Abstract
The CD31 monoclonal antibody, LYP21, binds to the CD31 domain 6 and inhibits the human mixed-lymphocyte reaction (MLR) in a specific and dose-dependent fashion. A synthetic CD31 peptide based on human CD31 epitope (amino acids 551 to 574) recognized by LYP21 is equally effective in inhibiting the MLR. In this study, we used the murine homolog of CD31 peptide 551 to 574 and a control peptide to study the role of CD31 molecule on T-cell activation. In vitro, CD31 peptide inhibited the MLR across several major and minor histocompatibility differences in a specific and dose-dependent fashion, similar to the results observed in the human system. Maximal inhibition was achieved at a dose of 200 μg/mL. In the cytotoxic T-lymphocyte (CTL) assay, CD31 peptide inhibited CTL responses by 97%. To study the in vivo effect of this peptide, graft-versus-host disease (GVHD) across minor histocompatibility barriers was induced in the B10.D2 (H-2d) → BALB/c (H-2d) model. BALB/c recipients received CD31 peptide (100 μg/d), or phosphate-buffered saline (PBS), or control peptide (100 μg/d) intraperitoneally (IP) for the first 5 weeks. CD31 peptide delayed onset of graft-versus-host disease and significantly increased long-term survival. Twelve of 14 mice receiving CD31 peptide survived more than 100 days after transplantation, as compared with none of 10 mice receiving PBS and none of five mice receiving control peptide (P = .0001). Long-term engraftment of allogeneic bone marrow was documented in all transplanted mice by polymerase chain reaction (PCR) analysis of microsatellite region in the interleukin (IL)-1β gene. Our data suggest that the CD31 molecule has an important functional role in T-cell activation in vitro and in vivo.
CD31 IS A MEMBER of the Ig gene superfamily of adhesion molecules. It is expressed on vascular endothelium, platelets, monocytes, neutrophils, and lymphocytes.1-4 In T lymphocytes, the highest surface expression of CD31 is associated with the CD8+CD45RA+ naive subset.5-7 Lower level expression of CD31 has been observed on CD8+CD45RO+ memory cells and CD4+ cells and B lymphocytes. CD31 also has a role in intercellular adhesion, as demonstrated by transfection of CD31 cDNA into mouse L cells. This transfection resulted in temperature- and calcium-dependent aggregation, which was inhibitable by anti-CD31 monoclonal antibodies.8-11 CD31 is localized to intercellular contact points in confluent vascular endothelial cells. Polyclonal antibodies against CD31 prevented endothelial confluence in tissue cultures.12,13 Moreover, CD31 antibodies interfered with leukocytes egress from the vasculature to sites of inflammation in animal models.14,15 The role of CD31 in cell activation has been suggested by the demonstration that lymphocyte, endothelial cell, and platelet activation results in immediate phosphorylation of CD31 by a protein kinase C–dependent mechanism.4 Following lymphocyte activation, CD31 mRNA is downregulated at the transcriptional level and there is increased surface turnover of CD31 protein.4
One CD31 monoclonal antibody, LYP21, inhibited the human mixed-lymphocyte reaction (MLR) in a specific and dose-dependent fashion. The LYP21 epitope was localized to the sixth immunoglobulin domain of CD31. A CD31 peptide (amino acids 551 to 574) based on the epitope was also found to strongly inhibit the human MLR.16 The cDNA cloned for murine CD31 indicated 70% to 80% homology to the human sequence17 and murine CD31 has a tissue distribution similar to that of human CD31.18 In this current study, the murine homolog of CD31 peptide 551 to 574 and a control peptide were synthesized to study the effect of CD31 molecule on lymphocyte activation in vitro and in vivo, specifically in graft-versus-host disease (GVHD).
MATERIALS AND METHODS
Animals.B10.D2/nSnJ (H-2d), BALB/c (H-2d), C57BL/6 (H-2d), and C3H (H-2k) mice were obtained from the breeding colony of the Department of Comparative Medicine at the Stanford University School of Medicine. PL/J (H-2u) and CBA (H-2k) mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Bone marrow transplantation.For the induction of GVHD, 1.25 × 106 B10.D2/nSnJ bone marrow cells and 5 × 106 B10.D2/nSnJ spleen cells were injected intravenously into lethally irradiated (8.0 Gy) BALB/c recipients' tail vein. B10.D2/nSnJ donor and BALB/c recipient mice (both H-2d) differ at multiple minor histocompatibility loci.19 Recipient mice were 12 to 13 weeks old at the time of transplant.
Peptides.CD31 peptide (SSMRTSPRSSTLAVRVFLAPWK) and a control peptide (PVAAALVPLASVTSLSLILAIHA) were synthesized on a Milligen Biosearch 9050 Peptide Synthesizer (Milligen Biosearch, Bedford, MA) by the Beckman Protein and Nucleic Acid Facility at Stanford. Peptides were purified by high-performance liquid chromatography, and were checked by amino acid analysis and mass spectroscopy.
Peptide treatment.Recipient BALB/c mice received 100 μg of CD31 peptide, 100 μg of control peptide, or phosphate-buffered saline (PBS) intraperitoneally (IP) daily for the first 5 weeks after transplant. Once a week, 100 μg of peptides was administered with incomplete Freund's adjuvant as a depot dose. Treatment was discontinued on day 36. The total dose of peptide per mouse was 3.5 mg.
Assessment of GVHD.Mice were monitored for 100 days after transplant for both clinical and histologic evidence of GVHD. Clinical GVHD was monitored by mortality, loss of body weight, and hair loss.20 Histologic changes were assessed as previously described.20
Polymerase chain reaction (PCR) analysis.Engraftment of donor bone marrow was documented by PCR analysis of a polymorphic microsatellite region within the murine interleukin-1β (IL-1β) gene. DNA was prepared from peripheral blood mononuclear cells or spleen cells 80 to 100 days after transplant according to standard protocols.21 Primer sequences are as follows: 5′-CCAAGCTTCCTTGTGCAAGTA-3′ and 5′-AAGCCCAAAGTCCATCAGTGG-3′.22 Oligonucleotides were synthesized on a 391 DNA synthesizer (Applied Biosystems, Foster City, CA). PCR conditions were as follows: 25 μL total volume with 250 ng of genomic DNA as template, 25 pmol primers, 0.4 mmol/L each dNTP, 3 mmol/L Mg2+, 2.5 μL 10 × PCR buffer, and 1.0 U AmpliTAQ DNA-polymerase (Perkin-Elmer, Emeryville, CA). Amplification was performed for 30 cycles, with 1 minute of denaturation at 94°C, 1 minute of annealing at 57°C, and 1 minute of elongation at 72°C.
Primary MLR across major histocompatibility barriers.A total of 2.5 × 105 responder spleen cells was plated with 5 × 105 irradiated (30 Gy, cesium source) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H]thymidine per well for an additional 16 hours. Results are mean counts per minute (cpm) from triplicate cultures.23
Secondary MLR across major histocompatibility barriers.Secondary MLRs across major histocompatibility barriers were set up as previously described.24 Briefly, 5 × 106 BALB/c spleen cells were primed with 5 × 106 C57BL/6 irradiated spleen cells in each well of 24-well plates with final volume of 1.5 mL medium per well in the presence of CD31 peptide or control peptide (200 μg/mL) or in the absence of peptide. The culture medium was RPMI 1640, supplemented with 10% human AB serum, 2 mmol/L glutamine, 50 μmol/L 2-mercaptoethanol, 100 U penicillin/mL, and 100 μg streptomycin/mL. Cells were cultured at 37°C in a 5% CO2 atmosphere for 4 days, harvested on day 5 and washed with RPMI 1640 three times, and resuspended in fresh culture medium without CD31 peptide at 3 to 5 × 106 cells/mL, then cultured in T-25 flask for an additional 3 days. Rested cells were harvested on day 9 and counted with Trypan-blue. A total of 2.5 × 105 primed cells was restimulated with 5 × 105 fresh stimulator C57BL/6 cells or 5 × 105 fresh stimulator CBA cells or concanavalin A (ConA); (2 μg/mL). IL-2 (200 IU per well) was added to the additional wells, where primed cells were restimulated with irradiated BALB/c stimulator.
Primary MLR across minor histocompatibility barriers.A total of 1 × 106 nylon wood–purified responder T cells was plated with 1 × 106 irradiated (10 Gy) spleen cell stimulators25 in the absence or presence of CD31 peptide or control peptide. After 5 days' incubation, cells were harvested and counted (cultures were pulsed with 1 μCi [3H]thymidine per well for 16 hours before harvest). Results are the mean cpm from triplicate cultures.
Cytotoxic T-lymphocyte (CTL) assay.Lyt2+-AR1 mouse CTL line is restricted to class I H-2d and was originally derived from C57BL/6 mice (kindly provided by Dr I.L. Weissman, Stanford, CA). AR1 cells were cocultured with irradiated BALB/c spleen cells for 6 days before the assay. AR1 cells were washed and plated at increasing effector to target (E:T) ratios (0.5:1, 1:1, 2.5:1, 5:1, 10:1, and 20:1) with 51Cr-labeled ConA-activated cells (for 36 to 48 hours) as targets in a standard 4-hour 51Cr-release assay. The percentage of specific 51Cr release was calculated according to the following equation: % Specific Release = [(Test Release) − (Spontaneous Release)]/[(Maximal Release) − (Spontaneous Release)] × 100%.
Spontaneous release was obtained by incubating target cells in medium alone. Maximal release was obtained after treatment with 2% Nonidet P-40 (Sigma, St Louis, MO).
1934.4 hybridoma assay.The Ac1-11 [4A]-reactive I-Au restricted T-cell hybridoma 1934.4 had been prepared by fusion of T-cell clone PJR-2526 with the AKR thymoma BW 5147 cells27 and was a kind gift of Dr L. Steinman, Stanford, CA. A total of 5 × 104 1994.4 hybridoma cells was incubated with 5 × 105 PL/J spleen cells and various concentrations (0.01 to 1,000 μmol/L) of Ac1-11 [4A] for 24 hours in the presence or absence of CD31 peptide. The IL-2 production of the Ac1-11[4A]-reactive hybridoma 1994.4 was used as a readout system for antigen presentation, and IL-2 production was measured by proliferation of the IL-2–dependent cell line HT-2 in the presence of supernatants from 1994.4 cultures.27 Results are expressed as the mean cpm from triplicate cultures.
Immunofluorescence analysis.Single-color flow cytometric analysis was performed using FACStar (Becton Dickinson, Mountain View, CA). Cells (1 × 106) were stained with phycoerythrin (PE)-conjugated rat antimouse CD31 or isotype control PE-conjugated rat IgG2a (Pharmingen, San Diego, CA) at 4°C for 30 minutes. Subsequently, the cells were washed twice and resuspended in RPMI 1640 containing 4% fetal calf serum. The data were expressed in histogram form, with the Y-axis representing cell number and x-axis fluorescence intensity. The percentage of cells considered positive was determined by threshold set by negative isotype staining.
Statistical analyses.Group comparisons of the onset of GVHD were made by Fisher's exact test. Survival data were analyzed by Mann-Whitney log-rank analysis. Probability (P ) values less than .05 were considered significant.
RESULTS
Inhibition of MLR across major and minor histocompatibility barriers.The role of CD31 was tested in MLR across major histocompatibility complex (MHC) barriers. T-cell activation was assessed in three different MHC-disparate strain combinations (Fig 1). CD31 peptide inhibited the MLR in a specific and dose-dependent fashion. Maximum inhibition (up to 96%) was found at peptide concentrations of 200 μg/mL. Higher concentrations of CD31 peptide did not increase inhibition (data not shown). Control peptide did not inhibit the MLR. Similar data were generated across minor histocompatibility barriers using B10.D2/nSnJ (H-2d) cells as responders and BALB/c (H-2d) as stimulators. This strain combination is the same as the in vivo system. CD31 peptide inhibited the MLR, whereas the control peptide had no effect (Fig 2).
Inhibition of MLR across major histocompatibility barriers. (A) BALB/c (H-2d) × C57BL/6 (H-2b); (B) BALB/c × PL/J (H-2u); (C) BALB/c × C3H (H-2k). A total of 2.5 × 105 responder spleen cells were plated with 5 × 105 irradiated (30 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine per well for an additional 16 hours. Results are the mean cpm from triplicate cultures and representative of 3 separate experiments.
Inhibition of MLR across major histocompatibility barriers. (A) BALB/c (H-2d) × C57BL/6 (H-2b); (B) BALB/c × PL/J (H-2u); (C) BALB/c × C3H (H-2k). A total of 2.5 × 105 responder spleen cells were plated with 5 × 105 irradiated (30 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine per well for an additional 16 hours. Results are the mean cpm from triplicate cultures and representative of 3 separate experiments.
Inhibition of MLR across minor histocompatibility barriers (B10.D2/nSnJ [H-2d], responder; BALB/c [H-2d], stimulator). This combination is the same as in the in vivo system. 1 × 106 nylon wood–purified responder T cells were plated with 1 × 106 irradiated (10 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 5 days' incubation, cells were harvested and counted (cultures were pulsed with 1 mCi [3H]thymidine per well for 16 hours before harvest). Results are the mean cpm from triplicate cultures and are representative of 2 separate experiments.
Inhibition of MLR across minor histocompatibility barriers (B10.D2/nSnJ [H-2d], responder; BALB/c [H-2d], stimulator). This combination is the same as in the in vivo system. 1 × 106 nylon wood–purified responder T cells were plated with 1 × 106 irradiated (10 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 5 days' incubation, cells were harvested and counted (cultures were pulsed with 1 mCi [3H]thymidine per well for 16 hours before harvest). Results are the mean cpm from triplicate cultures and are representative of 2 separate experiments.
Effect of CD31 peptide on the secondary MLR.CD31 peptide was able to block T-cell proliferative responses to alloantigen in the primary MLR. To evaluate whether there is a long-lasting blocking effect during the initial exposure to antigen, we performed restimulating experiments. BALB/c spleen cells were cultured with irradiated C57BL/6 spleen cells for 4 days in the medium with or without 200 μg/mL CD31 peptide. Cells were harvested and washed to remove peptide, cultured in the fresh medium for additional 3 days, and then restimulated with irradiated spleen cells either from BALB/c or CBA. Cells primed in the absence of CD31 peptide showed accelerated secondary responses to the restimulators. In contrast, cells primed in the presence of CD31 peptide showed decreased responses to restimulation. Exogenous IL-2 did not enhance responsiveness induced by priming T cells in the presence of CD31. ConA stimulation was not significantly affected (Table 1). Cells primed in the presence of control peptide showed similar results to the cells primed without peptide (data not shown)
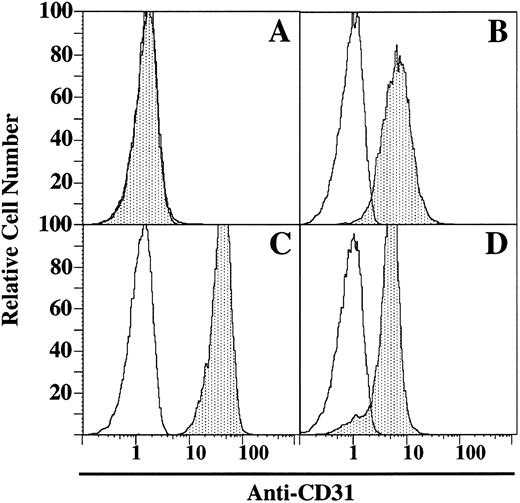
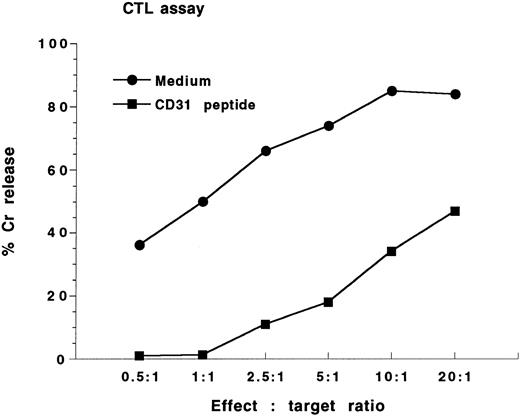
Expression of CD31 and effect of CD31 peptide on the inhibition of AR1 CTL response.The Lyt2+-AR1 mouse CTL line (specificity to class I H-2d; originally derived from C57BL/6 mice) was cocultured with irradiated BALB/c spleen cells for 6 days. Targets were ConA-activated BALB/c spleen cells. Immunofluorescence analysis showed that AR1 cell line was CD31− (Fig 3A), whereas BALB/c spleen cells were 95% CD31+ (Fig 3B). For the CTL assay, AR1 cells were washed and plated at increasing E:T ratios (0.5:1, 1:1, 2.5:1, 5:1, 10:1, and 20:1) with 51Cr-labeled ConA-activated BALB/c spleen cells as targets in the presence or absence of CD31 peptide (200 μg/mL). Figure 4 shows the results of the standard 4-hour 51Cr-release assays. Maximum lysis of BALB/c targets in the absence of CD31 peptide was 85% at an E:T ratio of 10:1. Addition of the CD31 peptide resulted in near complete inhibition of lysis.
Immunofluorescence analysis. Expression of CD31 on Lyt2+-AR. CTL line (A), ConA-activated BALB/c spleen cells (B), 1934.4 hybridoma (C), PL/J spleen cells (D). Open areas are isotope control MoAb; shaded areas are reactivity of CD31 MoAb. Results are representative of 3 separate experiments.
Immunofluorescence analysis. Expression of CD31 on Lyt2+-AR. CTL line (A), ConA-activated BALB/c spleen cells (B), 1934.4 hybridoma (C), PL/J spleen cells (D). Open areas are isotope control MoAb; shaded areas are reactivity of CD31 MoAb. Results are representative of 3 separate experiments.
CTL assay. The Lyt2+-AR1 mouse CTL line was cocultured with irradiated BALB/c spleen cells for 6 days. AR1 cells were washed and plated at increasing E:T ratios (0.5:1, 1:1, 2.5:1, 5:1, 10:1, 20:1) with 51Cr-labledBALB/c as targets in the presence or absence of CD31 peptide (200 μg/mL). Results are expressed as mean percent specific 51Cr release from triplicate wells and are reprecentative of 3 separate experiments.
CTL assay. The Lyt2+-AR1 mouse CTL line was cocultured with irradiated BALB/c spleen cells for 6 days. AR1 cells were washed and plated at increasing E:T ratios (0.5:1, 1:1, 2.5:1, 5:1, 10:1, 20:1) with 51Cr-labledBALB/c as targets in the presence or absence of CD31 peptide (200 μg/mL). Results are expressed as mean percent specific 51Cr release from triplicate wells and are reprecentative of 3 separate experiments.
Expression of CD31 and effect of CD31 peptide on antigen presentation to the 1934.4 hybridoma.1934.4 hybridoma was shown 100% CD31+ (Fig 3C), and PL/J spleen cells were 90% CD31+ (Fig 3D). For the 1934.4 hybridoma assay, IL-2 production (as gauged by HT-2 proliferation) of the Ac1-11 [4A] peptide-reactive hybridoma 1934.4 was used as a readout system for antigen presentation. Figure 5 shows the IL-2 production of 1934.4 hybridoma to increasing concentrations of Ac1-11 [4A] (0.01 to 1,000 μmol/L) in the presence or absence of CD31 peptide (200 μg/mL). The addition of CD31 peptide did not have any effect on this system.
1934.4 hybridoma assay. 1934.4 hybridoma cells (5 × 104) were incubated with 5 × 105 PL/J spleen cells and various concentrations (0.01 to 1,000 μmol/L) of Ac1-11 [4A] for 24 hours in the presence of CD31 peptide (200 μg/mL) or the absence of peptide. The IL-2 production of the Ac1-11 [4A]-reactive hybridoma 1994.4 was used as a readout system for antigen presentation. IL-2 production was measured by proliferation of the IL-2–dependent cell line HT-2 in the presence of supernatants from 1994.4 cultures. Results are expressed as the mean cpm from triplicate cultures. Results are representative of 3 separate experiments
1934.4 hybridoma assay. 1934.4 hybridoma cells (5 × 104) were incubated with 5 × 105 PL/J spleen cells and various concentrations (0.01 to 1,000 μmol/L) of Ac1-11 [4A] for 24 hours in the presence of CD31 peptide (200 μg/mL) or the absence of peptide. The IL-2 production of the Ac1-11 [4A]-reactive hybridoma 1994.4 was used as a readout system for antigen presentation. IL-2 production was measured by proliferation of the IL-2–dependent cell line HT-2 in the presence of supernatants from 1994.4 cultures. Results are expressed as the mean cpm from triplicate cultures. Results are representative of 3 separate experiments
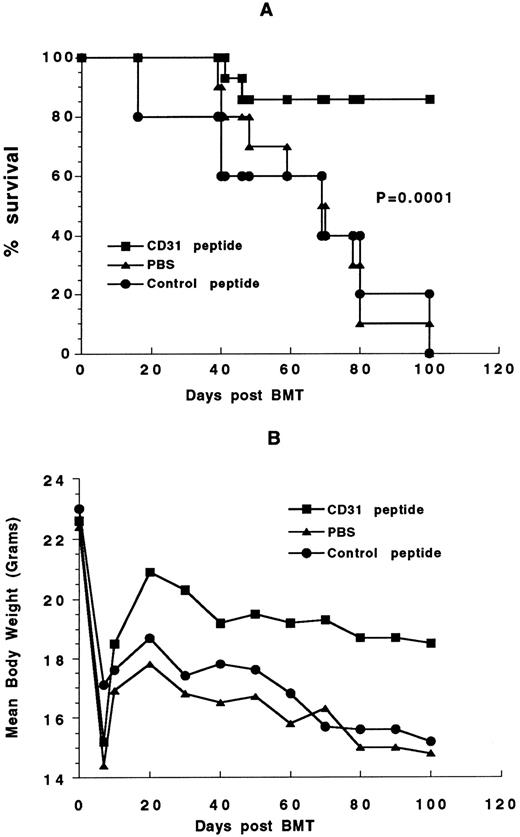
Effect of CD31 peptide on GVHD severity and mortality.Treatment with CD31 peptide for the first 5 weeks after transplantation reduced the incidence of GVHD on day 30 after transplantation from 100% (10/10 and 5/5) in the PBS and control peptide groups to 35.7% (5/14; P < .01; Table 2). In 11 of 14 mice treated with CD31 peptide, the onset of GVHD was delayed to median of 32 days (range, 26 to 60) after bone marrow transplantation compared with mice given PBS and control peptide who developed GVHD in a median of 19 days (range, 16 to 22). Moreover, the severity of the disease was also less as evidenced by a less severe reaction in the skin changes of mice with GVHD (data not shown). CD31 peptide significantly improved long-term survival from GVHD. As shown in Fig 6, 12 of 14 (86%) mice treated with CD31 survived more than 100 days after transplantation, compared with none of 10 of PBS-treated and with none of five of control peptide-treated mice. This difference was highly statistically significant at a P value of .0001 (data pooled from two similar experiments).
Effect of CD31 peptide on GVHD-related mortality and disease severity. B10.D2 bone marrow cells (1.25 × 106) and 5 × 106 spleen cells were transplanted into lethally irradiated (8.0 Gy) BALB/c recipients. Recipient mice received 100 μg/d of CD31 peptide or control peptide 100 μg/d or PBS (IP) for the first 5 weeks after transplantation. (A) Survival curve; (B) mean body weight curve. Date are pooled from 2 similar experiments.
Effect of CD31 peptide on GVHD-related mortality and disease severity. B10.D2 bone marrow cells (1.25 × 106) and 5 × 106 spleen cells were transplanted into lethally irradiated (8.0 Gy) BALB/c recipients. Recipient mice received 100 μg/d of CD31 peptide or control peptide 100 μg/d or PBS (IP) for the first 5 weeks after transplantation. (A) Survival curve; (B) mean body weight curve. Date are pooled from 2 similar experiments.
Documentation of chimerism.PCR analysis was performed to document long-term engraftment of allogeneic bone marrow cells. DNA polymorphism based on length variation in tandem repeat sequences of a microsatellite in the murine IL-1β gene was used as marker to differentiate between donor derived B10.D2 and recipient BALB/c cells. Long-term engraftment of allogeneic bone marrow was found in all transplanted mice (Fig 7).
Engraftment of donor bone marrow in the B10.D2 → BALB/c transplant documented by PCR amplification of a polymorphic microsatallite region within the IL-1β gene. Long-term engraftment of allogeneic bone marrow was found in all transplanted mice. This figure demonstrates one representative analysis on an ethidium bromide–stained 1.5% agarose gel. Lane 1, DNA marker φx174 digested with HaeIII (marker sizes: 1,353, 1,078, 872, 603, 310, 281, 271, 243, and 194 bp; GIBCO BRL, Gaithersburg, MD); lane 2, negative control (PCR without DNA); lane 3, recipient (BALB/c) standard; lane 4, donor (B10.D2) standard; lanes 5, 6, 7, and 8, recipients given CD31 peptide (ID no. 669, 670, 672, and 674); lane 9, recipient given control peptide (ID no. 691); lanes 10 and 11, recipients given PBS (ID no. 773 and 774).
Engraftment of donor bone marrow in the B10.D2 → BALB/c transplant documented by PCR amplification of a polymorphic microsatallite region within the IL-1β gene. Long-term engraftment of allogeneic bone marrow was found in all transplanted mice. This figure demonstrates one representative analysis on an ethidium bromide–stained 1.5% agarose gel. Lane 1, DNA marker φx174 digested with HaeIII (marker sizes: 1,353, 1,078, 872, 603, 310, 281, 271, 243, and 194 bp; GIBCO BRL, Gaithersburg, MD); lane 2, negative control (PCR without DNA); lane 3, recipient (BALB/c) standard; lane 4, donor (B10.D2) standard; lanes 5, 6, 7, and 8, recipients given CD31 peptide (ID no. 669, 670, 672, and 674); lane 9, recipient given control peptide (ID no. 691); lanes 10 and 11, recipients given PBS (ID no. 773 and 774).
DISCUSSION
T lymphocytes play a central role in GVHD. GVHD across minor histocompatibility barriers is induced by transplanted T cells recognizing multiple minor antigens of host as “nonself”.21,28-30 The onset of acute GVHD is related to the time required for these T cells to proliferate and differentiate.28 Both CD4+ and CD8+ T cells have been shown to contribute to the GVHD process in the B10.D2 → BALB/c system.19,23 Depletion of both CD4+ and CD8+ T cells with anti-CD4+ anti-CD8+ monoclonal antibodies prevents lethal GVHD.31 32 Our studies demonstrate that, in vitro, a peptide derived from the sixth domain of CD31 inhibited the MLR in a specific and dose-dependent fashion, similar to results using the homologous human peptide in the human MLR. The inhibition was observed over several different histocompatibility barriers, which suggests that this CD31 peptide inhibits a common pathway in T-cell activation.
These encouraging results from the in vitro studies led to in vivo studies using the CD31 peptide to prevent acute GVHD, a T-cell–mediated disease process. CD31 peptide was administered for a short time period following transplantation in the B10.D2 → BALB/c model. Mice that received this peptide appeared healthier and had delayed onset of GVHD. Moreover, mice that received CD31 peptides for only the first 5 weeks following bone marrow transplantation had a significantly improved long-term survival. These data demonstrate that peptides based on the sixth immunoglobulin domain of the CD31 molecule interfere with T-cell activation in vitro and in vivo. These data are consistent with a functional role of CD31 in the immune response and suggest that modulation of this signal leads to a significant prolongation of survival in this murine model.
The direct mechanism of CD31's involvement in T-cell activation is not yet clear, although the molecular basis for the various pathways of T-cell activation have been intensely studied in recent years33 and a number of cell-surface receptors have been implicated in the costimulation or augmentation of T-cell activation. Efficient T-cell activation requires triggering of an antigen-specific T-cell receptor (TCR), which delivers the first signal, and a costimulatory molecule, which delivers the second signal.34,35 Signal 1 is mediated by the interaction of TCR with antigenic peptide fragments presented by self class I or class II molecules encoded by the MHC.36 Administration of peptides with high binding affinity for the respective class II MHC molecules after transplantation is capable of preventing the development of GVHD.23 Signal 2 for T-cell activation is delivered by antigen-presenting cells35 and requires cell-to-cell contact.37 Signal 2 determines the outcome of the first signal, leading to either complete activation,34 partial activation,38,39 or a long-lasting state of antigen specific unresponsiveness.40,41 The most well-characterized adhesion proteins as candidate molecules for potent costimulation of T cells are CD28/B7, VCAM-1/VLA-4, and ICAM/LFA-1. B7 has been demonstrated to be the main costimulatory molecule for activated CD4+ T cells.42,43 In contrast, resting CD4+ T cells preferentially response to costimulatory signal(s) provided by either VCAM-1 or ICAM-1.42,43 In vivo blockade of CD28/B7 and/or ICAM/LFA-1 or VCAM-1/VLA-4 has been used to diminish GVHD. Our previous study demonstrated that inhibition of T-cell costimulation by VCAM-1 prevents murine GVHD in B10.D2 → BALB/c model.23 In vivo blockade of B7:CD28 interaction with CTLA4-Ig reduces lethal murine GVHD in C57BL/6 → B10.BR model,32 and coblockade of LFA-1/ICAM and CD28/B7 has a better effect on preventing acute GVHD.44 CD31-ligand(s) interactions could represent yet another costimulatory member for the delivery of a second stimulatory signal. One member of the integrin family, αvβ3 , has been reported to be a ligand of CD31.45 Like ICAM-1 and ICAM-2 binding to β-2 integrins,46 or VCAM-1 binding to VLA-4, a β-1 integrin,47 the CD31-integrin ligand-receptor interaction might function as a costimulatory signal. Our secondary MLR demonstrates that CD31 peptide-ligand interaction induces global hyporesponsiveness to allogeneic or IL-2 stimulation. Moreover, this hyporesponsiveness can be overcome by the addition of ConA. These results suggest that this peptide is not acting in an antigen-specific manner.
Further analyses demonstrated that the CD31 peptide significantly inhibited CD8+-mediated CTL responses in vitro. Immunofluorescent staining and analysis showed that AR1 CTL line was CD31−, while target BALB/c spleen ConA blast cells were 95% positive. On the other hand, CD31 peptide did not inhibit antigen presentation in a system testing CD4 hybridoma 1994.4 response to a specific peptide (Ac 1-11 [4A]) presented by PL/J spleen antigen-presenting cells (APC). 1934.4 hybridoma cells were CD31+ and PL/J spleen cells were 90% positive. These results suggest that this CD31 peptide may prevent CD31−-CD31+ heterophilic interaction in the CTL response, but does not block CD31+-CD31+ homophilic reaction, at least in the system we tested. In recent years, several reports have demonstrated that CD31 can participate in both homophilic7,48 and heterophilic adhesion,8,9 similar to other molecules in the immunoglobulin superfamily.49 A recent report by Prager et al50 demonstrated that interaction of CD31 with a heterophilic counterreceptor is involved in downregulation of human T-cell responses. Therefore, we postulate that the CD31− AR1 cell line may express a heterotypic ligand that is blocked by this CD31 peptide. The CD31+ 1934.4 hybridoma prepared from PJR-25 T cell clone, which is different from heterogenous mononuclear cells, may not have a heterotypic ligand, resulting in lack of blocking effect by the CD31 peptide. The other possibility is that other costimulating molecules overcome the CD31 peptide-blocking effect in this specific antigen presentation model.
As a member of the immunoglobulin superfamily of adhesion molecules, CD31 itself also participates in intercellular adhesion. Therefore, prevention of CD31 binding may simply prevent the egress of alloreactive cells from the circulation. Our data from the in vivo experiments suggest that CD31 peptide has a dual effect. It seems to delay the onset of GVHD, suggesting that it interferes with T-cell responses. The marked improvement in survival suggests that while alloreactive cells do develop, these mice treated with the CD31 peptide have a marked improvement in outcome, perhaps because the cells are unable to reach the GVHD target organs. This possibility is corroborated by the evidence that there were fewer infiltrating cells in the skin biopsy of mice with GVHD that had also received this peptide.
Our study demonstrates that administration of CD31 peptide can significantly decrease the onset of GVHD across minor histocompatibility barriers and increase the survival from GVHD. It is the first demonstration that in addition to inhibiting lymphocyte responses in vitro, peptides based on the sixth immunoglobulin domain of CD31 have in vivo immune-modulating effects. These data raise the possibility of a CD31-derived peptide as a novel immunomodulatory or immunosuppressive agent. Defining the CD31 ligand in T-cell activation and the functional role of CD31 in the immune response remain important questions in understanding the biologic function of CD31.
Supported by a Stanford University School of Medicine, Dean's Postdoctoral Fellowship Award (1995/97, Y.C.)
Address reprint requests to Nelson J. Chao, MD, Division of Bone Marrow Transplantation, Room H-1353, Stanford University Medical Center, 300 Pasteur Dr, Stanford, CA 94305.

![Fig. 1. Inhibition of MLR across major histocompatibility barriers. (A) BALB/c (H-2d) × C57BL/6 (H-2b); (B) BALB/c × PL/J (H-2u); (C) BALB/c × C3H (H-2k). A total of 2.5 × 105 responder spleen cells were plated with 5 × 105 irradiated (30 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 96 hours of incubation, cultures were pulsed with 1 μCi [3H] thymidine per well for an additional 16 hours. Results are the mean cpm from triplicate cultures and representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1452/4/m_bl_0014f1.jpeg?Expires=1767727419&Signature=cfY2Ds05qs9i~xk7YR34JM7MtrOCokGfPjJUWDmAMHITWpIFxfYXyJVWU0URraMQzxysoCf70wODoI8oFCNPlwizMETIYUUxgKi1AjU016jpwAjUJEEYpGnhBRm0LAJBmujVQbuHypWzX~U7c5v~gHa5OKboyiI4LHlmr1CTT4j5-vtO~yb3SNVyLXvK8RM8D9Gy7CZr2G4FJ6nkUtrcnk66O8xPn2jfZY-WawnHnhx2l4ThXdTHcYJCgcMWlI7qVZk0EjxBuae99DrgIsm0KA2ijLMhakNJNkaMO2535x64XYpQTSZHwQSG5kEqxQkts3SJrymi9Xz~zVh0tyh0Gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Inhibition of MLR across minor histocompatibility barriers (B10.D2/nSnJ [H-2d], responder; BALB/c [H-2d], stimulator). This combination is the same as in the in vivo system. 1 × 106 nylon wood–purified responder T cells were plated with 1 × 106 irradiated (10 Gy) spleen cell stimulators in the absence or presence of CD31 peptide or control peptide. After 5 days' incubation, cells were harvested and counted (cultures were pulsed with 1 mCi [3H]thymidine per well for 16 hours before harvest). Results are the mean cpm from triplicate cultures and are representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1452/4/m_bl_0014f2.jpeg?Expires=1767727419&Signature=n4~lMuztx3uumi6eL7DXdLBYoZqvjXNvScQrBW2b~8X3-ug90z4Y4rBmsV0i3ZGsbxA4~3m7I0zUBzNizLFIB25bL9-VJ19043yKElKSpXId-0uHufoVV2mBEUKlDfKKfyaNI7A2OdVa9zAoQ-~FaQq-IOGSreX4Ytr-tYf~vfJWQT7Z2Z97afZfLNB4lBRviriS3dwmRBjchTiq3jOIgKgBSLVXZeC~1-SQqnhAegxtJdXp45971ncvYripHid96u3VFBpkBRbaKfC4IDzrxXseS4MttCnDhIGKZtuXllfourV0AuUEI3VQNkdatVtCDr1zXSwVP5ZFF2jTvcdRtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. 1934.4 hybridoma assay. 1934.4 hybridoma cells (5 × 104) were incubated with 5 × 105 PL/J spleen cells and various concentrations (0.01 to 1,000 μmol/L) of Ac1-11 [4A] for 24 hours in the presence of CD31 peptide (200 μg/mL) or the absence of peptide. The IL-2 production of the Ac1-11 [4A]-reactive hybridoma 1994.4 was used as a readout system for antigen presentation. IL-2 production was measured by proliferation of the IL-2–dependent cell line HT-2 in the presence of supernatants from 1994.4 cultures. Results are expressed as the mean cpm from triplicate cultures. Results are representative of 3 separate experiments](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1452/4/m_bl_0014f5.jpeg?Expires=1767727419&Signature=2BbQShnsiL8A~JhjjtTOtoyGwOJsV5xkPmwDOzSwf-EfLaFcVnD5qtT4EygYl24tX3WKdP3HwNHCLWU7SHGYT~7wfYdNu-2f~HIvovNtkhcpGOpDtzLWX~iQZ9LPHinqKSCy05ATEzjQ2ijppvuQPaLMztCqZuxGFuY6lzxcG43NrPxZrDIO9SE5rY2-zUAGE5cR5F18q05MmwQC066boqH5FH7NQTN0IU~L7a6LNxspQ3ZiBB4HleJGFQDVnICuIdXp6eLF9ejgxfjPwA9LoP3HNthtFBrcqQlaDNjM30ufrl0hm6G4oZujd8Ag4Idva1aw~ECualkSXfMDWbO8ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)