This phase 2 trial assessed high-dose IV ascorbic acid in TET2 mutant clonal cytopenia. Eight of 10 patients were eligible for response assessment, with no responses at week 20 by International Working Group Myelodysplasia Syndromes/Neoplasms criteria. This trial was registered at www.clinicaltrials.gov as #NCT03418038.
TO THE EDITOR:
Clonal cytopenia(s) of undetermined significance (CCUS) is defined as persistent cytopenia(s) with myeloid neoplasm (MN)–associated somatic mutations (MTs) in hematopoietic stem and progenitor cells (HSPCs), not meeting the diagnostic criteria for MN.1 Individuals with CCUS have a 2-year cumulative incidence of progression to MN of 2.8% to 12.6%.2,3 Currently, no US Food and Drug Administration–approved therapies exist,4 and the inclusion, exclusion, and response criteria for CCUS clinical trials remain undefined.5
Although oral ascorbic acid (AA) exhibited minimal anticancer activity, high-dose IV AA at pharmacologic concentrations demonstrates anticancer effects through hydrogen peroxide–induced oxidative stress and DNA demethylation via TET activation.6-8 TET2, a methylcytosine (mC) dioxygenase, oxidizes 5 mC to 5 hydroxymethylcytosine, resulting in DNA demethylation.9 AA is a critical cofactor for TET2, binding to its catalytic domain and enhancing TET activity.10,11 Given the high prevalence of truncating and hypomorphic TET2MT in CCUS, we hypothesized that IV AA could induce epigenetic changes by enhancing TET activity, primarily acting on the wild-type allele and by exploiting functional redundancies in TET1 and TET3, thus restoring DNA methylation, with potential to improve cytopenias.
This investigator-initiated phase 2 trial (NCT03418038) evaluated the safety and preliminary efficacy of IV AA in patients with high-risk CCUS, who are likely to progress to MNs. Eligibility criteria required TET2MT and at least 1 clinically significant cytopenia to be considered as high risk: hemoglobin (Hb) ≤10 g/dL, absolute neutrophil count (ANC) ≤1 × 109 per liter, and platelet count (PLT) ≤100 × 109 per liter.12 Bone marrow biopsy slides were reviewed at Mayo Clinic, confirming CCUS diagnosis, while excluding bona fide MNs based on the fourth edition World Health Organization criteria (2016).13 IV AA (1 g/kg; maximum 100 g in 1 liter of sterile water) was administered thrice weekly at 0.75 to 1.0 g per minute for 12 weeks via a central venous line. Responses were evaluated at 20 and 52 weeks (supplemental Figure 1, available on the Blood website). The primary end point was hematologic response at week 20, per Myelodysplasia Syndromes/Neoplasms (MDS) International Working Group 2018 criteria,12 reported as hematologic improvement in erythropoietic cells, platelets, and neutrophils. Secondary end points included safety and adverse events (AEs), graded by NCI-CTCAE v4.03.14 Exploratory end points included clonal dynamics and progression to MNs. Correlative studies assessed longitudinal changes in TET2MT variant allele fraction (VAF) and DNA methylation/hydroxymethylation changes in peripheral blood mononuclear cells (see the supplemental Material for methods).15
Ten patients were enrolled; 9 were eligible for baseline characteristic analysis (patient 7 was enrolled with a diagnosis of TET2, SRSF2, and KRAS mutant CCUS but in hindsight met the criteria for chronic myelomonocytic leukemia [CMML]); and 8 were eligible for response assessment (patient 5 came off study during cycle 1 due to a central line–associated thrombosis). The median age was 71.7 years (range, 65-79); 78% were male. Among 11 TET2 variants, 64% were truncating, and the remainder were hypomorphic. The median number of somatic MTs was 2 (range, 1-3), with 89% having co-MTs. All but 1 patient had a normal karyotype (Table 1).
At baseline, all patients had normal Hb, except patient 6 who was red blood transfusion dependent. Four patients (44%) had PLT <100 × 109 per liter, including 2 (patients 4 and 9) with PLT ≤50 × 109 per liter; 56% had ANC <1 × 109 per liter, including 2 (patients 8 and 10) with ANC <0.5 × 109 per liter.
IV AA was well tolerated, with the most common AEs being transient (resolved within 48 hours of completing therapy) polyuria (56%), and polydipsia (44%). Grade 1 constipation, headaches, and dyspepsia were reported in 2 patients (22%). One patient discontinued therapy after cycle 1 due to central line–associated thrombosis. No grade 3 or 4 treatment-related AEs or deaths occurred. No patients developed oxalate nephrolithiasis, a known complication of high-dose IV AA.19
The median follow-up duration was 31.6 months (range, 17.6-37). No significant differences in laboratory values (Hb, ANC, and PLT) or TET2MT VAF were observed at baseline, week 20, and week 52, as assessed by repeated measures analyses (supplemental Figures 2-4). Two patients (22%) acquired additional somatic MTs: patient 8 (RUNX1 p. Ser322∗) and patient 9 (CBL p. Cys384Arg and p. Thr406Asnfs∗26). VAF changes in other MTs are shown in supplemental Table 2.
At week 20, no responses were observed per International Working Group 2018 MDS response criteria. Three patients (33%; patients 4, 8, and 10) developed bona fide MN (2 MDS and 1 CMML). Two patients (patients 4 and 9) had baseline AA deficiency that normalized after therapy. Although patient 9 had stable disease at week 20, patient 4 progressed to CMML-1 at 52 weeks. No significant differences in clinical characteristics were found between stable and progressing patients (supplemental Table 1). All patients were alive at the last follow-up.
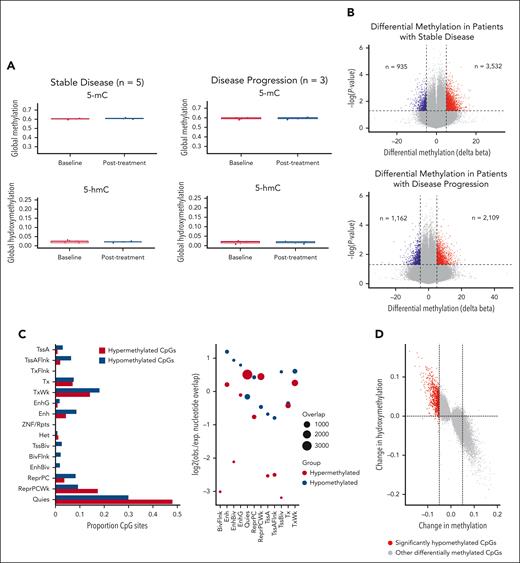
To quantify the impact of IV AA on TET activity, we profiled DNA methylation and hydroxymethylation in blood samples before and after treatment. Using the Illumina MethylationEPIC array paired with oxidative bisulfite treatment, we identified differentially methylated and hydroxymethylated regions. Initial analysis of global levels of 5 mC and 5 hydroxymethylcytosine before and after treatment across all patients revealed no significant differences (supplemental Figure 5). This finding persisted when patients were grouped by stable disease (n = 5) or disease progression (n = 3) (Figure 1A). However, given TET2's defined role at enhancer regions, global methylation averages may not fully capture its activity in TET2MT cases. Therefore, we next focused on site-specific methylation changes before (cycle 1, day 1 predose) and after treatment (end of cycle 3, postdose). Significant methylation changes were observed in both stable disease and disease progression groups (Figure 1B). Using peripheral blood mononuclear cells genome annotations from the Roadmap Epigenomics project,20 we examined whether AA treatment affected methylation at different genomic regions. In patients with stable disease, hypomethylated cytosine-phosphate-guanine sites (CpGs) were enriched at enhancer sites (enhancer [Enh]; bivalent enhancer [EnhBiv], and genic enhancer [EnhG]), whereas hypermethylated CpGs were enriched in quiescent and repressed regions (Quies and ReprPCWk; Figure 1C). This enhancer enrichment was absent in patients with disease progression (supplemental Figure 6). In stable disease patients, we integrated methylation changes with observed hydroxymethylation changes after treatment and found that hypomethylation correlated with increased hydroxymethylation (Figure 1D), suggesting these CpGs as potential targets of IV AA–enhanced TET activity.
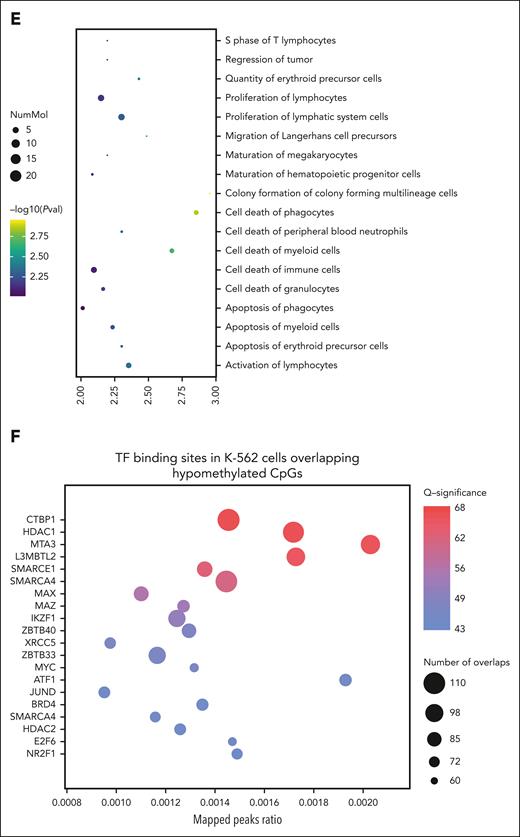
Pathway analysis revealed that hypomethylated sites in patients with stable disease are involved in the development, function, and survival of HSPCs (Figure 1E). Previous studies suggest TET2MT could predispose to malignancy through methylation-driven disruption of transcription factor (TF) binding.21 To assess this, we overlapped the differentially methylated regions identified with chromatin immunoprecipitation sequencing data from the K-562 cell line for 413 TF available through ReMap.22 This highlighted key TFs involved in HSPC differentiation and function, such as IKZF1 and MAZ, and others regulating transcriptional repression and chromatin remodeling, including CTBP1, HDAC1, SMARCA4, and SMARCE1 (Figure 1F).23,24 These findings suggest that, in select cases, IV AA may enhance TET activity to counteract methylation dysregulation at enhancer regions.
This study assessed single-agent high-dose IV AA in patients with TET2MT CCUS, demonstrating safety, while longitudinally monitoring for responses, including genetic and epigenomic changes. Although there were no clinical responses based on MDS criteria, the efficacy of high-dose IV AA may have been hindered by the clonal complexity of enrolled patients. Despite the absence of significant changes in TET2 mutational allele burdens over time, we observed an epigenetic impact on critical enhancer regions in HSPCs among patients with stable disease, likely due to augmented TET2/TET3 activity. These findings justify further investigation of IV AA, potentially in combination with other epigenetic agents.25 As a next step, we have initiated a pilot study (NCT03418038, Arm E), combining high-dose IV AA with standard-dose decitabine in patients with TET2MT CMML.
This study was approved by the Mayo Clinic Institutional Review Board.
Acknowledgments
The authors extend their heartfelt gratitude to the patients who participated in this clinical trial. Their invaluable contribution and commitment to advancing medical knowledge have been instrumental in the progress of the clonal cytopenia(s) of undetermined significance research. Z.X. acknowledges training from the American Society of Hematology Clinical Research Training Institute.
This study was supported in part by the Predolin Foundation Biobank at Mayo Clinic and received the generous support of Mayo Clinic Philanthropy. M.M.P. acknowledges support from the National Institutes of Health, National Cancer Institute (grant R01 CA272496-02) and SPORE (grant CA97274).
Authorship
Contribution: Z.X., M.M.P., and T.E.W. wrote the clinical trial protocol and led the clinical trial; Z.X. and B.R.L. led the data collection and analysis; Z.X. and M.M.P. contributed to the interpretation of results and wrote the first draft; T.L., C.F., and J.F. conducted the methylation analysis; M.A., A.A.M., N.G., and M.E. actively participated in patient recruitment; K.K.R. performed the immunohistochemistry stain; K.B.M. contributed to data collection; and all authors collaboratively drafted and revised the manuscript, bringing together their unique expertise to produce a cohesive and impactful contribution to this work.
Conflict-of-interest disclosure: N.G. served on the advisory boards for Disc Medicine and Agios. M.M.P. has received research funding from Kura Oncology, Epigenetix, Polaris, Solu Therapeutics, and Stemline Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Mrinal M. Patnaik, Hematology Division, Mayo Clinic, 200 First St SW, Rochester, MN 55901; email: patnaik.mrinal@mayo.edu; and Zhuoer Xie, Department of Malignant Hematology, H. Lee Moffitt Cancer Center, Tampa, FL 33612; email: zhuoer.xie@moffitt.org.
References
Author notes
Z.X. and J.F. are joint first authors.
All relevant clinical and methylation data can be made available on request from the corresponding authors, Zhuoer Xie (zhuoer.xie@moffitt.org) and Mrinal M. Patnaik (patnaik.mrinal@mayo.edu).
The online version of this article contains a data supplement.