Abstract
Agonist signals delivered through cell surface Fas induce apoptosis. However, the apoptotic program can be modulated by signals from the environment, and in particular, by signals delivered through adhesion molecules. Because neutrophil functional activity in inflammation is contingent on cell survival, and because circulating neutrophils normally die rapidly through a constitutively expressed apoptotic program, we evaluated Fas-mediated apoptosis in resting and inflammatory human neutrophils. We show that normal neutrophils respond to Fas engagement with accelerated rates of apoptosis, but cross-linking of β2 integrins or priming with bacterial lipopolysaccharide (LPS) prevents this increase. Adhesion molecule cross-linking results in increased intracellular glutathione (GSH). Augmentation of intracellular GSH with exogenous GSH or N-acetylcysteine is sufficient to reduce the Fas-triggered increase in apoptotic rates. Prevention of the activation induced GSH increase by buthionine sulfoximine, a cell permeable inhibitor of GSH biosynthesis, restored Fas responsiveness in activated neutrophils, an effect that could be blocked with exogenous GSH. Taken together, these data show that Fas-induced signaling for neutrophil apoptosis is blocked in a redox sensitive manner by costimulatory signals delivered through β2 integrins or activation by LPS, and provide a biologic explanation for sustained neutrophil survival in the inflammatory environment.
FAS (CD95/APO-1), A MEMBER of the tumor necrosis factor (TNF )/nerve growth factor receptor family, is a type 1 transmembrane receptor that is expressed constitutively on a variety of cell types.1 Ligation of Fas by agonistic antibody or by its physiologic ligand, Fas ligand (FasL), transduces a signal leading to apoptotic cell death.2 Although Fas itself lacks a discrete intracellular signaling domain, it aggregates with a family of membrane-associated molecules3 that, in turn, generate a death signal either directly,4,5 or through the downstream activation of proteases of the ICE/CED3 family.6
Fas-mediated signaling for apoptotic cell death is centrally involved in the deletion of autoreactive T cell clones,7 and is the mechanism for elimination of transformed cells by natural killer cells.8 Following their release from bone marrow stores, neutrophils are induced to die a rapid death by apoptosis, with the result that the entire circulating population is eliminated within 72 hours.9 Since neutrophils express both Fas and its physiologic ligand, it has been proposed that autocrine Fas/FasL interactions mediate the constitutive apoptosis of this cell population.10
Rapid expression of programmed cell death in the neutrophil may serve to limit nonspecific tissue injury resulting from the release of proteases and oxygen intermediates by activated neutrophils. The apoptotic program in the neutrophil is exquisitely sensitive to the influence of factors in the local inflammatory environment. Neutrophil apoptosis is delayed, for example, by bacterial lipopolysaccharide (LPS),11 or host-derived cytokines including granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interleukin-8 (IL-8), IL-1, and interferon-γ.11-13 Its expression is similarly delayed by the process of transmigration into an inflammatory focus, an effect mediated in part by cross-linking of the β2 integrins, CD11a and CD11b.14 Conversely, the phagocytosis of Escherichia coli,15 and cytokines such as IL-4, transforming growth factor-β (TGF-β), and TNF-α16-18 accelerate expression of the apoptotic program.
Fas engagement signals for the programmed cell death of a number of hematopoietic cells, including lymphocytes,19 eosinophils,20 monocytes,21 and neutrophils.9 Fas-triggered signaling in the monocyte can be modulated by intracellular reactive oxygen intermediates.21 Therefore, we sought to evaluate the role of Fas-triggered signaling for apoptosis in the neutrophil, and to determine its susceptibility to modulation during neutrophil activation. We show that resting neutrophils respond to Fas engagement with accelerated rates of apoptosis, but that activated neutrophils are refractory to signals delivered through Fas. Moreover, the studies suggest that this state of nonresponsiveness to Fas-mediated signals results from elevated levels of intracellular thiols.
MATERIALS AND METHODS
Reagents.Dulbecco's modified Eagle's medium (DMEM), penicillin, and streptomycin solution, L-glutamine, phosphate-buffered saline, and fetal calf serum (FCS) were purchased from GIBCO Life Technologies Ltd (Burlington, Ontario, Canada). Dextran T500 and Ficoll were purchased from Pharmacia (Baie D'Urfe, Quebec, Canada). The endothelial cell line, ECV-304, was supplied by American Type Culture Collection (ATCC, Rockville, MD). LPS from E coli 0111:B4 was purchased from Difco Laboratories (Detroit, MI) and dihydrorhodamine 123 from Molecular Probes (Eugene, OR). All other chemicals, unless otherwise stated, were purchased from Sigma Chemical Co (San Diego, CA). E-Lyse was supplied by Cardinal Associates Inc (Santa Fe, NM).
Antibodies.Murine antibodies to human CD11b (Bear-1) and Fas (CH-11), a mouse IgM negative control, fluorescein isothiocyanate (FITC)-conjugated murine antihuman Fas (UB2), FITC-labeled goat antimouse IgG, and F(ab′ )2 were purchased from Immunotech (Burlington, Ontario, Canada). Murine antihuman CD11b (Bear-1) and L-selectin (Dreg-56) FITC-conjugated antibodies were purchased from Becton Dickinson (Mountain View, CA).
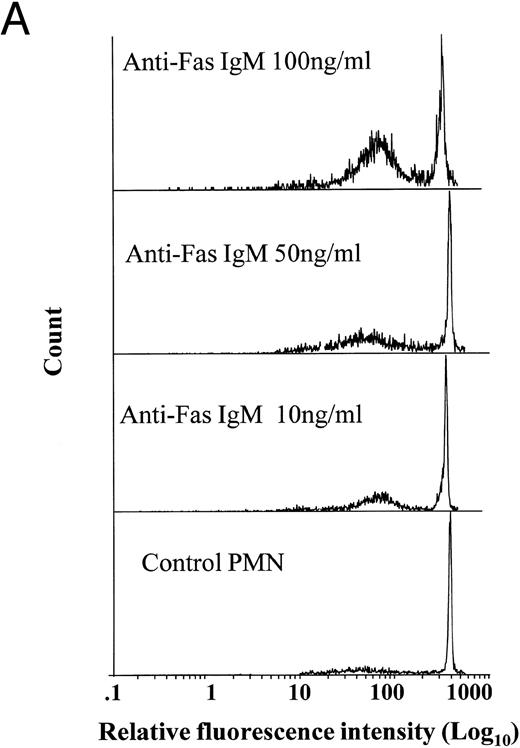
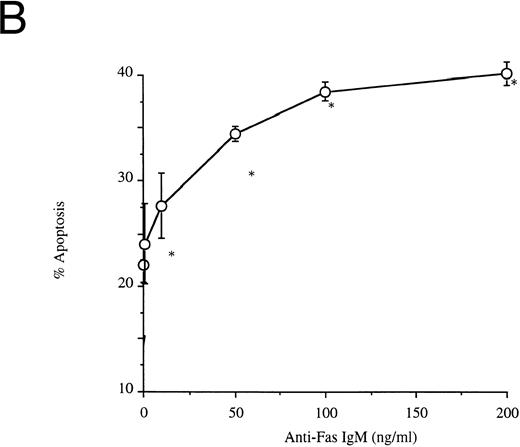
Effects of anti-Fas IgM ligation on neutrophil apoptosis. Human neutrophils were incubated with increasing concentrations of anti-Fas IgM, CH-11, and assessed for apoptosis after 18 hours. Apoptosis was measured by flow cytometry using PI DNA staining with representative histograms shown in (A) and the percentage of cells with apoptotic DNA plotted in Fig 1B. (*P < .05 v ng/mL anti-Fas). The high-intensity peak at a relative fluorescence intensity of 500 Ln represents normal DNA, whereas the lower peak between 10 and 100 Ln intensity indicates apoptotic or fragmented DNA with reduced uptake of propidium iodide. Results are the mean ± SD for n = 5 experiments with three separate donors of neutrophils.
Effects of anti-Fas IgM ligation on neutrophil apoptosis. Human neutrophils were incubated with increasing concentrations of anti-Fas IgM, CH-11, and assessed for apoptosis after 18 hours. Apoptosis was measured by flow cytometry using PI DNA staining with representative histograms shown in (A) and the percentage of cells with apoptotic DNA plotted in Fig 1B. (*P < .05 v ng/mL anti-Fas). The high-intensity peak at a relative fluorescence intensity of 500 Ln represents normal DNA, whereas the lower peak between 10 and 100 Ln intensity indicates apoptotic or fragmented DNA with reduced uptake of propidium iodide. Results are the mean ± SD for n = 5 experiments with three separate donors of neutrophils.
Isolation of human neutrophils.Blood from 10 healthy volunteers was collected in heparinized tubes. Neutrophils (PMN) were isolated by dextran sedimentation and centrifugation through a discontinuous ficoll gradient; red blood cells were lysed using E-Lyse.10 The PMN pellet was resuspended in DMEM supplemented with 10% FCS, 1% glutamine, and 1% penicillin/streptomycin solution at a concentration of 1 × 106 cells/mL. Cells were incubated in polypropylene tubes to prevent adherence. PMN purity was greater than 95% as assessed by size and granularity on flow cytometry.
Quantification of PMN apoptosis.Neutrophil apoptosis was quantitated using the technique of Nicoletti et al.22 Briefly, PMN cell suspensions were centrifuged at 200g for 10 minutes. The cell pellets were gently resuspended in 1 mL of hypotonic fluorochrome solution (50 μg/mL PI, 3.4 mmol/L sodium citrate, 1 mmol/L Tris, 0.1 mmol/L EDTA, 0.1% Triton X-100 [Sigma]) and stored in the dark at 4°C for 3 to 4 hours before they were analyzed using a Coulter EPICS XL-MCL cytofluorometer (Miami, FL). A minimum of 5,000 events were collected and analyzed. Apoptotic nuclei were distinguished from normal nuclei by their hypodiploid DNA; debris was excluded from analysis by raising the forward threshold. All measurements were performed under the same instrument settings.
Neutrophil migration.Neutrophil transmigration was performed as previously described.14 Briefly, transwell chambers, 6-well size (Costar, Cambridge, MA), with polycarbonate membranes of 3.0 μm pore size were coated for 1 hour with fibronectin (50 μg/mL), then seeded with the endothelial cell line ECV-304 (1 × 106 /mL). After 24-hours incubation at 37°C and 5% CO2, plates were washed three times in assay medium. Confluence of ECV-304 monolayer with tight cell junctions was confirmed by the ability of the monolayer to prevent diffusion of radiolabeled 125I-labeled albumin diffusion. Control PMN, and PMN incubated with LPS (1 μg/mL for 1 hour) (1 × 106 /mL) were added to the top chamber and allowed to migrate in response to FMLP (10−7 mol/L), for 45 minutes at 37°C and 5% CO2. Migrated PMN were collected from the bottom chamber, washed, and resuspended at 1×106 PMN/mL, a concentration that necessitated pooling cells from 2- to 3-bottom chambers.
Cross-linking.To cross-link β2-integrin receptors, PMN (2 × 106/200 μL) were incubated at 4°C for 20 minutes with differing concentrations of monoclonal antibody, then washed twice in cold Hanks' balanced salt solution (HBSS) without Mg2+/Ca2+. Cells were then incubated with F(ab′ )2 fragments for 20 minutes at 4°C, to cross-link the primary antibody. Washed PMN were resuspended at 1 × 106/mL and incubated at 37°C before study. To confirm cross-linking, FITC-conjugated F(ab′ )2 fragment was used to cap the primary antibodies, then intensity of binding was analyzed by flow cytometry.14
Respiratory burst activity.Generation of reactive oxygen intermediates was assessed by the technique of Smith et al.23 Briefly, neutrophils (5 × 105 /mL) were incubated with 1 μmol/L dihydrorhodamine 123 for 5 minutes at 37°C and then stimulated with PMA (5 nmol/L) for 10 minutes. Neutrophil fluorescence intensity was assessed by flow cytometry and expressed as Ln mean channel fluorescence (LnMCF ).
Chemiluminescence.Whole cell chemiluminescence was assessed by the method of Allen et al.24 Briefly, 20 μL of cells at a concentration of 5×106 /mL was added to 400 μL of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) balanced salt solution. PMA (5 nmol/L) stimulated chemiluminescence was followed over a 60-minute period using an Automat LB 953 (Wildbad, Germany). Total chemiluminescence during this interval was integrated using software provided by the manufacturer.
Assay of intracellular glutathione (GSH).GSH was assessed using a DTNB (5,5′-dithiobis ([2-nitrobenzoic acid])-based assay as described by Jocelyn et al.25 Isolated PMN (5 × 106 /mL) were sonicated for 30 seconds in 300 μL of 5% 5-sulfosalicylic acid, and centrifuged for 10 minutes at 10,000g. The resultant acid thiol extract was assayed for nonprotein sulfhydryls by quantitating the reduction of DTNB through its conversion to TNB (5-thiol-2 nitrobenzoic acid) at 412 nm using a spectrophotometer. Sample values were then calculated from a standard curve generated using known amounts of GSH and expressed as nmol/107 PMN.
Statistics.Statistical analysis was performed using ANOVA with Scheffe's correction; significance was assumed for values of P < .05.
RESULTS
Ligation of Fas induces neutrophil apoptosis.Incubation of resting neutrophils with an agonistic anti-Fas antibody (anti-Fas IgM), CH-11, caused a dose-dependent increase in rates of spontaneous apoptosis in vitro (Fig 1A and B). Neutrophils incubated with anti-Fas IgM (CH-11, 100 ng/mL) and washed after 1 hour also showed the characteristic morphologic features of apoptosis when examined by light microscopy (data not shown).
Surface expression of Fas on the neutrophil was evaluated using an FITC-conjugated murine anti-Fas IgM. Resting neutrophils express Fas with a relative fluorescence intensity of 8.2 ± 0.3 LnMCF, significantly higher than the binding of an isotype-matched mouse IgM negative control (data not shown).
Activated neutrophils are resistant to Fas-triggered apoptosis.We have previously shown that rates of spontaneous apoptosis are retarded in neutrophils that have migrated into an inflammatory focus; this refractory state can be reproduced in vitro by exposure to LPS, by transmigration across an endothelial cell monolayer in response to fMLP, or by cross-linking of cell surface β2 integrins.14 Each of these maneuvers resulted in functional and phenotypic changes of activation, evidenced by increased levels of intracellular reactive oxygen intermediates, upregulation of cell surface CD11b, and shedding of L-selectin; none, however, altered Fas expression (Table 1). The increased levels of reactive oxygen intermediates, although statistically significant, were biologically modest: PMA treatment of resting neutrophils (5 nmol/L for 10 minutes) generated levels of reactive oxygen intermediates of 495 ± 48 LnMCF, or 14-fold higher than those resulting from LPS stimulation.
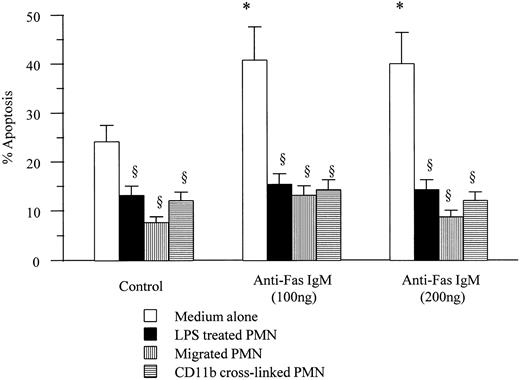
Neutrophils incubated with LPS (1 μg/mL) for 1 hour showed a significant delay in rates of spontaneous apoptosis. Unlike resting neutrophils, LPS primed neutrophils were refractory to the proapoptotic effects of incubation with anti-Fas IgM at doses of up to 200 ng/mL. Similarly transmigrated neutrophils and neutrophils in which the β2-integrin CD11b had been cross-linked failed to respond to anti-Fas IgM engagement with increased rates of apoptosis (Fig 2).
Effects of anti-Fas IgM on apoptotic rates of activated neutrophils. Neutrophils (1 × 106/mL) incubated with LPS (1 μg/mL), migrated through an endothelial cell/fibronectin monolayer, or cross-linked with antibodies to CD11b, were incubated with anti-Fas IgM (100 or 200 ng/mL) for 1 hour before being washed. Apoptosis was assessed after 18-hour incubation in medium alone by flow cytometry. *P < .05 versus control, ρP < .05 versus medium alone. Results are the mean ± SD for n = 5 experiments with four separate donors of neutrophils.
Effects of anti-Fas IgM on apoptotic rates of activated neutrophils. Neutrophils (1 × 106/mL) incubated with LPS (1 μg/mL), migrated through an endothelial cell/fibronectin monolayer, or cross-linked with antibodies to CD11b, were incubated with anti-Fas IgM (100 or 200 ng/mL) for 1 hour before being washed. Apoptosis was assessed after 18-hour incubation in medium alone by flow cytometry. *P < .05 versus control, ρP < .05 versus medium alone. Results are the mean ± SD for n = 5 experiments with four separate donors of neutrophils.
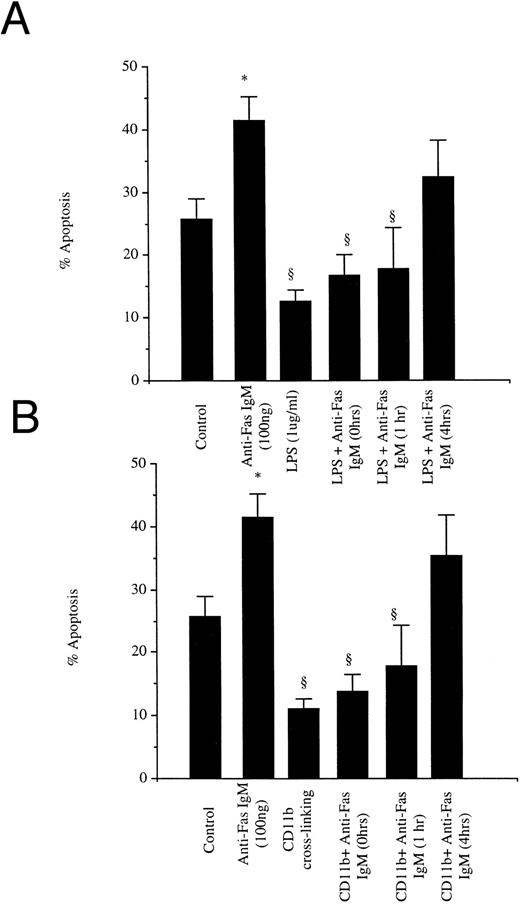
The refractoriness of activated neutrophils to anti-Fas IgM-triggered apoptosis was a transient, reversible phenomenon. By 4 hours after exposure to LPS or cross-linking of CD11b, cultured cells again responded to anti-Fas IgM engagement with an accelerated apoptotic rate (Fig 3). The restoration of susceptibility to anti-Fas antibody required that the LPS and CD11b antibody be washed out of the culture plates.
Temporal course of responsiveness to anti-Fas IgM ligation. Neutrophils (1 × 106/mL) were incubated with LPS (1 μg/mL) (A) or cross-linked with antibodies to CD11b (8 μg/mL) (B) for 0, 1, or 4 hours, washed, and then incubated with anti-Fas IgM (100 ng/mL for 1 hour). Apoptosis was assessed by flow cytometry at 18 hours after incubation with LPS or CD11b ligation. *P < .05 versus control, ρ P < .05 versus Fas (100 ng/mL). Results are the mean ± SD for n = 5 experiments with four separate donors of neutrophils.
Temporal course of responsiveness to anti-Fas IgM ligation. Neutrophils (1 × 106/mL) were incubated with LPS (1 μg/mL) (A) or cross-linked with antibodies to CD11b (8 μg/mL) (B) for 0, 1, or 4 hours, washed, and then incubated with anti-Fas IgM (100 ng/mL for 1 hour). Apoptosis was assessed by flow cytometry at 18 hours after incubation with LPS or CD11b ligation. *P < .05 versus control, ρ P < .05 versus Fas (100 ng/mL). Results are the mean ± SD for n = 5 experiments with four separate donors of neutrophils.
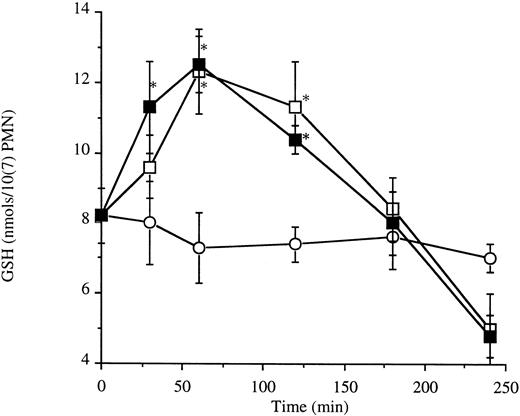
Fas engagement does not alter levels of intracellular reactive oxygen intermediates or thiols.Depletion of endogenous antioxidant thiols induces apoptosis of resting neutrophils26; conversely, superoxide anion has been shown to inhibit anti-Fas IgM-induced apoptosis in a TNFR p55-Fas transfected M14 cell line.27 Activated neutrophils manifest a rapid but transient increase in levels of intracellular reactive oxygen intermediates and GSH (Table 1 and Fig 4). Therefore, we hypothesized that altered responsiveness to anti-Fas IgM in activated neutrophils might occur through a redox-sensitive mechanism.
Neutrophil intracellular glutathione levels. Neutrophils (10 × 106/5 mL) were incubated with LPS (1 μg/mL) (□) or cross-linked with antibodies to CD11b (8 μg/mL) (▪). *P < .05 versus control (○). Results are the mean ± SD for n = 3 experiments with three separate donors of neutrophils.
Neutrophil intracellular glutathione levels. Neutrophils (10 × 106/5 mL) were incubated with LPS (1 μg/mL) (□) or cross-linked with antibodies to CD11b (8 μg/mL) (▪). *P < .05 versus control (○). Results are the mean ± SD for n = 3 experiments with three separate donors of neutrophils.
Engagement of Fas had no effect on levels of intracellular reactive oxygen intermediates as measured by oxidation of dihydrorhodamine or chemiluminescent response to PMA (5 nmol/L) (Table 2); similarly, Fas engagement alone had no effect on GSH levels (Table 2).
Increased levels of GSH inhibit Fas-induced apoptosis.Although Fas induced apoptosis did not require changes in the intracellular redox state, refractoriness to Fas-induced apoptosis in activated neutrophils was associated with increased levels of reactive oxygen intermediates and GSH. We have previously showed that depletion of GSH induces neutrophil apoptosis, an effect that can be reversed by the antioxidant, NAC (N-acetylcysteine). Therefore, we hypothesized that elevated GSH levels following neutrophil activation might block Fas-mediated apoptotic signaling.
The antioxidant NAC is rapidly deacylated and converted to glutathione after its uptake by the cell.28 Augmentation of intracellular GSH levels by incubation of neutrophils with either authentic GSH (25 mmol/L) or NAC (25 mmol/L) raised intracellular concentrations of GSH to levels observed in activated neutrophils (Fig 5A), and reduced the percentage of cells undergoing apoptosis in response to anti-Fas IgM (Fig 5B). Although apoptotic rates were reduced, they did not return to baseline levels. Having shown that elevation of intracellular GSH was sufficient to inhibit Fas-triggered apoptosis of resting neutrophils, we then sought to determine whether elevation of GSH was necessary to Fas-triggered apoptosis of activated cells.
Effects of the antioxidants GSH and NAC on neutrophil GSH levels (A) and Fas induced apoptosis (B). (A) Neutrophils (10 × 106/mL) were incubated for 1 hour with GSH (25 mmol/L) or NAC (25 mmol/L), washed extensively, then assayed for intracellular GSH levels as described in Materials and Methods. (B) Neutrophils (1 × 106/mL) were incubated for 1 hour with GSH (25 mmol/L) or NAC (25 mmol/L), then incubated with anti-Fas IgM (100 ng/mL) for 1 hour before washing. Apoptosis was assessed by flow cytometry 18 hours after the addition of anti-Fas IgM. *P < .05 versus control; ρP < .05 versus control PMN and medium alone. Results are the mean ± SD for n = 4 experiments with four separate donors of neutrophils.
Effects of the antioxidants GSH and NAC on neutrophil GSH levels (A) and Fas induced apoptosis (B). (A) Neutrophils (10 × 106/mL) were incubated for 1 hour with GSH (25 mmol/L) or NAC (25 mmol/L), washed extensively, then assayed for intracellular GSH levels as described in Materials and Methods. (B) Neutrophils (1 × 106/mL) were incubated for 1 hour with GSH (25 mmol/L) or NAC (25 mmol/L), then incubated with anti-Fas IgM (100 ng/mL) for 1 hour before washing. Apoptosis was assessed by flow cytometry 18 hours after the addition of anti-Fas IgM. *P < .05 versus control; ρP < .05 versus control PMN and medium alone. Results are the mean ± SD for n = 4 experiments with four separate donors of neutrophils.
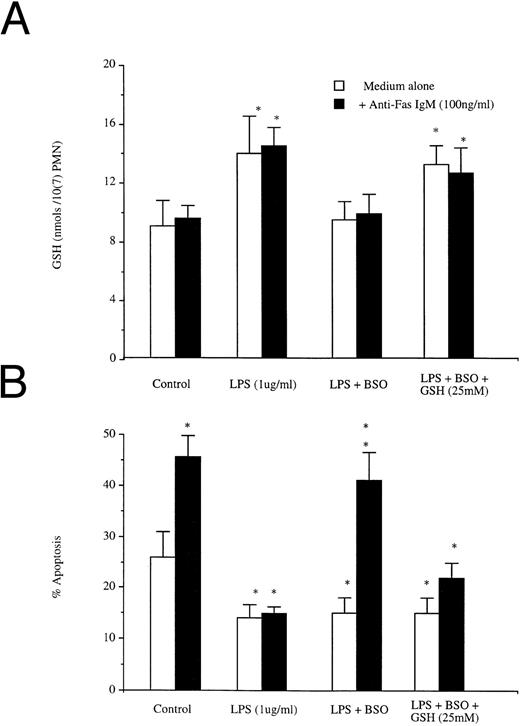
In resting cells, Fas ligation triggered apoptosis without a corresponding increase in intracellular GSH; LPS induced both a rise in concentrations of intracellular GSH and a reduction in apoptotic rates that was not altered by ligation of Fas (Fig 6A). Buthionine sulfoximine (BSO) is a specific inhibitor of GSH biosynthesis, but has no effect on extant levels of preformed protein.29 BSO pretreatment did not alter GSH levels or Fas-induced apoptosis in resting neutrophils (data not shown). Pretreatment of neutrophils with 50 mmol/L BSO before LPS exposure prevented the LPS-induced increase in GSH (Fig 6A), but not the LPS-induced reduction in apoptosis (Fig 6b). However, blockade of the LPS-triggered increase in GSH resulted in restoration of normal responsiveness to Fas (Fig 6B). Restoration of GSH levels after BSO and LPS treatment with exogenous GSH (25 mmol/L) was associated again with a resistance to Fas-induced apoptosis.
Effects of BSO on (A) LPS induced increases in GSH and (B) LPS-induced blockade of anti-Fas IgM induced neutrophil apoptosis. Neutrophils (1 × 106/mL) were preincubated with or without BSO (50 mmol/L) and then incubated with LPS (1 μg/mL) or LPS (1 μg/mL) + GSH (25 mmol/L), all groups were incubated after 1 hour with or without Fas (100 ng/mL). Intracellular GSH and apoptosis were assessed at 1 hour and 18 hours, respectively. *P < .05 versus control. Results are the mean ± SD for n = 3 experiments with three separate donors of neutrophils.
Effects of BSO on (A) LPS induced increases in GSH and (B) LPS-induced blockade of anti-Fas IgM induced neutrophil apoptosis. Neutrophils (1 × 106/mL) were preincubated with or without BSO (50 mmol/L) and then incubated with LPS (1 μg/mL) or LPS (1 μg/mL) + GSH (25 mmol/L), all groups were incubated after 1 hour with or without Fas (100 ng/mL). Intracellular GSH and apoptosis were assessed at 1 hour and 18 hours, respectively. *P < .05 versus control. Results are the mean ± SD for n = 3 experiments with three separate donors of neutrophils.
Together these studies show that augmentation of intracellular GSH concentrations is both necessary and sufficient to prevent Fas-mediated apoptosis of activated neutrophils.
DISCUSSION
Engagement of Fas or the p55 component of the receptor for TNF (TNFR1) results in rapid cell death through the controlled process of apoptosis.1 Such a response has clear adaptive benefit to the host in facilitating the elimination of transformed cells,8 or terminating an activated inflammatory response.9 Yet the interaction of TNF with its cellular receptor can also activate NF-κB,30 leading to enhanced proinflammatory gene transcription, a response that supports antimicrobial activity in the inflammatory microenvironment. Fas engagement has similarly been reported to activate NF-κB leading to IL-6 synthesis in an SV80 fibroblast cell line,31 although not in an L929 cell line stably transfected with Fas cDNA.32 Thus, a single signal delivered through a common receptor can produce fundamentally divergent responses: cellular activation or cellular death; the mechanism underlying the preferential expression of one response or other is unknown.
A variety of biologic functions are altered as a consequence of the adherence of neutrophils to biologic substrata,33 and cell surface adhesion molecules are now recognized to function as signaling molecules, altering gene expression in response to stimuli originating external to the cell,34 and, in particular, modulating the expression of programmed cell death.35 We have shown that engagement of adhesion molecules involved in the delivery of neutrophils to an inflammatory focus alters the temporal expression of spontaneous neutrophil apoptosis, L-selectin engagement accelerating rates and β2-integrin engagement retarding them.14 The present study sought to determine whether cell activation or adhesion molecule engagement could alter apoptotic signals delivered through the death receptor Fas.
We confirmed that quiescent neutrophils that die in vitro by spontaneous apoptosis are induced to die more rapidly when Fas is engaged with an agonist monoclonal antibody.36 It has recently been shown that neutrophils express Fas ligand constitutively, and further, that they can release soluble Fas ligand in tissue culture.37 The autocrine interaction of neutrophil Fas with soluble or cell-associated Fas ligand provides an attractive model to explain the rapid process of spontaneous apoptosis that occurs in normal neutrophils.10 Our data show that this process is susceptible to modulation during the process of neutrophil activation.
The development of a state of refractoriness to Fas-induced death signals was a transient, reversible phenomenon. It could not be explained by alterations in cell surface expression of Fas, since expression was similar in both activated and nonactivated cells. Neutrophil activation by LPS, endothelial transmigration, or CD11b cross-linking led to a rapid increase in levels of reactive oxygen intermediates and a later increase in GSH levels. Clement et al27 showed that increased superoxide levels inhibit the Fas-mediated death of melanoma cells, indicating that alterations in the redox potential of the cell influence the ability of Fas to induce apoptosis.
We have previously reported that the depletion of intracellular thiols by diethylmaleate results in increased rates of spontaneous neutrophil apoptosis, a phenomenon that can be reversed by the anti-oxidants GSH and NAC,26 although these exert no intrinsic effect on rates of spontaneous apoptosis. In the present study we found that these same antioxidants reduced the Fas-induced apoptosis of resting neutrophils. This finding is in keeping with the observation that mutant T cells expressing increased levels of intracellular glutathione as a result of augmented activity of γ-glutamylcysteine synthetase acquire resistance to Fas-mediated death signals,38 and suggests a role for intracellular thiols in cellular resistance to apoptotic stimuli. Consistent with the hypothesis that augmented levels of GSH are necessary for the inhibition of Fas-mediated apoptosis in activated neutrophils, we found that prevention of GSH synthesis in response to LPS by BSO permitted normal expression of Fas-induced signaling. Taken together, these observations show that augmentation of intracellular GSH is both necessary and sufficient to block Fas-mediated death signals in activated human neutrophils. Conversely, it can be hypothesized that depletion of GSH following neutrophil activation may again render the cell susceptible to Fas-induced apoptosis, and favor the resolution of an inflammatory response once the inciting stimulus has been removed. In keeping with the hypothesis we have previously shown that neutrophil ingestion of E coli results in the induction of apoptosis through an oxygen dependent mechanism associated with a reduction in intracellular thiol levels induced by the oxidant stress, since the effect is inhibited by the antioxidants GSH and NAC.15
Reconciliation of the divergent imperatives of cell growth and activation on the one hand, and programmed cell death and quiescence on the other is critical to the maintenance of normal immunologic homeostasis. Mice of the MRL/lpr strain, lacking functional Fas, develop an autoimmune disorder characterized by nephritis and arthritis,39 and are particularly sensitive to septic shock resulting from Staphylococcal enterotoxin B.40 In contrast, administration of an agonistic antibody to Fas induces lethal hepatitis and neutropenia in mice.41 An optimal response to an acute infectious challenge necessitates the sequential expression of cell activation and cell quiescence. The observations reported here suggest that the cell surface molecules whose upregulation accompanies neutrophil activation serve a costimulatory function, and block the apoptotic response to death signals delivered by Fas. Because this costimulatory role is dependent on augmentation of intracellular thiols, the removal of an inflammatory stimulus, may permit the return of normal apoptosis in response to Fas, and so permit the normal resolution of inflammation.
Supported in part by a Grant from the Canadian Liver Foundation (Toronto, Ontario), the Surgical ICU Research Group, the Toronto Hospital, and the Medical Research Council (Ottawa, Ontario, Canada).
Address reprint requests to John C. Marshall, MD, Eaton N 9-234, Toronto Hospital, General Division, 200 Elizabeth St, Toronto, Ontario, Canada M5G 2C4.