombinant CRT (rCRT) cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. Representative overlay histograms confirm the exposure of surface CRT in the different conditions (right), as assessed by flow cytometry. (C) Phagocytosis assay of BTZ-treated (7.5 nM; 16 hours) or -untreated stained KMS11 WT or GABARAPOE cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. (D) BTZ-treated (16 hours) or -untreated U266 either WT or GABARAPKO cells were cocultured with HLA-matched DCs and T cells from the same healthy donors. After 5 days, T cells were negatively selected from all 4 coculture conditions (α, WT untreated; β, WT treated with BTZ, δ, GABARAPKO untreated; and γ, GABARAPKO treated with BTZ) and then cultured for 24 hours with new U266 cells at 1:5 target:effector (T:E) ratio, followed by 7-AAD staining and quantification of MM cell lysis by flow cytometry. Shown in the graph is the fold change increase of MM cell lysis induced by the T cells retrieved from the treated conditions versus the untreated ones. For panels A-D, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). GFP, green fluorescent protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/25/10.1182_blood.2023022777/2/m_blood_bld-2023-022777-gr3.jpeg?Expires=1765170734&Signature=LcytInFZ-yr2helc8kfZOXMWxDwHr0tSBZdo-PrApz1-2QuhRAfmeCeYcVuqdHXgBrkt-zMRg1TxL3Z2rNjO29DaSDKIsvV10utB6n6jIsGFKVe~kJlRcYKP6BwcRTonu-tdKWFUeh6tWqgT~GnnLD9GsEpsLI0s4GnSr2elcCrZ8O~88VCpqt9cr9CGzoVYecu6xJYQYXWs8zc3eSm3Fc4bL5bpAkou7VsVaRA1bWL9FhwqOi9VOHP3EhR~NhKGyHkGCrZqCUdXJrPFQtFvIT5UsUGrMTj1TspkIHtXcMmgcKSZWwYld7daDKQFzhQGS8GAP7o-~fr6giyWuLI7Bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![GABARAP is a clinically relevant binding partner of CRT. (A) Schematic representation of the analysis combining proteomic and transcriptomic data. (B-C) Prognostic relevance (overall survival [OS] [B] or progression-free survival [PFS] [C]) of low GABARAP level estimated in patients enrolled in the IFM/DFCI. P value was calculated with a log-rank test. (D-E) Same analysis as in panels B and C but excluding patients from the IFM/DFCI carrying 17p deletion. P value was calculated with a log-rank test. (F-G) Immunoblot of GABARAP, CRT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on total protein lysates or proteins bound to CRT or Immunoglobulin G isotype control in AMO1 cells untreated or treated with BTZ (5 nM; 10 hours) (F) or CFZ (10 nM, 16 hours) (G). (H) Representative confocal images of coimmunofluorescence of intracellular staining of GABARAP (green) and CRT (red) in AMO1 WT cells untreated or treated with BTZ (5 nM; 10 hours). DAPI (4′,6-diamidino-2-phenylindole) was used to stain nuclei. An enlargement of the squared area shows colocalization with yellow fluorescence due to colocalizing signals; scale bars, 25 μm; enlargement scale bar, 10 μm. (I) Immunoblot of GABARAP, CRT, streptavidin, and GAPDH on total protein lysates and biotin pull-down proteins before and after doxycycline treatment (1 μg/mL; 24 hours) in AMO1, H929, and U266 CRT-3xHA-TurboID cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/25/10.1182_blood.2023022777/2/m_blood_bld-2023-022777-gr1.jpeg?Expires=1765350509&Signature=gsaKkzfAvbIyDShgQTbf4GcXT75TF-uMEqrO66BD7-mCpikLqk4-FjEgzKtm~j~EzZ3hThTnahqHmTQZj9DpqsFyCObz3UVhUzrv1Hss~1qA-naYtUYV1DKR3q-AQCllMnzexEUNv6oaWose0T90C-Ba6QlBzvGVwhDavb~InfISUA45ccecabXOx650TtN1atM50GCJffYeUCsacwTnThBoqTwD9HmdBHB85bgd6beYPjpV46bhNznrm2Ou9QH9l30aD0asCrrhDWmHnIdxPQozSWWvoHBvoZA8LoCqQHhF7U8LZzRPUIPSX4d0-QBlykWvIBr06GdzBs-wvI4~NA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Loss of GABARAP impairs ICD-induced phagocytosis and antitumor T-cell activation. (A) For phagocytosis assay, MM cells and DCs were prestained with different dyes (either far-red or carboxyfluorescein diacetate succinimidyl ester [CFSE]). Dye-stained AMO1, H929, and 5TGM1 cells either WT or GABARAPKO were left untreated or treated with BTZ (5, 2.5, and 7.5 nM, respectively) for 16 hours. Then, they were cocultured with dye-stained DCs. Analysis was performed after 4 hours. Shown in the graph is the fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells, as assessed by flow cytometry. (B) Phagocytosis assay of BTZ-treated (5 nM; 16 hours) or -untreated stained AMO1 WT, GABARAPKO, and GABARAPKO with the addition of exogenous recombinant CRT (rCRT) cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. Representative overlay histograms confirm the exposure of surface CRT in the different conditions (right), as assessed by flow cytometry. (C) Phagocytosis assay of BTZ-treated (7.5 nM; 16 hours) or -untreated stained KMS11 WT or GABARAPOE cocultured with stained DCs for 4 hours. Fold increase in the percentage of double-positive DCs in treated cells compared with untreated cells is shown. (D) BTZ-treated (16 hours) or -untreated U266 either WT or GABARAPKO cells were cocultured with HLA-matched DCs and T cells from the same healthy donors. After 5 days, T cells were negatively selected from all 4 coculture conditions (α, WT untreated; β, WT treated with BTZ, δ, GABARAPKO untreated; and γ, GABARAPKO treated with BTZ) and then cultured for 24 hours with new U266 cells at 1:5 target:effector (T:E) ratio, followed by 7-AAD staining and quantification of MM cell lysis by flow cytometry. Shown in the graph is the fold change increase of MM cell lysis induced by the T cells retrieved from the treated conditions versus the untreated ones. For panels A-D, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (unpaired Student t test). GFP, green fluorescent protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/25/10.1182_blood.2023022777/2/m_blood_bld-2023-022777-gr3.jpeg?Expires=1765350509&Signature=SeQ5G882VJs0B-OPFpeHBeZPqTuP9HslV1CACSbtvdOin7YcDo7puplKFWVQfyyXxrg4PHdXqD~IoB8JnC6vWYYTvctcCzrdf8iH1ptAGkyHs0BLdQXllVMzPx8oZEnmDvYQSc1qxY0F2biBt5xsXz4Gk54yvG2WUSYOL5w4UIDweHmk83e98ZvvLDLY92zzegJqwBaXeaO7vceHc-kD0Se-uTp7AE~zDgM-5d3wlbPNKlDcRZnvFjn37pOaTwemMhKyX25z7WBT3pSWgoGSu2KkJrMxWab2QOfSL06bhj8DBwXQgrLJaQNX1LsJwOc8x26rw~WsrSyMJpCA3x9A~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)