Abstract
Interleukin-15 (IL-15) is a potent T-cell stimulating factor, which has recently been used for pre-clinical in vivo immunotherapy. Here, the IL-15 effect on CD3-stimulated peripheral human T cells was investigated. IL-15 induced a significant T-cell proliferation and upregulated CD25 expression. IL-15 significantly enhanced T-cell production of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-10. Between 10- and 100-fold greater concentrations of IL-15 were necessary to reach a biological effect equivalent to that of IL-2. Blockade of IL-2 binding to the high-affinity IL-2 receptor did not affect the IL-15 effects, suggesting that IL-15 did not act by inducing endogenous IL-2. Exogenously administered IL-10 significantly reduced the IL-15 and IL-2–mediated IFN-γ and TNF-α production, whereas T-cell proliferation and CD25 expression were not affected. The inhibitory effects of exogenously administered IL-10 on T-cell cytokine production appeared indirect, and are likely secondary to decreased IL-12 production by accessory cells. Inhibition of endogenous IL-10 binding to the IL-10 receptor significantly increased IFN-γ and TNF-α release from T cells. These data suggest that endogenous IL-10 can regulate activated T-cell production of IFN-γ and TNF-α via a paracrine negative feedback loop. The observations of this study could be of relevance for the therapeutic use of IL-15 in vivo.
INTERLEUKIN-15 (IL-15) has recently been cloned from the simian kidney epithelial cell line CV1/EBNA.1 Although IL-15 shows no structural homology with IL-2, both cytokines exert functional similarities. Using specific receptor antibodies and cells transfected with different IL-2R subunits, the IL-15 signal has been shown to be transduced by an intact IL-2R β-γ chain complex, followed by activation of the same pattern of cytoplasmic kinases as are stimulated by IL-2.2-10 The ligand binding protein of IL-15, however, is distinct from the IL-2Rα chain.11 This may account for divergent functional activities such as activation of pre-T-cell lines by IL-2 but not IL-15, as well as a different capacity to induce cytokine production by T cells.2,8 10
Adoptive immunotherapy with IL-2 has been used extensively in the treatment of adult renal cell carcinoma, melanoma, colon carcinoma, and pediatric sarcomas.12-15 More recently, IL-15 has been introduced into anticancer therapy. In mice dose-response curves showed that an about twofold dose of IL-15, compared to IL-2, was necessary to reach the same effect. However, the dose of IL-15 required to induce severe side effects such as pulmonary capillary leakage syndrome was six times higher than that required for IL-2.16 These differences might be caused by differential regulation of IL-2– and IL-15–induced cytotoxic effects. However, the mechanism by which IL-15 regulates cytotoxic cytokines remains widely elusive.
IL-10 is a negative regulator of T-cell activation and proliferation. Recently the inhibition of interferon-γ (IFN-γ) production in LPS treated MNCs has been shown to correlate with an inhibition of IL-12 production. Addition of exogenous IL-12 could partially overcome this inhibition.17 Besides these indirect effects, some minor directly inhibitory effects of IL-10 on T-cell function such as inhibition of IL-2 and other cytokines have also been described.18,19 In contrast to IL-2, IL-15 is produced by macrophages as well as by a variety of other cells and tissues, but not by T cells.20,21 IL-10 has been shown to be able to upregulate the expression of IL-15 in mouse macrophages,22 suggesting that the role of IL-10 in IL-15–mediated immune responses might be different from that in IL-2–mediated immune responses. To add to the understanding of the interaction between IL-15 and IL-10 in T-cell immunity, CD3-activated T cells were stimulated with both cytokines.
MATERIALS AND METHODS
Reagents. Recombinant human cytokines as well as cytokine enzyme-linked immunosorbent assay (ELISA) were purchased from R&D SYSTEMS (Minneapolis, MN), Biosource (Camarillo, CA), and Peprotech (Frankfurt a.M., Germany). Monoclonal antibodies (MoAbs) for fluorescence-activated cell sorting (FACS) analysis were supplied by Becton Dickinson (Heidelberg, Germany). Sheep erythrocytes were obtained from Behring (Marburg, Germany). For stimulation of T cells a purified anti-CD3 MoAb (clone X 35, mouse IgG2a; Immunotech, Hamburg, Germany) was used. Function of endogenous IL-12 or binding of endogenous IL-10 or IL-2 to their receptors were blocked by a neutralizing anti-IL-12 MoAb (clone 24910.1, mouse IgG1; R&D Systems), an inhibitory anti-IL-10R MoAb (clone 37607.11, mouse IgG1; R&D Systems) or an inhibitory antibody to the high-affinity IL-2R (clone 22722.2, mouse IgG1). All experiments with neutralizing or blocking MoAbs were controlled for by performing identical experiments with nonreactive control MoAbs (anti-CD20 MoAb [clone B9E9, mouse IgG2a, Immunotech], mouse IgG2a MoAb [clone 20102.1, R&D Systems], mouse IgG1 MoAb [clone 11711.11, R&D Systems]) prepared under identical conditions to experimental MoAbs.
Isolation and stimulation of T cells. T cells were obtained from healthy donor buffy coat preparations by ficoll density centrifugation and subsequent positive selection using neuraminidase-treated sheep erythrocytes as described previously.19 T-cell preparations contained 90% to 95% CD3+ T cells, about 2% of each B cells, monocytes, or CD56+ natural killer (NK) cells as determined by immunofluorescence staining and FACS analysis. Using trypan blue staining, viability was checked to be >95% before stimulation. T cells were stimulated with anti-CD3 MoAb (100 ng anti-CD3/106 cells) on ice for 30 minutes and resuspended in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum and penicilline/streptomycine at a density of 106/mL. T cells were then stimulated as indicated with IL-15, IL-10, or combinations thereof.
In some experiments ultrapure T-cell preparations were used. Therefore, T cells were isolated from mononuclear cells (MNCs) by twofold rosetting. Thereafter, B cells, NK cells, and monocytes were depleted by incubation of the cell suspension with magnetic bead-antibody-bound anti-CD20, anti-CD56, and anti-CD33 antibodies and cell extraction with a MiniMACS separation unit (Milteny, Berjind-Gladlach, Germany). FACS analysis of these T-cell fractions revealed T-cell purity to be greater than 97% with less than 0.5% of B cells or monocytes and about 1% NK cells. These T-cell fractions were then stimulated by immobilized anti-CD3 in the presence or absence of IL-2 or IL-15. Therefore 96-well flat-bottom full area plates (Falcon, Lincoln Park, NJ) were coated with 4 μg/mL of anti CD3 diluted in carbonate buffer pH 9.8 according to the description of Taga et al.23 After 1 hour at 37°C, excess antibody was removed and plates were washed and then stored at 4°C until usage. The purity of the T cells was further shown by only marginal CD25 expression and lack of significant T-cell cytokine production after incubation with unbound anti-CD3 MoAb (data not shown). In contrast, stimulation with immobilized anti-CD3–mediated significant T-cell activation as shown by significantly enhanced expression of CD25 (data not shown) and T-cell cytokine production (see Results). Taken together these data indicate that virtually no reactive antigen-presenting cells (APCs) were present in these T cells.
Cytokine ELISA. IL-10, IL-12 (heterodimer), tumor necrosis factor-α (TNF-α) and IFN-γ were detected by commercially available ELISA as has been described previously.19 Therefore cells were stimulated for 72 hours. Cell-free supernatants were procured and stored at −70°C until determination of cytokines.
FACS analysis. T cells were stained with fluorescence labeled anti-CD56, anti-CD3, anti-CD20, anti-CD33, anti-CD25, or isotype control antibodies suspended in phosphate-buffered saline (0.2 mol/L phosphate buffer pH 7.2 and 50 mmol/L NaCl) containing 0.1% bovine serum albumin (BSA) and 0.1% sodium azide (PBSBA). After 20-minutes incubation on ice, cells were washed in PBSBA and immunofluorescence was measured by FACScan (Becton Dickinson).
T-cell proliferation. T cells were cultured with medium or cytokines after anti-CD3 stimulation at a density of 105/mL. After 66 hours, H3-thymidine (0.1 μCi) was added. Six hours later cells were procured and thymidine incorporation was determined by a beta counter.
Detection of β-actin, IL-10, and IFN-γ mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR). Total RNA isolation was performed by RNAzol preparation as described previously.24 Briefly, 1 × 106 cells were lysed in 200 μL RNAzol (Wak-Chemie, Bad Homburg, Germany) and 20 μL chloroform. The suspension was mixed by vortexing and chilled on ice for 10 minutes. After centrifugation at 12,000g and 4°C for 15 minutes, the aqueous phase was transferred to an Eppendorf tube (Eppendorf, Hamburg, Germany) and reconstituted with an equal volume of isopropanol. RNA was precipitated at −20°C for 16 hours. Precipitates were pelleted at 12,000g and 4°C, then washed twice in 70% ethanol. Air-dried pellets were resuspended in 20 μL RNase-free water. First strand cDNA synthesis was performed at 37°C for 60 minutes. Heat-denatured RNA of 106 cells was mixed with 11 μL Bulk-Mix (Moloney Murine Leukemia Virus reverse transcriptase, RNAguard, RNase/DNase-free BSA, deoxyadenosine triphosphate, deoxycytidine triphosphate, deoxyguanosine triphosphate, and deoxythymidine triphosphate in aqueous solution; Pharmacia, Uppsala, Sweden), 1 μL dithiotritol (DTT) (200 mmol/L aqueous solution, Pharmacia), 1 μL random hexamer primers (random hexadeoxynucleotides at 0.2 μg/μL in aqueous solution, Pharmacia). Diethylpyrocarbonate (DEPC)-treated water was added to a final volume of 50 μL. First strand cDNA (1 μL, 0.1 μL, 0.01 μL) was added to 45 μL of PCR mix containing 5 μL 10 × buffer (100 mmol/L Tris-HCl pH 8.3; 500 mmol/L KCl, 15 mmol/L MgCl2 ; 0.01 % wt/vol gelatin), 2.4 mL deoxynucleotide triphospahte (2.5 mmol/L each, Pharmacia), 33.45 μL sterile water, 2 μL of each primer (10 μmol/L), and 0.15 μL of AmpliTaq DNA polymerase (Roche Molecular Systems, Inc, Grenzach-Wyhlen, Germany). The first-strand cDNA was amplified using a Biometra thermal cycler (Biometra, Göttingen, Germany) for 25 (β-actin) or 32 (IFN-γ and IL-10) cycles. The temperature profile used for cytokine mRNA amplification was 94°C for 30 seconds for denaturation, 62°C for 45 seconds for annealing, and 72°C for 50 seconds for primer extension. The temperature profile for amplification of β-actin mRNA was 94°C for 30 seconds for denaturation, 64°C for 40 seconds for annealing, and 72°C for 45 seconds for primer extension. Semiquantitative determination of PCR products was performed similar to previous description.25,26 Initially, nonsaturable PCR conditions were excluded by using three different concentrations of template (1, 0.1, 0.01 μL) for the amplification of β-actin cDNA. PCR products were separated on ethidium bromide-stained 1.6% agarose gels (Sigma Chemical Co, Munich, Germany). The gels were photographed under UV light and the band intensities were measured on polaroid films using a ELS 400-SM densitometer (Hirschmann, Munich, Germany). Subsequently, β-actin band intensities were used to extrapolate the RNA content of the different samples. cDNA was entered into the PCR at amounts that would yield similar β-actin band intensities and would thus allow direct comparison of the different cytokine PCR products. RT samples to which no cellular RNA had been added served as templates for RT-PCR negative control reaction. Cytokine specific primers and β-actin primers were synthesized by MWG-Biotec (Freiburg, Germany). All primers were RNA specific and the structure of β-actin primers has previously been reported.27 The structures of the IL-10 primers were : 5′ACA GCT GCA CCC ACT TCC 3′(sense), 5′CCC AGG GAG TTC ACA TGC g3′(antisense); IFN-γ (5-GTT ACT GCC AGG ACC CAT ATG 3′(sense), 5′-GAC AGT TCA GCC ATC ACT TGG 3′ (antisense). Primer sequences were compared with Genbank Heidelberg and with previously published data.28
Statistical analysis. The t-test was used for statistical evaluation of the data.
RESULTS
IL-15– and IL-2–induced T-cell proliferation and activation. Highly enriched T cells were stimulated with medium, anti-CD20, a nonspecific isotype control antibody, anti-CD3 alone, or anti-CD3 + IL-15 or IL-2 for 3 days. Stimulation with medium or isotype control antibodies did not induce significant T-cell proliferation. IL-15 and IL-2 induced a significant and dose-dependent increase of T-cell proliferation compared with basal levels or T cells activated by anti-CD3 alone (data not shown, P < .01). However, about 10 times higher IL-15 concentrations were necessary to reach the same degree of proliferation as with IL-2, which is consistent with previous reports.1,29 Addition of IL-10 at a high concentration (10 ng/mL) did not suppress IL-2– or IL-15–mediated T-cell proliferation, consistent with previously reported results.30
Analysis of T-cell activation by measuring CD25 expression showed that stimulation of T cells with medium or isotype control antibodies did not induce significant T-cell activation (Fig 1). Stimulation with anti-CD3 in the presence or absence of IL-15 or IL-2 induced a significant increase in CD25 expression (Fig 1, P < .01). Again, about 10-fold higher concentrations of IL-15 were necessary to reach effects equivalent to those mediated by IL-2 (Fig 1). At a high concentration, exogenously administered IL-10 (10 ng/mL) did not suppress the IL-2– or IL-15–mediated CD25 expression on activated T cells.
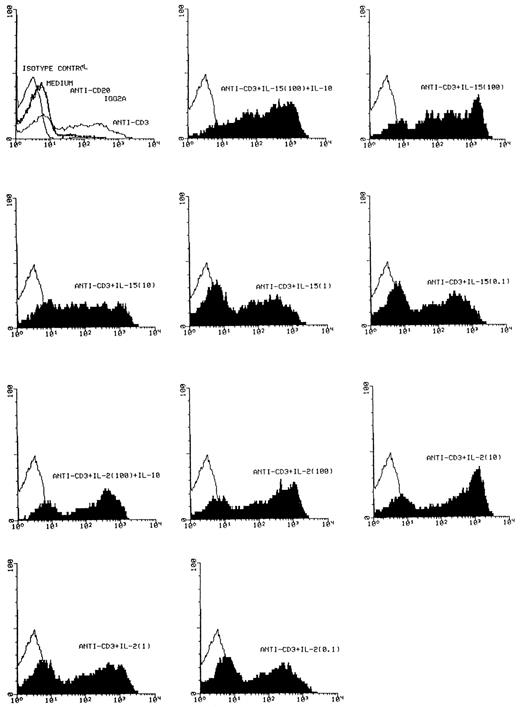
Effect of IL-15 on T-cell activation. FACS analysis of CD25 expression was performed with a PE-conjugated antihuman CD25 MoAb on T cells, which had been cultured as indicated with medium, anti-CD20, a nonspecific IgG2a isotype control antibody, anti-CD3, anti-CD3 + IL-15 (100 ng/mL, 10 ng/mL, 1 ng/mL, 0.1 ng/mL), anti-CD3 + IL-15 (100 ng/mL) + IL-10 (10 ng/mL), anti-CD3 + IL-2 (100 ng/mL, 10 ng/mL, 1 ng/mL, 0.1 ng/mL and anti-CD3 + IL-2 (100 ng/mL) + IL-10 (10 ng/mL) for 72 hours. Control profiles using PE-conjugated mouse IgG-1 are shown on each panel (light areas). Shown are the results from one representative experiment. A total of four experiments has been performed. Depicted are cell number (Y-axis) and fluorescence intensity of the CD25 staining (X-axis).
Effect of IL-15 on T-cell activation. FACS analysis of CD25 expression was performed with a PE-conjugated antihuman CD25 MoAb on T cells, which had been cultured as indicated with medium, anti-CD20, a nonspecific IgG2a isotype control antibody, anti-CD3, anti-CD3 + IL-15 (100 ng/mL, 10 ng/mL, 1 ng/mL, 0.1 ng/mL), anti-CD3 + IL-15 (100 ng/mL) + IL-10 (10 ng/mL), anti-CD3 + IL-2 (100 ng/mL, 10 ng/mL, 1 ng/mL, 0.1 ng/mL and anti-CD3 + IL-2 (100 ng/mL) + IL-10 (10 ng/mL) for 72 hours. Control profiles using PE-conjugated mouse IgG-1 are shown on each panel (light areas). Shown are the results from one representative experiment. A total of four experiments has been performed. Depicted are cell number (Y-axis) and fluorescence intensity of the CD25 staining (X-axis).
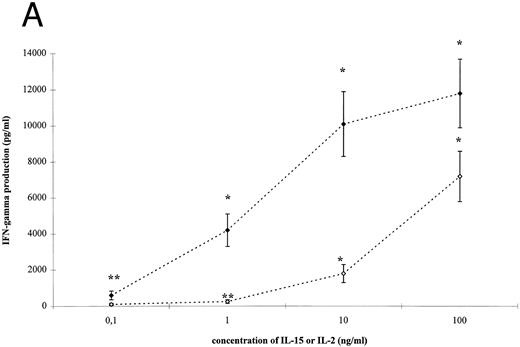
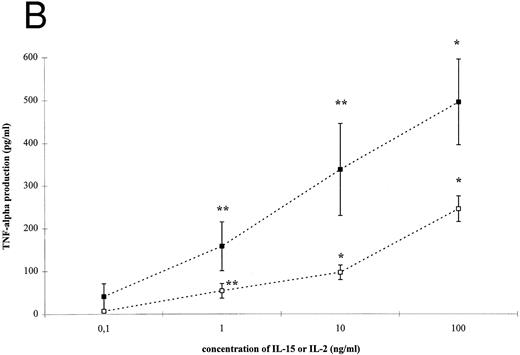
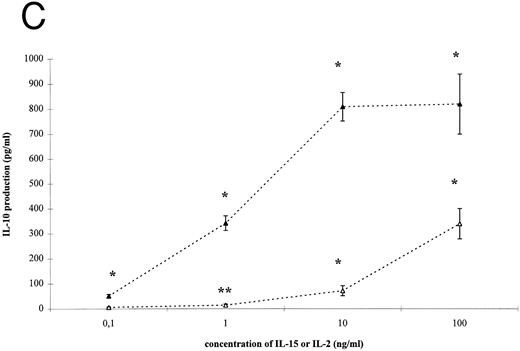
IL-15– and IL-2–induced cytokine production by T cells. To analyze the effect of IL-15 on T-cell–derived cytokine production, T cells were cultured with the respective stimuli for 3 days. T-cell activation by IL-15 and IL-2 was accompanied by a significant and dose-dependent increase of IL-10, IFN-γ and TNF-α production by T cells (Fig 2, P < .01). However, between 10- and 100-fold higher concentrations of IL-15 were necessary to induce cytokine concentrations approximately equivalent to those yielded by IL-2 stimulation.
IL-15–induced cytokine production by T cells. (A) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for IFN-γ. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (⋄) or IL-2 (♦) compared to anti-CD3 alone (248 ± 58 pg/mL) is indicated by * (P < .01) or ** (P < .05). (B) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for TNF-α. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (□) or IL-2 (▪) compared to anti-CD3 alone (65 ± 9 pg/mL) is indicated by * (P < .01) or ** (P < .05). (C) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for IL-10. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (▵) or IL-2 (▴) compared to anti-CD3 alone (2.5 ± 1.6 pg/mL) is indicated by * (P < .01) or ** (P < .05).
IL-15–induced cytokine production by T cells. (A) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for IFN-γ. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (⋄) or IL-2 (♦) compared to anti-CD3 alone (248 ± 58 pg/mL) is indicated by * (P < .01) or ** (P < .05). (B) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for TNF-α. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (□) or IL-2 (▪) compared to anti-CD3 alone (65 ± 9 pg/mL) is indicated by * (P < .01) or ** (P < .05). (C) T cells were isolated by rosetting and then stimulated as indicated. After 72 hours cell-free supernatants were procured and assayed for IL-10. Depicted are mean and SEM of six independent experiments. Significantly altered cytokine production induced by anti-CD3 + IL-15 (▵) or IL-2 (▴) compared to anti-CD3 alone (2.5 ± 1.6 pg/mL) is indicated by * (P < .01) or ** (P < .05).
The role of endogenous IL-2 in IL-15–mediated cytokine production was further evaluated by using an inhibitory antibody against the high-affinity IL-2 receptor. This antibody could significantly reduce IL-2 (used at 1 or 10 ng/mL, P < .01) mediated induction of IL-10, IFN-γ, or TNF-α. However, this antibody did not significantly reduce IL-15–mediated cytokine production (Table 1).
Interaction between IL-15 or IL-2 and IL-10. In this study the effect of IL-10 on IL-15 or IL-2–mediated cytokine production by purified T cells was analyzed. Therefore, T cells were stimulated with anti-CD3 + IL-15 or IL-2 (each used at 100 ng/mL) in the presence or absence of IL-10. IL-10 significantly reduced the IFN-γ and TNF-α protein synthesis in a dose-dependent fashion (Fig 3, P < .01). At a concentration of 10 ng/mL IL-10 suppressed IFN-γ production by more than 90% and TNF-α production by more than 70%. At this concentration, IL-10 also inhibited IL-2 and IL-15 induced IL-10 and IFN-γ mRNA expression as determined by semiquantitative RT-PCR (data not shown). At lower concentrations of IL-2 or IL-15 (1 or 10 ng/mL, data not shown), IL-10 showed the same degree of inhibition of cytokine production as for concentrations of 100 ng/mL (Fig 3).
Inhibitory effect of IL-10 on IL-2– or IL-15–mediated cytokine production. (A) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-15 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%. (B) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-2 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%.
Inhibitory effect of IL-10 on IL-2– or IL-15–mediated cytokine production. (A) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-15 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%. (B) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-2 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%.
Previously IL-10 had been reported to suppress IFN-γ production by MNCs via inhibition of IL-12 synthesis, and very low concentrations of IL-12 had been shown to be able to induce significant cytokine release from MNCs.17 Therefore, we performed a series of experiments with ultra-pure T cells to better understand the role accessory cell-produced IL-12 may have in mediating the observed suppression of T-cell cytokine production by IL-10.
T cells obtained after rosetting with sheep erythrocytes contained a low number of APCs. Stimulation of four different T-cell populations with high concentrations of IL-2 and IL-15 (each 100 ng/mL) induced low but significant concentrations of the IL-12 heterodimer (P < .025), which is the biologically active form of this cytokine.31 IL-10 could reduce the IL-2–induced IL-12 production from 3.9 ± 0.8 pg/mL to below 1 pg/mL (P < .025). Similarily, IL-15–mediated IL-12 release was reduced from 3.0 ± 0.7 pg/mL to below 1 pg/mL (P < .025).
Using a neutralizing antibody against IL-12, the role of endogenous IL-12 in IL-2– and IL-15–mediated cytokine production was analyzed. At a concentration where this antibody inhibited at least 1 ng/mL of IL-12, IL-2, and IL-15–induced cytokine production was significantly reduced (Table 2). However, the extent to which the different cytokines were inhibited varied somewhat. The greatest inhibition was obtained for IFN-γ, while inhibition of IL-10 and TNF-α production was less pronounced (Table 2).
To analyze the role of endogenous IL-12 in the IL-10–induced suppression of IL-2– and IL-15–mediated cytokine production, two approaches were taken: (1) the inhibitory effect of IL-10 was assessed in the absence of endogenous IL-12 and (2) the effect of exogenous IL-12 on IL-10–mediated cytokine suppression was determined.
Using ultra-pure T cells, which were virtually completely void of APCs, IL-15 and IL-2 each induced significant (P < .01) amounts of IFN-γ and TNF-α in CD3-activated T cells compared to stimulation with anti-CD3 alone (data not shown). In addition, IL-15 stimulated the release of 978 ± 118 pg/mL of IL-10 from CD3-activated T cells compared with 389 ± 134 pg/mL after stimulation with anti-CD3 alone (P < .01). Similarly IL-2 stimulated also a significant IL-10 release from CD3-activated T cells (1,354 ± 223 pg/mL; P < .01). Under these experimental conditions, IL-2– and IL-15–mediated cytokine production were not significantly affected by anti-IL-12 or IL-10, suggesting that IL-12 is not produced by ultra-pure T-cell populations and that the suppressive effects of IL-10 on IL-2/15 activated T-cell cytokine production are not directly mediated.
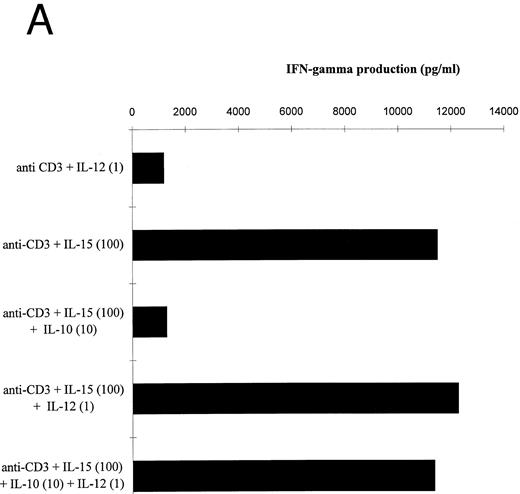
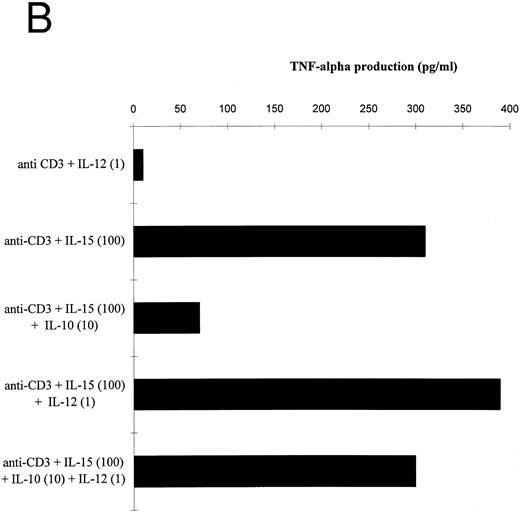
IL-10 significantly reduced the IL-15–mediated IFN-γ and TNF-α production by T cells stimulated in the presence of accessory cells (Fig 4A and B). IL-12 prevented the IL-10–induced suppression of IFN-γ and TNF-α by IL-15–activated T cells. The IL-12 effect on the IFN-γ production exceeded the effect on TNF-α release, probably because IL-12 participated more strongly in the production of IFN-γ than of TNF-α (Table 2). Similar results were obtained with IL-2 (data not shown).
Effect of IL-12 on IL-15–mediated cytokine production by T cells. (A and B) T cells were isolated by rosetting and then stimulated as indicated. Depicted are the increments in IFN-γ (A) or TNF-α (B) production obtained by stimulation with anti-CD3 + IL-15 (100 ng/mL) over stimulation with anti-CD3 alone of one representative experiment. At total of four experiments have been performed.
Effect of IL-12 on IL-15–mediated cytokine production by T cells. (A and B) T cells were isolated by rosetting and then stimulated as indicated. Depicted are the increments in IFN-γ (A) or TNF-α (B) production obtained by stimulation with anti-CD3 + IL-15 (100 ng/mL) over stimulation with anti-CD3 alone of one representative experiment. At total of four experiments have been performed.
Kinetics of IL-2– and IL-15–mediated cytokine release. Kinetic analyses of IFN-γ, TNF-α, and IL-10 production showed that IL-15– and IL-2–induced IL-10 release peaked on day 2, while maximum concentration of IFN-γ and TNF-α levels were only reached after 3 to 5 days, when IL-10 levels were already beginning to decline. In detail, on day 2 mean IL-10 response was above 85% of the maximum response compared to less than 60% of IFN-γ and TNF-α (P < .01, data not shown). In contrast, on day 5 mean IFN-γ and TNF-α release was above 80%, of the maximum response while mean IL-10 levels had already dropped to less than 65% (P < .01, data not shown).
The role of endogenous IL-10 in IL-2– and IL-15–mediated cytokine production. To evaluate the inhibitory potential of IL-15– and IL-2–induced IL-10 production the role of endogenously produced IL-10 on cytokine release was assessed. Stimulation of T cells by IL-15 or IL-2 in the presence of an IL-10R blocking MoAb significantly upregulated the release of IFN-γ and TNF-α (Table 3), suggesting that endogenous IL-10 could affect the IL-2/IL-15–mediated immune responses through negative feedback regulation. Similarly, blocking of IL-10 binding to its receptor by use of the inhibitory MoAb also lead to an increase of detectable IL-12. However, these differences were not statistically significant due to high interexperimental variation of the results. This might be explained by technical reasons, because IL-12 concentrations were detected at the lower detection limit of our assay. Since transforming growth factor-β (TGF-β) is another suppressive cytokine also produced by T cells, we investigated whether endogenous TGF-β release might also act on IL-2/IL-15–activated T-cell cytokine production. However, stimulation of T cells by anti-CD3 and IL-2 or IL-15 failed to induce immunoreactive TGF-β (data not shown). Consequently, a neutralizing anti-TGF-β MoAb did not affect cytokine release from activated T cells when compared with an isotype control antibody (data not shown).
DISCUSSION
In this study we report that IL-15– and IL-2–activated purified human T cells. Previously, Kanegane et al32 had observed that, similar to IL-2, IL-15 could induce proliferation and activation of memory and naive T cells, when stimulating a mononuclear cell fraction. We also showed that IL-10 could not suppress IL-2– or IL-15–mediated T-cell proliferation or CD25 expression. This is in agreement with previous observations that IL-10 downmodulates mitogen or anti-CD3 induced T-cell proliferation in the presence or absence of accessory cells via inhibition of endogenously produced IL-2.23,30 In these experiments addition of IL-2 could compensate the inhibitory effect of IL-10, suggesting that IL-2–mediated T-cell proliferation was not dependent on the secretion of accessory cell-derived factors such as IL-12, as has been demonstrated for anti-CD2–activated MNCs.33
The comparison between IL-2– and IL-15–mediated T-cell activation revealed that between 10- to 100-fold greater concentrations of IL-15 were necessary to reach equivalent biological effects as with IL-2. These results are similar to those reported by Carson et al29 who investigated NK-cell activation by IL-15 and IL-2. The difference in potency might be explained by the different number of IL-15/IL-2 high-affinity receptors expressed on NK cells. Similarily, anti-CD3–stimulated T cells expressed 5 to 7 times less high-affinity IL-15 receptors than IL-2 receptors.34 However, this might not completely explain the striking difference between IL-2 and IL-15 concentrations. In addition to a different number of high-affinity binding sites, Kumaki et al35 also found that binding of IL-15 significantly reduced the binding capacity of IL-15 to B and T cells by about 80%.
Since activated T cells produce their own IL-2, we explored the role of endogenous IL-2 in IL-15–mediated T-cell activation. Stimulation of anti-CD3 activated T cells by IL-15 was not affected by an inhibitory MoAb to the high-affinity IL-2R, which significantly reduced the effect of IL-2, suggesting that the IL-15 effect on cytokine release from T cells was not mediated by inducing endogenous IL-2. These results are in keeping with those reported by Mori et al.36 They showed that IL-15–mediated IL-5 production by human T-cell clones or stimulated PBMC was not dependent on IL-2 and that IL-15 could replace the function of IL-2.
In contrast to the effects on IL-15/IL-2–mediated T-cell proliferation and CD25 expression, IL-10 could significantly suppress IL-15– and IL-2–induced production of IFN-γ and TNF-α. IL-10 did not only inhibit protein release but also mRNA expression, suggesting that IL-10 downregulated protein de novo synthesis stimulated by IL-15 and IL-2. Taga et al30 showed that in the presence of accessory cells IL-10 could inhibit both IFN-γ production and proliferation of T cells after anti-CD3 stimulation. However, IL-2 could restore T-cell proliferation, while IFN-γ production remained suppressed even in the presence of exogenously added IL-2. Thus, in contrast to IL-2–mediated T-cell proliferation, IL-2– as well as IL-15–induced cytokine production seem to be dependent on the production of certain accessory cell derived factors.
Therefore, we investigated the role of IL-12 in IL-2– and IL-15–mediated cytokine production by T cells and the role of endogenous IL-12 in the IL-10– mediated inhibition of IL-2 and IL-15 effects. We found that stimulation of T cells by IL-2 and IL-15 in the presence of accessory cells mediated low concentrations of IL-12 heterodimer, which is the biologically active form of IL-12.31 In addition, an anti-IL-12 antibody could significantly inhibit the IL-2– and IL-15–mediated production of IFN-γ and TNF-α. In addition, anti-IL-12 also inhibited the release of IL-2– and IL-15–induced IL-10, although IL-12 had originally been described as a Th-1 cell stimulating cytokine.37 However, Meyaard et al33 showed that IL-12 could also facilitate the production of IL-10, a Th-2 derived cytokine, and that IL-2 and IL-12 had a synergistic effect on IL-10 release from T cells and T-cell clones.38
Armant et al39 described that IL-2–mediated T-cell activation in the presence of APCs but not involving TCR stimulation is dependent on endogenous IL-12 production. The mechanism by which IL-12 is induced seems to be mediated by an interaction between CD40L and CD40. IL-2 has been shown to significantly upregulate IL-12 production, probably by enhancing T-cell–APC interaction. In this study we could show for the first time that IL-2– and IL-15–mediated stimulation of TCR-activated T cells is dependent on the production of endogenous IL-12. By two lines of evidence we could also show that the inhibition of this IL-12 production might be a major mechanism by which IL-10 suppresses IL-2– and IL-15–mediated T-cell activation: (1) the significant inhibitory effect of IL-10 on IL-2/IL-15–activated T-cell cytokine production was not directly mediated because it was not observed in APC-free preparations and (2) addition of exogenous IL-12 prevented IL-10–induced suppression of cytokine production by IL-2/IL-15–activated T cells. However, it also appeared that IL-12 was not the only factor involved, because anti-IL-12 could not completely inhibit the IL-2– and IL-15–mediated cytokine production and exogenous IL-12 could not completely overcome the inhibitory effect of IL-10. D'Andrea et al17 found that IL-10 inhibited IFN-γ release by MNCs after stimulation with lipopolysaccharide or Staphylococcus aureus Cowan I. The inhibitory effect was closely related to a suppression of IL-12 production, but the inhibitory effect of IL-10 could be fully compensated only by a combination of IL-1β and IL-12.
T cells produce substantial amounts of IL-10. Inhibition of endogenous IL-10 binding to the IL-10R significantly upregulates IL-15– and IL-2–mediated T-cell cytokine production. Since the inhibitory effect of IL-10 on T-cell cytokine production appeared to be indirect, involving decreased IL-12 production by accessory cells, these data suggest that endogenous IL-10 can regulate T-cell production of IFN-γ and TNF-α via a paracrine negative feedback loop.
This negative feedback loop by endogenous IL-10 might be important for the kinetics of cytokine responses induced by IL-15 and IL-2. IL-15– and IL-2–induced IL-10 production peaked earlier than IFN-γ and TNF-α. These results are in keeping with those of McHugh et al40 who found that stimulation of peripheral blood mononuclear cells with phytohemagglutinin first induced an IL-10 secretion, followed by a release of IFN-γ and TNF-α.
In contrast to its effect on T cells, IL-10 augmented IL-2–mediated proliferation of human NK cells, and exacted additive effects on cytotoxic activity. Production of IFN-γ, granulocyte-macrophage colony-stimulating factor, and TNF-α by IL-2–stimulated NK cells was significantly enhanced by IL-10. Thus, the induction of IL-10 by IL-2 and IL-15 during T-cell activation might not only exhibit a negative feedback control mechanism in T-cell immunity, but can also facilitate cross-talk with NK cells, which might lead to enhanced NK-cell activity.41
IL-15 has been used for anticancer activities in vivo and in vitro16,42 and it seems to exhibit a therapeutic index superior to IL-2.16 The cytotoxic effects of IL-2 and probably of IL-15 are mediated by cellular mechanisms including the activation of Fas and Perforin as well as by the release of soluble mediators such as IFN-γ and TNF-α.43 The results of this study show that IL-15 could significantly induce the release of cytotoxic cytokines from T-cell receptor activated T cells via an IL-2–independent mechanism. In addition, exogenous and endogenous IL-10 inhibited IL-2– and IL-15–mediated T-cell derived cytokine production. In contrast, IL-12 prevented the inhibitory action of IL-10 on IL-2/IL-15–mediated T-cell cytokine production. Thus, future concepts of anticancer immunotherapy should consider the use of IL-15 alone or in combination with IL-12.
Supported by DFG (Bonn, Germany) Grant Ko 971/3-2, DFG Sonderforschungsbereich 503 (projects B-2, C-3, C-4, and C-6), and Elterninitiative Kinderkrebsklinik Düsseldorf e.V.
Parts of this paper have been presented at the 38th Annual Meeting of the American Society of Hematology, Orlando, FL, December 1996. Data presented in this paper are part of the thesis of U.B.
Address reprint requests to Priv. Doz. Dr Dieter Körholz, Department of Pediatric Hematology and Oncology, Heinrich-Heine University Medical Center, Moorenstr. 5, 40225 Düsseldorf, Germany.

![Fig. 3. Inhibitory effect of IL-10 on IL-2– or IL-15–mediated cytokine production. (A) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-15 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%. (B) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-2 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4513/3/m_bl_0040f3a.jpeg?Expires=1769080572&Signature=faeFkpCCAdw-QcAZk0FoCXkJzsjJrw-gnV6x2fjB3UwU14lRav-kWPqvTPxYQBn9nojbqKQSeAEvyN4~wR80oW~fib1-KXM2bzE7wpAZ1bUTBkw3SnVgPuQJKD5c1lB-GagJp5PzS-ihQyc0Hy9UTB41jbTHwNRw0pwMlZESVJ7Mv9HzCqW4f76pWSsvuk9Un-9Z7K5p0epzis0lSE2LB5NXLZppqNFvoOy3RrAHkjIwe877JeEdmbeZDplhN~f17sMYocmc1j9XVGHLUslCEXzKWRgttGR-kqlFjPPBZdHqKNoklJ19EwArj9HZtvhFfzO6IbYjgVTq8omWSsNLCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Inhibitory effect of IL-10 on IL-2– or IL-15–mediated cytokine production. (A) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-15 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%. (B) T cells were isolated by rosetting and then stimulated by anti-CD3 + IL-2 (100 ng/mL) in the presence or absence of IL-10 (0.1 to 10 ng/mL). Depicted are mean and SEM of four independent experiments showing inhibition of cytokine-induced IFN-γ or TNF-α production. % Inhibition of cytokine production by IL-10 was calculated by (1 − [cytokine production in the presence of IL-10]/[cytokine production in the absence of IL-10]) × 100%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4513/3/m_bl_0040f3b.jpeg?Expires=1769080572&Signature=22QgY-VCNNfks-cGKcqYNx4lREfwqNck33uTF9DsCLd28EnO1bhwyTf0jUST9ASSWnz-Js7Ks0k1THlmio1cuzC34qYLsqRatkrfM5lwkebbhny6KsH4bm7khDjvsdn74BOL~3uVs3I3H0IXRy73Ynwwu-WLKA8poR9Lmwla84Op~zclsxQ56DpsuGyncc9c~H7WrnrOSBoysDwU46F5GJxvu70ksrEoLwh~RTkwsER8ZF0oa3lNPqEEQBBRFR~YnXz~sdomhYeTUbatQ1EepgghqOgR0FN1KUe~VLWAvc5yEx2rpbA7d6UTQFYoHBWERJ2UzvWdOBacp5zkqb0dbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)