Abstract
The mechanisms of tissue necrosis and vascular damage characteristics of certain Epstein-Barr virus (EBV)-associated lymphoproliferative disorders are unknown. The CXC chemokines interferon-γ–inducible protein-10 (IP-10) and the monokine induced by interferon-γ (Mig) caused tissue necrosis and vascular damage in Burkitt's lymphoma tumors established in nude mice. We report higher levels of IP-10 and Mig gene expression in tissues with necrosis and vascular damage from EBV-positive lymphomatoid granulomatosis and nasal or nasal-type T/natural killer (NK)-cell lymphomas compared with tissues with lymphoid hyperplasia, which lacked tissue necrosis and vascular damage. By immunohistochemistry, Mig and IP-10 proteins localized with similar patterns in viable tissue surrounding dead tissue, mostly within endothelial cells, monocyte/macrophages, and lymphocytes. Circulating levels of IP-10 were abnormally elevated in patients with EBV-positive lymphomatoid granulomatosis and nasal or nasal-type T/NK-cell lymphomas. These experiments provide the first description of the presence of Mig in any human normal or diseased tissue and the first description of IP-10 in certain lymphoproliferative lesions. These data suggest that Mig and IP-10 play an important role in the pathogenesis of tissue necrosis and vascular damage associated with certain EBV-positive lymphoproliferative processes.
CELL AND TISSUE DEATH are conspicuous histological features of many Epstein-Barr virus (EBV)-associated lymphoproliferative disorders (LPDs), including lymphomatoid granulomatosis (LYG) and nasal or nasal-type T/natural killer (NK)-cell lymphomas.1 LYG and nasal or nasal-type T/NK-cell lymphomas share many histological and clinical features. The zonal distribution pattern of tissue death and the histological evidence of vascular damage have suggested a vascular pathogenesis to tissue death in both of these disorders.2 The preferred localization of tumor cells around and within blood vessels in LYG and nasal or nasal-type T/NK-cell lymphoma may cause destruction of the blood vessel wall leading to poor tissue perfusion.1-4 However, the absence of histological evidence for angioinvasion in many of these cases has suggested the possible participation of other factors, including tumor necrosis factor-α (TNF-α).1
Mig, the monokine induced by interferon-γ (IFN-γ), and IP-10, the IFN-γ–inducible protein-10, are structurally related chemokines of the C-X-C (or α subfamily of chemokines with the first two of four conserved cysteines separated by one amino acid residue) that share a receptor, CXCR3.5 Studies in vitro have shown that Mig and IP-10 are active as chemotactic factors for stimulated T and NK cells6-9 and inhibit colony formation by hematopoietic cells.10,11 In vivo, Mig and IP-10 inhibit neovascularization and exert antitumor effects.12-15 Recently, we found expression of Mig and IP-10 genes to be significantly higher in mice bearing Burkitt's lymphoma undergoing regression after treatment with EBV-immortalized B cells compared with mice bearing progressively growing Burkitt's tumors.12,16 17 Inoculation of either Mig or IP-10 into progressively growing Burkitt's tumors in athymic mice produced tumor tissue necrosis. Histologically, Burkitt's tumors treated with EBV-immortalized B cells, Mig, or IP-10 exhibited a similar pattern of zonal necrosis and widespread vascular damage.
To explore the role of IP-10 and Mig in tissue necrosis induced by EBV, we elected to study cases representative of the following conditions: (1) LYG, an EBV-positive, usually monoclonal B-cell LPD18 and (2) nasal T/NK-cell lymphoma, an EBV-positive T-cell or NK-cell lymphoma. The patterns of vascular damage in LYG and nasal T/NK-cell lymphoma are very similar, both previously being linked under the heading of “angiocentric immunoproliferative syndromes.”4 We now report production and tissue distribution of Mig and IP-10 in LYG and nasal T/NK-cell lymphoma.
MATERIALS AND METHODS
Case selection. Patient cases were retrieved from the files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health (NIH), Bethesda, MD and from the consultation files of one of the authors (E.S.J.). Cases included LYG, 10 cases; nasal or nasal-type T/NK-cell lymphoma, 7 cases; reactive lymphoid hyperplasia, 9 cases; non-Hodgkin's B-cell lymphoma, 4 follicle center and 3 diffuse large-cell cases; and Burkitt's lymphoma, 7 cases. All patients were human immunodeficiency virus negative. Malignant lymphomas, classified according to the Revised European American Lymphoma (REAL) classification,4 were immunophenotyped by immunohistochemistry. A B-cell phenotype was established by staining of neoplastic cells for L26 (CD20), and a T/NK-cell phenotype was established by staining for CD3 and CD56, respectively.
EBV in situ hybridization. In situ hybridization used an EBV probe specific for EBV-encoded small RNAs (EBER), as described previously19 using an automated system (Ventana Medical System, Inc, Tucson, AZ).
Reverse transcriptase-mediated polymerase chain reaction (RT-PCR). RNA extraction from paraffin-embedded tissue was performed as previously described with modifications.20 Briefly, six to ten 20-μm paraffin sections were deparaffinized three times in xylene (with heating at 55°C for 10 minutes), twice in 100% ethanol and twice in 70% ethanol. Sections were resuspended in 1 mL extraction buffer (10 mmol/L NaCl, 50 mmol/L Tris-HCL, pH 7.4, 20 mmol/L EDTA, 1% sodium dodecyl sulfate) at 55°C overnight with 500 μg/μL proteinase K. After addition of 1 mL of RNA Trizol (GIBCO/BRL, Life Technologies, Gaithersburg, MD) and 200 μL of chloroform followed by centrifugation, the aqueous phase was combined with an equal volume of isopropanol. The precipitated pellet was washed with 70% ethanol and resuspended in diethylpyrocarbonate (DEPC)-treated water.
RT-PCR was performed as previously described with modifications for highly degraded RNA obtained from paraffin-embedded tissues.17,21 For semiquantitative results, PCR products were detected by quantitating incorporated 32P (α-dCTP) radioactivity. The amount of each RNA sample used was selected on the basis of equivalent amounts of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA amplified from each sample. Variability of results from different experiments was minimized by use of a standard control RNA preparation from cultured IFN-γ–stimulated endothelial cells. RNA samples were DNase treated (GIBCO/BRL, Life Technologies) then subjected to an initial assay for amplifiable contaminating genomic DNA using primers specific for G3PDH mRNA. Positive samples were retreated with DNase and negative samples (2 to 5 μg) were reverse transcribed using an RNase H- RT (Superscript; GIBCO/BRL, Life Technologies). The resultant cDNA (25 to 100 ng) was amplified as previously described with modifications.21 Primers, listed in Table 1, were designed for amplification of short amplicons (80 to 130 bp) from highly degraded RNA and spanned at least one splice junction. Genomic DNA could be distinguished from mRNA or cDNA. Amplifications were performed in a thermocycler (Stratagene Robocycler, La Jolla, CA) adding 1.25 U Taq polymerase (GIBCO/BRL) after heating at 94°C for 3 minutes (“Hot Start”); followed by 31 or 35 amplification cycles (94°C for 45 seconds; primer annealing temperature as specified in Table 1, and extension at 72°C); and maintained at 4°C until analysis. The entire amplification reaction (50 μL) was analyzed by electrophoresis on 8% acrylamide (Long Ranger; AT Biochem, Malvern, PA) Tris-borate EDTA gels (polyacrylamide gel electrophoresis [PAGE]) followed by autoradiography and quantitation by phosphorimage analysis using ImageQuantTM v3.3 software (Molecular Dynamics, Sunnyvale, CA). Band integrations were obtained as the sum of values for all pixels after subtraction of background (areas around each sample). Integrated volumes were then normalized to the standard positive control (IFN-γ–stimulated endothelial cells). Standard normalized integrated values for each sample were then normalized for the results of RT-PCR amplification for G3PDH. The results of RT-PCR analysis are presented as absolute numbers of normalized arbitrary units (pixels)/sample.
Measurement of IP-10 in serum. Serum samples from untreated patients with LYG, nasal or nasal-type T/NK-cell lymphoma, and normal adults were tested for the presence of IP-10 protein by a sandwich enzyme-linked immunosorbent assay (ELISA). The IP-10 ELISA used a goat antihuman IP-10 antiserum (R&D Systems, Minneapolis, MN) diluted at 1:250 and a purified rabbit antihuman IP-10 polyclonal antiserum (a gift of Dr D. Taub, National Cancer Institute, Frederick, MD) to capture bound IP-10 followed by an alkaline phosphatase-conjugated goat antirabbit antibody (Sigma, St Louis, MO). After development with substrate, the optical density was read at 450 nm. The lowest limit of sensitivity for the assay corresponded to 10 pg/mL of a highly purified recombinant human IP-10 standard preparation (Pepro Tech Inc, Rocky Hill, NJ). The assay was specific for IP-10 and failed to recognize other human cytokines, including interleukin-1α (IL-1α), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, IFN-γ , TNF-α, and transforming growth factor-β1.
IP-10 and Mig immunohistochemistry in tissue sections. Tissue sections were treated with either 0.01 mol/L citrate buffer (pH 6.0) containing 0.1% Tween-20 (Sigma) or a Target Retrieval Solution (DAKO Corp, Carpenteria, CA) in a microwave pressure cooker (Nordic Ware, Minneapolis, MN) at maximum power (800 W) for 40 minutes as previously reported.22 Sections were incubated with primary antibodies including either a rabbit antihuman IP-10 purified antibody (1:2,000 dilution; a gift from Dr D. Taub, National Cancer Institute) or a rabbit antihuman Mig antiserum (1:5,000 dilution; a gift of Dr J. Farber, National Institute on Allergy and Infectious Diseases [NIAID], Bethesda, MD) overnight at room temperature. Primary antibodies were detected with a biotin-conjugated universal secondary antibody formulation, which recognizes rabbit and mouse immunoglobulins (Ventana Medical Systems, Inc, Tucson, AZ). After addition of an avidin-horseradish peroxidase conjugate, the enzyme complex was visualized with 3,3′-diaminobenzdine tetrachloride and copper sulfate.
Statistical analysis. Geometric means and standard errors of the mean used conventional formulas. Student's t-test was used to evaluate the significance of group differences. Kendall Tau and Sperman Rho tests were used to examine correlations of results.
RESULTS
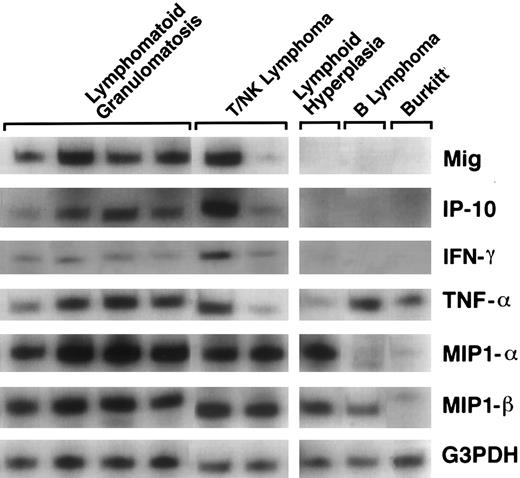
Detection of Mig and IP-10 gene expression. Total RNA was extracted from formalin-fixed, paraffin-embedded tissues representative of LYG (10 cases), and nasal T/NK-cell lymphoma (7 cases) that were EBV positive and displayed evidence of tissue necrosis and vascular damage. Patient characteristics and histopathologic changes are shown in Table 2. Control RNA was extracted from tissues representative of reactive lymphoid hyperplasia (9 cases), follicle center or diffuse large B-cell lymphoma (7 cases), and Burkitt's lymphoma (7 cases), which displayed no histological evidence of tissue necrosis and vascular damage. Reactive hyperplasias and large B-cell lymphomas were EBV negative as determined by in situ EBER-1 hybridization. Burkitt's lymphomas were EBV positive, but were negative for latent membrane protein-1 (LMP-1) protein expression. The cytokine/chemokine mRNA profiles were examined by a modified RT-PCR analysis. The PCR products for the chemokines Mig and IP-10 were more abundant in tissues representative of EBV-positive LYG, and nasal T/NK-cell lymphoma (referred to collectively as EBV-LPD) than in tissues representative of lymphoid hyperplasia, follicle center and large B-cell lymphoma, and Burkitt's lymphoma. Also, IFN-γ expression was noted in those samples that expressed both Mig and IP-10. In contrast, the PCR products for the cytokine TNF-α were variable in study and control cases, whereas those for the chemokines MIP-1α and MIP-1β were detected at similar levels in study cases but at variable levels in control cases (Fig 1).
Patterns of cytokine and chemokine mRNAs in lymphoproliferative disease tissues shown by RT-PCR analysis. Total cellular RNA, extracted from paraffin-embedded tissues representative of lymphomatoid granulomatosis, nasal T/NK-cell lymphoma, lymphoid hyperplasia, malignant B-cell lymphoma, and Burkitt's lymphoma, was subjected to RT-PCR analysis using appropriately designed primers.
Patterns of cytokine and chemokine mRNAs in lymphoproliferative disease tissues shown by RT-PCR analysis. Total cellular RNA, extracted from paraffin-embedded tissues representative of lymphomatoid granulomatosis, nasal T/NK-cell lymphoma, lymphoid hyperplasia, malignant B-cell lymphoma, and Burkitt's lymphoma, was subjected to RT-PCR analysis using appropriately designed primers.
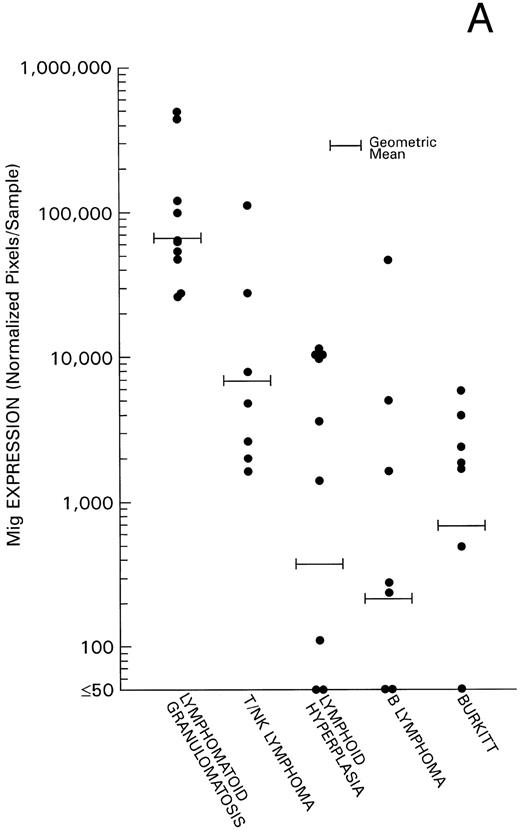
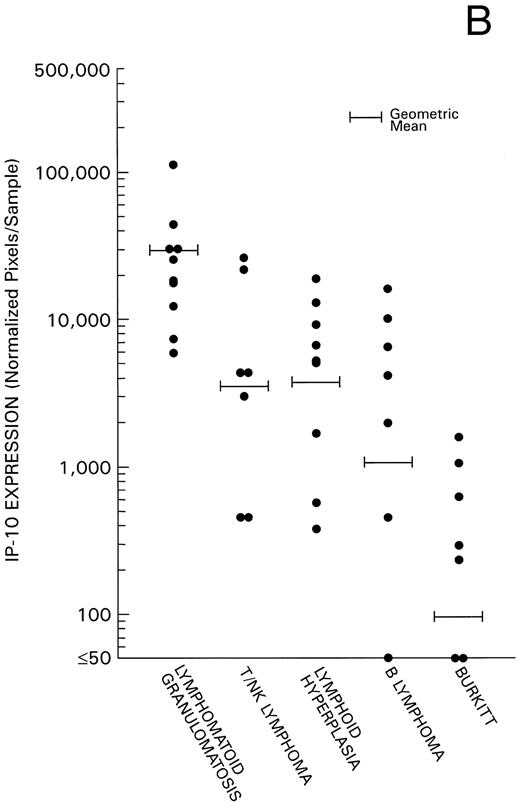
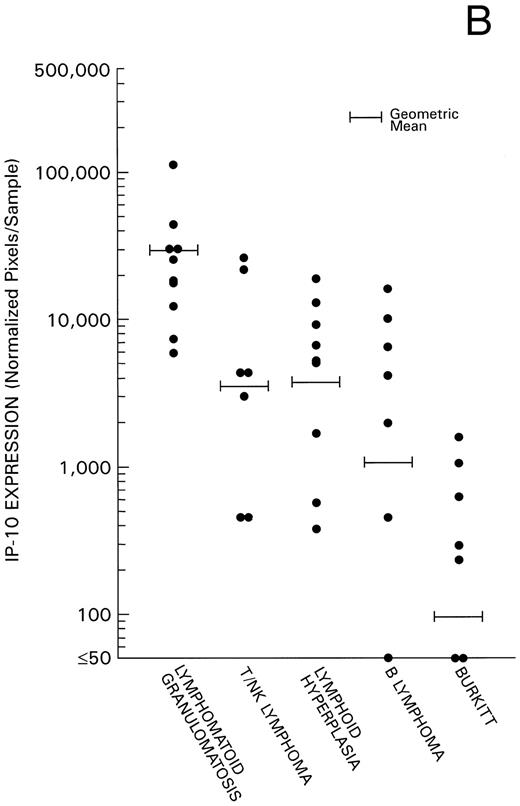
Levels of Mig and IP-10 expression in all 40 cases were estimated by phosphorimage analysis of RT-PCR–derived products and subsequent normalization for RT-PCR products of the housekeeping G3PDH gene. Experimental variability was minimized by the use of a standard RNA preparation derived from IFN-γ–stimulated endothelial cells throughout the experiments. We found levels of Mig and IP-10 mRNA expression (Fig 2) to be significantly higher in the 17 cases of EBV-LPD associated with histological evidence of tissue and cell death (10 cases of LYG, and 7 cases of nasal or nasal-type T/NK-cell lymphoma) compared with the 9 cases of reactive lymphoid hyperplasia without histological evidence of tissue and cell death (P = .004 and P = .029, respectively). In addition, we found a significant direct correlation between levels of Mig and IP-10 expression in these 17 cases of EBV-LPD (Kendall Tau = 0.43, P = .004). Levels of Mig and IP-10 expression in the 7 cases of malignant B-cell lymphoma and levels of Mig expression in the 7 cases of Burkitt's lymphoma were found to be similar to those of the 9 cases of reactive hyperplasia (P = .6 all comparisons). In contrast, levels of IP-10 expression in the lymphoid hyperplasia group were significantly higher than in the Burkitt's lymphoma group (P = .008), indicating that regulation of expression of the IP-10 and Mig gene differ. The low level or failure to amplify IP-10 mRNA from Burkitt's tissue as opposed to lymphoid hyperplasia raises the possibility that IP-10 is expressed in lymphoid tissues during certain inflammatory processes reflected histologically as lymphoid hyperplasia but not in lymphoid tissues that are replaced by Burkitt's lymphoma.
Mig and IP-10 mRNA expression in EBV-associated lymphoproliferative diseases. Total cellular RNA, extracted from paraffin-embedded tissues diagnosed with either LYG, nasal T/NK-cell lymphoma, lymphoid hyperplasia, malignant B-cell lymphoma, or Burkitt's lymphoma, was subjected to semiquantitative RT-PCR. After normalization to a standard RNA preparation and to G3PDH, the results of Phosphorimage analysis are shown as absolute numbers of normalized arbitrary units (pixels)/sample. (A) Mig mRNA expression; (B) IP-10 mRNA expression.
Mig and IP-10 mRNA expression in EBV-associated lymphoproliferative diseases. Total cellular RNA, extracted from paraffin-embedded tissues diagnosed with either LYG, nasal T/NK-cell lymphoma, lymphoid hyperplasia, malignant B-cell lymphoma, or Burkitt's lymphoma, was subjected to semiquantitative RT-PCR. After normalization to a standard RNA preparation and to G3PDH, the results of Phosphorimage analysis are shown as absolute numbers of normalized arbitrary units (pixels)/sample. (A) Mig mRNA expression; (B) IP-10 mRNA expression.
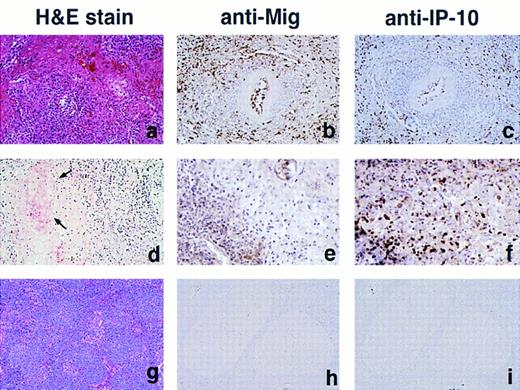
Immunohistochemical localization of Mig and IP-10. Paraffin-embedded sections of EBV-LPD tissues, sequential to those used for RNA extractions were stained with a rabbit antiserum to either human Mig or IP-10 (Fig 3). All EBV-LPD tissue specimens studied, representative of LYG (8 samples) and nasal T/NK-cell lymphoma (3 samples), stained positively for both Mig and IP-10. The staining for Mig was, in general, more intense than for IP-10, although the pattern of Mig and IP-10 staining was generally indistinguishable. Staining for these chemokines was mostly restricted to viable tissue surrounding areas of tissue necrosis, but isolated clearly positive cells were also identified within the necrotic tissue. The pattern of staining was mostly intracellular, suggesting that Mig and IP-10 are synthesized by these cells. Positively staining cells were identified morphologically as endothelial cells lining capillary vessels, lymphocytes, macrophages, and few fibroblasts. Occasional extracellular staining was also noted, which is consistent with the secreted nature of these chemokines. In contrast, minimal to absent staining for Mig and IP-10 was observed in 6 cases of Burkitt's lymphoma. Although staining for Mig was generally absent in 12 cases of lymphoid hyperplasia, rare scattered histiocytes, lymphocytes, and endothelial cells of capillaries showed faint staining for IP-10 in 3 of 12 of these cases. These results are consistent with the data of IP-10 and Mig gene expression as shown in Fig 2A and B.
Immunohistochemical analysis of Mig and IP-10 in lymphoproliferative disease tissues representative of LYG, nasal T/NK-cell lymphoma, and lymphoid hyperplasia. (A, B, and C) LYG, lung; (D, E, and F ) nasal T/NK-cell lymphoma, nasal septum (D, arrows indicate vessel with fibrinoid necrosis); (G, H, and I) reactive lymphoid hyperplasia, lymph node. Paraffin-embedded tissue sections were stained with Hematoxylin and Eosin (A, D, and G), with an anti-Mig heteroantiserum (B, E, and H), and with an anti–IP-10 heteroantiserum (C, F, and I). Primary antibodies were detected with biotinylated antimouse/rabbit IgG followed by streptavidin-peroxidase complexes. (Original magnification × 40.)
Immunohistochemical analysis of Mig and IP-10 in lymphoproliferative disease tissues representative of LYG, nasal T/NK-cell lymphoma, and lymphoid hyperplasia. (A, B, and C) LYG, lung; (D, E, and F ) nasal T/NK-cell lymphoma, nasal septum (D, arrows indicate vessel with fibrinoid necrosis); (G, H, and I) reactive lymphoid hyperplasia, lymph node. Paraffin-embedded tissue sections were stained with Hematoxylin and Eosin (A, D, and G), with an anti-Mig heteroantiserum (B, E, and H), and with an anti–IP-10 heteroantiserum (C, F, and I). Primary antibodies were detected with biotinylated antimouse/rabbit IgG followed by streptavidin-peroxidase complexes. (Original magnification × 40.)
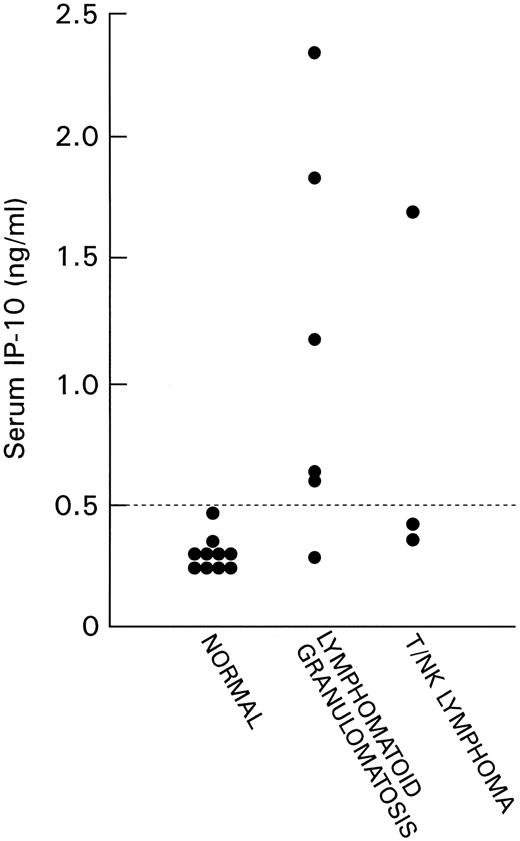
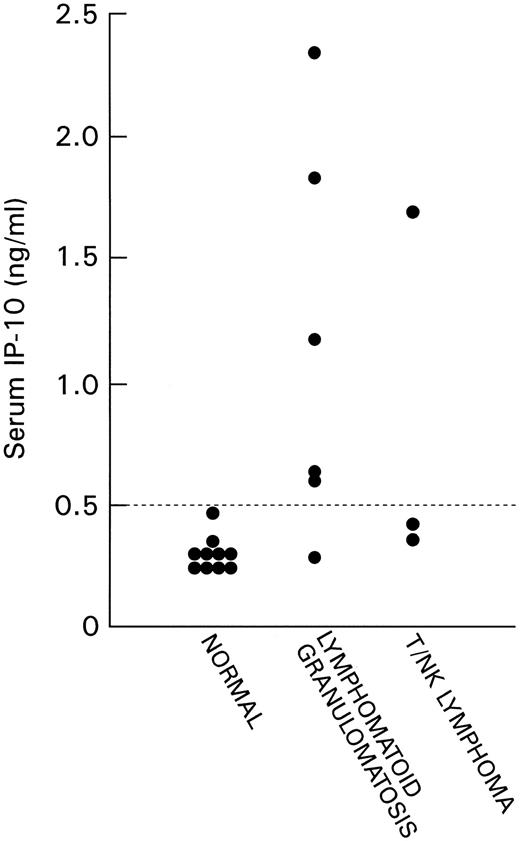
Detection of IP-10 in serum. We detected IP-10 at low levels in the serum of normal adult individuals (mean level 0.31 ng/mL, 95% confidence interval 0.26 to 0.35 ng/mL). IP-10 levels were measured in serum samples from six patients with LYG and three patients with nasal T/NK-cell lymphoma (Fig 4). All cases of LYG and nasal T/NK-cell lymphoma were confirmed histologically, and in three cases (case 7 with nasal T/NK-cell lymphoma in Table 2 and cases 11 and 12 with LYG in Table 2) paraffin-embedded tissues showed high levels of IP-10 and Mig expression. Serum IP-10 levels were found to be abnormally elevated in patients with LYG and nasal T/NK-cell lymphoma compared with sera from normal individuals (P = .006, Student's t-test).
IP-10 content in serum samples of normal patients and patients with LYG and nasal T/NK-cell lymphoma detected by sandwich ELISA. The lowest limit of detection was 10 pg/mL.
IP-10 content in serum samples of normal patients and patients with LYG and nasal T/NK-cell lymphoma detected by sandwich ELISA. The lowest limit of detection was 10 pg/mL.
DISCUSSION
This study shows the presence of the chemokines Mig and IP-10 in lymphoproliferative disease tissues from EBV-positive LYG and nasal T/NK-cell lymphoma. Tissue necrosis and vascular destruction are distinguishing and often prominent histopathologic features of these lymphoproliferative lesions.1 Previously, we detected high level expression of the murine IP-10 and Mig genes in lymphoblastoid cell line–treated experimental Burkitt's lymphoma undergoing regression with extensive tumor necrosis and vascular damage. In addition, we found that inoculation of IP-10 or Mig proteins into established Burkitt's tumors caused extensive tumor necrosis and vascular damage.12 17 Thus, Mig and IP-10 are likely to contribute to tissue necrosis and vascular damage detected in lymphoproliferative disease tissues from LYG and nasal T/NK-cell lymphoma.
Immunostaining of lymphoproliferative disease tissues from LYG and nasal T/NK-cell lymphoma localized Mig and IP-10 predominantly in viable tissue surrounding areas of necrosis within cells identified morphologically as endothelial cells, lymphocytes, macrophages, and occasional fibroblasts. These cell types were previously recognized as a source of Mig23 and IP-10.9,24 It is of interest that the atypical cells, presumably the tumor cells in LYG and nasal T/NK-cell lymphoma, were negative for these chemokines, suggesting that the reactive cells are the principal source of these chemokines. Studies in vitro and in vivo have indicated that IFN-γ is the only inducer of Mig in monocyte/macrophages, fibroblasts, and keratinocytes.25,26 By contrast, IP-10 is induced in monocytes by a variety of stimuli, including IFN-γ, lipopolysaccharide, and IFN-α/β.27 In recent studies with IFN-γ knockout mice, the induction of Mig but not IP-10 was found to be absolutely dependent on IFN-γ.26 Using RT-PCR analysis, we found evidence for IFN-γ expression in most EBV-positive lymphoproliferative disease tissues that also expressed Mig and IP-10, suggesting that IFN-γ is an important mediator for induction of Mig and IP-10 in these cases. Also, results of semiquantitative PCR showed a significant correlation between levels of expression of the Mig and IP-10 genes in these disorders suggesting a common or closely related pathway of induction. However, in lymph nodes showing reactive hyperplasia, levels of IP-10 expression were found to be significantly higher than those of Mig, and there was no evidence of IFN-γ expression by RT-PCR. This finding supports the notion that IP-10 but not Mig is inducible by signals other than IFN-γ, and it further suggests that concordant expression of the IP-10 and Mig genes points to an IFN-γ–dependent pathway of chemokine induction.
The present study has not established the mechanisms for induction of IFN-γ and, secondarily, IP-10 and Mig. Because IL-12 is a potent inducer of IFN-γ and IL-12 is secreted by certain EBV-infected cells, we looked for IL-12 expression in the lymphoproliferative disease tissues expressing IFN-γ. However, we could not reliably amplify IL-12 p35 or p40 mRNA from paraffin-embedded tissues, which prevented our assessment of IL-12 expression in the tissues examined.
The mechanisms by which IP-10 and Mig mediate tissue necrosis are unknown. In vitro, IP-10 and Mig have been shown to be potent T- and NK-cell chemoattractants, and IP-10 enhanced the release of granule-derived serine esterases.8 Granzymes and perforins have been shown to be expressed in nasal T/NK-cell lymphoma,28 in anaplastic large-cell lymphoma, and in Hodgkin's disease.22 Perhaps release of these enzymes contributes to tissue necrosis.
Previously, IP-10 was identified by immunohistochemistry in the epidermis and dermis during the delayed-type hypersensitivity response to purified protein derivative of tuberculin and in the lesions of tuberculoid leprosy and cutaneous Leishmaniasis.29 Keratinocyte staining for IP-10 was also reported in psoriatic plaques,30 in the skin of patients with early contact sensitivity,31 in fixed drug eruptions,32 and in cutaneous T-cell lymphoma.33 However, the presence of IP-10 in EBV-associated lymphoproliferative lesions has not been previously recognized, nor has the presence of Mig been previously reported in any human normal or diseased tissue. In addition, IP-10 was not previously recognized in human normal or diseased serum. Our preliminary studies suggested a close correlation between IP-10 levels in tissues and serum in patients with LYG and nasal T/NK-lymphoma (Fig 4).
This study is also the first to show the presence of Mig in human tissue and suggests a biological role for IP-10 and Mig in EBV-associated LPDs. The recent discovery that Mig and IP-10 share a receptor, CXCR3, combined with the concordant expression and localization of Mig and IP-10 in the lymphoproliferative tissues investigated here emphasizes the functional similarities between these chemokines.
Preclinical studies in rodents have provided evidence that IP-10 and Mig can prevent tumor growth, reduce tumor growth, cause tumor tissue necrosis, and limit metastasis.17 Our finding, that IP-10 and Mig are produced in the context of certain benign and malignant LPDs associated with tissue necrosis and vascular damage, raises the possibility that these chemokines may be part of natural responses designed to limit excessive or abnormal lymphoid cell growth.
ACKNOWLEDGMENT
The authors thank Patricia Duffey and Dr William C. Kopp at the National Cancer Institute-Frederick Cancer Research and Development Center for serum samples; Dr Adnan Mansoor for cases of Burkitt's lymphoma from Pakistan; Dr Cecilia Sgadari, Dr Barry Cherney, and Karen Jones for laboratory assistance; Dr Laszlo Krenacs and Sarah Cappello for technical advice and assistance with the immunohistochemical analysis; and Dr Joshua M. Farber for critically reading the manuscript.
Presented in part at the 38th Annual American Society of Hematology Meeting, December 10, 1996, in Orlando, FL.
Address reprint requests to Elaine S. Jaffe, MD, Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Building 10, Room 2N202, 10 Center Drive MSC 1500, Bethesda, MD 20892-1500.