Abstract
In autoimmune hemolytic anemia (AIHA), there is accumulating evidence for an involvement of FcγR expressed by phagocytic effector cells, but demonstration of a causal relationship between individual FcγRs and IgG isotypes for disease development is lacking. Although the relevance of IgG isotypes to human AIHA is limited, we could show a clear IgG isotype dependency in murine AIHA using pathogenic IgG1 (105-2H) and IgG2a (34-3C) autoreactive anti–red blood cell antibodies in mice defective for FcγRIII, and comparing the clinical outcome to those in wild-type mice. FcγRIII-deficient mice were completely resistent to the pathogenic effects of 105-2H monoclonal antibody, as shown by a lack of IgG1-mediated erythrophagocytosis in vitro and in vivo. In addition, the IgG2a response by 34-3C induced a less severe but persistent AIHA in FcγRIII knock-out mice, as documented by a decrease in hematocrit. Blocking studies indicated that the residual anemic phenotype induced by 34-3C in the absence of FcγRIII reflects an activation of FcγRI that is normally coexpressed with FcγRIII on macrophages. Together these results show that the pathogenesis of AIHA through IgG1-dependent erythrophagocytosis is exclusively mediated by FcγRIII and further suggest that FcγRI, in addition to FcγRIII, contributes to this autoimmune disease when other IgG isotypes such as IgG2a are involved.
AUTOIMMUNE hemolytic anemia (AIHA) is the oldest recognized autoimmune disease in humans. It is characterized by the production of pathogenic self-reactive antibodies causing anemia as a result of immune destruction of red blood cells (RBCs). The antibodies involved are classified as warm autoantibodies, cold agglutinins, and biphasic hemolysins which are cleared by distinct effector mechanisms.1 The predominant forms of AIHA involve warm IgG-autoantibodies accompanied with extravascular hemolysis. It is presumed that hemolysis of IgG opsonized RBCs in warm AIHA is largely mediated through either Fc and/or complement receptors expressed by phagocytic effector cells. Two recent studies in murine models of AIHA have suggested a more prominent role of Fc receptors than of complement activation. First, the treatment with recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), which normally enhances FcγR-dependent phagocytosis, also accelerates the progression of spontaneous AIHA in New Zealand Black (NZB) mice.2 A similar effect of GM-CSF in accelerating the clearance of IgG-coated RBCs has been noted in humans.3Second, experimentally induced AIHA occurs even in the absence of the complement components C3, C4, and C5, but requires the presence of Fc receptors in association with the common FcR γ-chain.4
There are three classes of murine receptors for IgG, FcγR on leukocytes: the high-affinity receptor FcγRI, and the two low-affinity receptors, FcγRII and FcγRIII.5,6 Although FcγRI is capable of binding monomeric IgG2a, both FcγRII and FcγRIII have been proposed to interact preferentially with murine IgG1 and IgG2b immune complexes.7,8 These receptors are structurally related consisting of similar ligand-binding domains, but differ in their transmembrane and intracellular domains. The FcγRII isoforms, termed b1 and b2, are single subunit receptors with inhibitory functions. FcγRI and FcγRIII are multimeric receptors in association with the common FcR γ-chain9-11 required for assembly and the triggering of various effector functions, including phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), and the release of inflammatory mediators.6 Knock-out (KO) mice deficient in FcγRI, FcγRII, FcγRIII, and FcR γ-chain allow dissecting the contribution of FcγRs to various normal and pathological immunological events.12-15 For instance, both FcR γ-chain–deficient mice (which are unable to function through both FcγRI and FcγRIII) and FcγRIII KO mice exhibit an impaired Arthus reaction indicating for FcγRIII as the essential Fcγ receptor in the initiation of IgG immune complex–triggered inflammation and autoimmune disease.14,16,17 FcR γ-chain–deficient mice also argue for an important role of FcγRs expressed on macrophages in the pathogenesis of AIHA.18 But the specific FcγR classes involved could not be identified yet. Furthermore, until this study, the relationship between specific FcγRs such as FcγRIII and IgG isotypes for disease development of AIHA has not been investigated.
In the present study, we tested the cytotoxic activities of IgG1 (105-2H) and IgG2a (34-3C) α murine RBC (MRBC) monoclonal antibodies (MoAbs) both reacting with the same autoantigen epitope identified as the erythrocyte anion channel band 3 on MRBCs19 20 in FcγRIII KO mice and their wild-type littermates. We show that mice lacking FcγRIII are protected to experimental AIHA determined through IgG1-dependent erythrophagocytosis. FcγRIII KO mice are not completely resistant to IgG2a-induced anemia indicating, in addition to macrophage FcγRIII, the contribution of FcγRI. These findings suggest that differences in IgG isotype specificities of individual FcγRs are critical in the disease process of AIHA.
MATERIALS AND METHODS
Mice.
FcγRIII-deficient and wild-type littermates were developed in collaboration with the group of Dr J.S. Verbeek (Utrecht, The Netherlands), as described previously.14 All mice were bred and maintained under dry barrier conditions in the animal facilities at the Hannover Medical School (Hannover, Germany). Mice were studied at 2 to 4 months of age. All experiments received institutional approval.
αMRBC and other antibodies.
105-2H (IgG1), and 34-3C (IgG2a) monoclonal αMRBC autoantibodies were obtained by fusion of spleen cells from unmanipulated NZB mice as described.19 Hybridoma cells were maintained in RPMI/10% fetal calf serum (FCS). Culture supernatants were concentrated by precipitation in 50% saturated ammonium sulfate followed by purification with protein A affinity chromatography (Pharmacia, Uppsala, Sweden) and dialysis against phosphate-buffered saline (PBS). Purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Concentrations of αMRBC MoAb were determined by Ig class-specific enzyme-linked immunosorbent assay (ELISA; DAKO, Hamburg, Germany). Other antibodies in use were: 2.4G2,21 which is directed against FcγRII/III (Pharmingen, San Diego, CA), M1/70 against Mac-1 (Pharmingen), the isotype-matched control antibodies 5E5 (murine IgG1), and W6/32 (murine IgG2a). Polyclonal rabbit-anti–rat-IgG (Z0494) and monoclonal APAAP (alkaline phophatase anti-alkaline phosphatase) rat IgG (D0488) were obtained from DAKO.
Phagocytosis of IgG-opsonized MRBCs by peritoneal macrophages.
Peritoneal macrophages elicited by intraperitoneal injection with 1 mL 3% thioglycolate (DIFCO Laboratories, Detroit, MI) were flushed out the peritoneal cavity on day 3 postinjection and suspended in PBS. Freshly isolated MRBCs were washed two times with ice-cold PBS by centrifugation at 1,600 rpm and processed for opsonization. Hereby, 10 μL of pelleted MRBCs were incubated at 4°C for 60 minutes with 10 μL of αMRBC MoAbs at saturating (determined by fluorescence-activated cell sorting [FACS] analysis) concentrations. Aliquots of 50 μL of 1% opsonized MRBC suspension were added to 50 μL peritoneal macrophages preparation and incubated at 37°C for 60 minutes. For some experiments, peritoneal macrophages were first incubated for 30 minutes at 4°C with the anti-FcγRII/III blocking antibody 2.4G2. Noningested extracellular MRBC were lysed by hypotonic shock, immediately followed by two washes with PBS. Peritoneal macrophages were conventionally stained with Giemsa/hematoxylin-eosin, and phagocytosis was determined by light microscopy. Peritoneal macrophages containing more than two MRBCs were considered as phagocytic.
Experimental AIHA.
Hemolytic anemia was induced by a single intraperitoneal (IP) injection of the pathogenic αMRBC autoantibodies 105-2H (450 μg) or 34-3C (120 μg) or the same amounts of isotype-matched control antibodies 5E5 (mIgG1) or W6/32 (mIgG2a). In some experiments mice received IP 10 μg naja naja cobra venom factor (Calbiochem, La Jolla, CA) 1 day before and 2 days after the injection of 34-3C. This treatment depletes serum levels of complement C3 as determined by CH50-measurements. For FcγRII and FcγRIII blockade 250 μg 2.4G2 MoAb was injected IP 24 hours before and 24 and 72 hours after administration of αMRBC 34-3C.22 Blood samples obtained from the retroorbital plexus were collected into heparinized microhematocrit capillary tubes and centrifuged for 5 minutes at 12,000 rpm in a microfuge. Hematocrits measured by the percentage of packed RBCs were directly determined after centrifugation.
Histopathology.
Mice were killed at day 2 after injection of pathogenic αMRBC, and major organs including spleens and livers were processed for histological examination. Tissues were fixed in 10% buffered formaline, embedded in paraffin, and stained with hematoxylin and eosin (H + E) according to conventional procedures. In further experiments tissues were prepared for immunocytochemical techniques. Mac-1+ cells were revealed by incubating cryostat sections for 30 minutes with the rat antibody M1/70 and then incubated with the bridging anitbody Z0494 and the rat-APAAP antibody complex (D0488) for 30 minutes. The last two steps were repeated for 15 minutes followed by visualization using naphthyl phosphate and Fast blue.23Ingested erythrocytes were shown by their endogeneous peroxidase using H2O2 and diaminobenzidine as substrates. Thus, Mac-1+ macrophages phagocytosing erythrocytes were seen as blue cells with brown content. The slides were counterstained with hematoxylin and mounted in glycergel (DAKO).
RESULTS AND DISCUSSION
Phagocytosis of IgG1 versus IgG2a-coated MRBCs in FcγRIII-deficient mice.
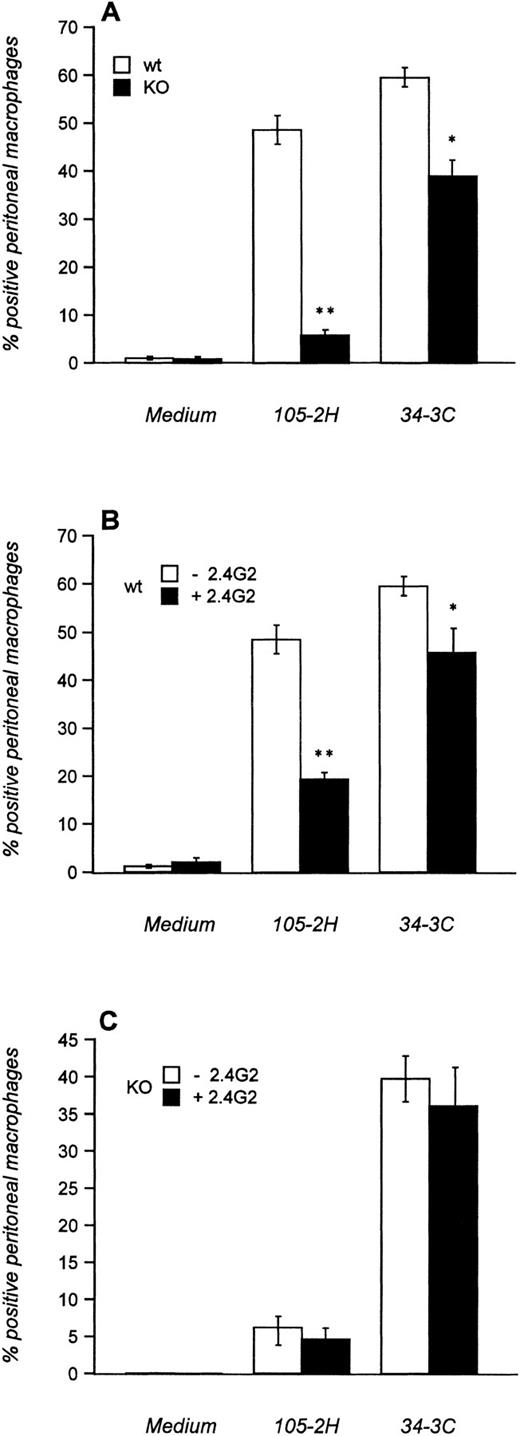
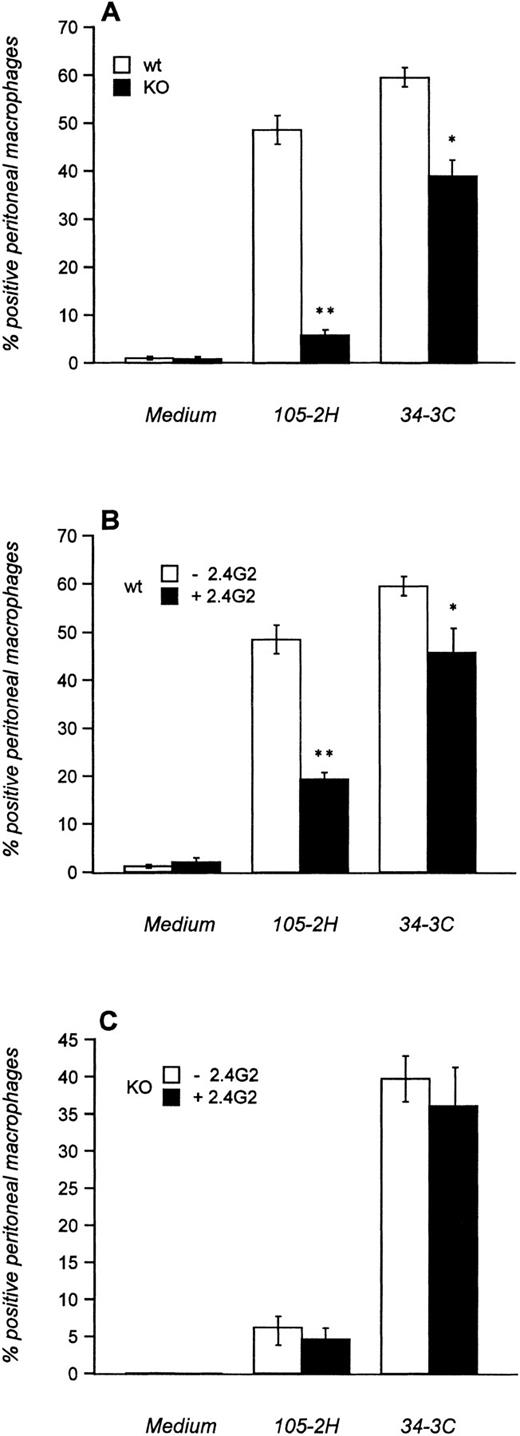
We first investigated the in vitro-phagocytosis of MRBCs opsonized with 105-2H (IgG1) and 34-3C (IgG2a) using thioglycolate-elicited peritoneal macrophages that normally express FcγRI, FcγRII, and FcγRIII. High levels of phagocytosis were evident with both 105-2H and 34-3C in wild-type mice (Fig 1A). The phagocytosis was either slightly (34-3C) or substantially (105-2H) diminished in the presence of the anti-FcγRII/III antibody 2.4G2 (Fig1B). This is consistent with our observation that FcγRIII KO mice lack phagocytosis of 105-2H opsonized MRBCs (Fig 1A), indicating an apparent specificity of IgG1 for FcγRIII. In case of the IgG2a αMRBC 34-3C the reduced phagocytosis in FcγRIII KO mice was not further decreased by 2.4G2 (Fig 1A and C). These data show that the specificity of complexed IgG2a for FcγRI15 is not absolute. It appears that FcγRIII contributes to some extent to the binding and phagocytosis of IgG2a-coated MRBCs.
Phagocytosis of IgG-opsonized MRBCs. Thioglycolate-elicited peritoneal macrophages from FcγRIII wild-type (□) and FcγRIII KO (▪) mice were incubated with MRBCs opsonized with (A) pathogenic MRBC MoAbs of the IgG1 (105-2H) and IgG2a (34-3C) isotypes or with medium alone. In addition, macrophages from FcγRIII wild-type (B) and FcγRIII KO (C) mice were first incubated with (▪) or without (□) the 2.4G2 antibody, which is directed against FcγRII and FcγRIII, and subsequently opsonized with the MRBC 105-2H and 34-3C. After 1 hour of incubation at 37°C, extracellular erythrocytes were lysed by hypotonic shock and the percentage of positive peritoneal macrophages that had ingested more than two erythrocytes was assessed microscopically. Results are expressed as the mean values ± SEM of five individual experiments. Significances are determined by Student’s t-test (*P< .05; **P < .001).
Phagocytosis of IgG-opsonized MRBCs. Thioglycolate-elicited peritoneal macrophages from FcγRIII wild-type (□) and FcγRIII KO (▪) mice were incubated with MRBCs opsonized with (A) pathogenic MRBC MoAbs of the IgG1 (105-2H) and IgG2a (34-3C) isotypes or with medium alone. In addition, macrophages from FcγRIII wild-type (B) and FcγRIII KO (C) mice were first incubated with (▪) or without (□) the 2.4G2 antibody, which is directed against FcγRII and FcγRIII, and subsequently opsonized with the MRBC 105-2H and 34-3C. After 1 hour of incubation at 37°C, extracellular erythrocytes were lysed by hypotonic shock and the percentage of positive peritoneal macrophages that had ingested more than two erythrocytes was assessed microscopically. Results are expressed as the mean values ± SEM of five individual experiments. Significances are determined by Student’s t-test (*P< .05; **P < .001).
FcγRIII-deficient mice are resistant to experimental AIHA induced by pathogenic IgG1 but not IgG2a αMRBC MoAbs.
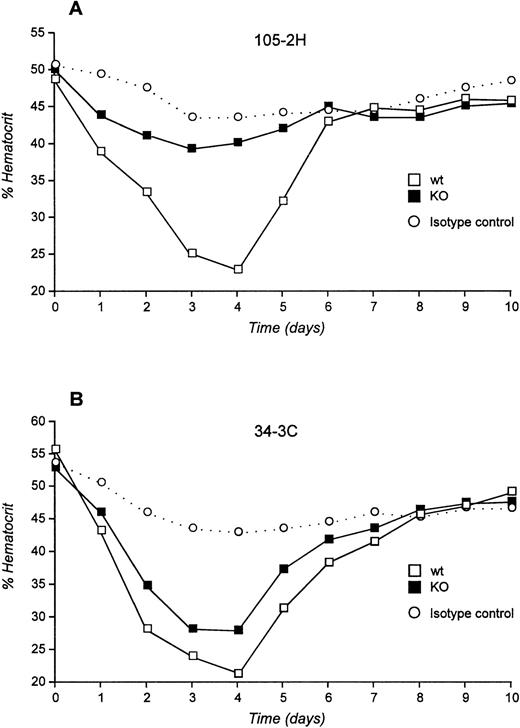
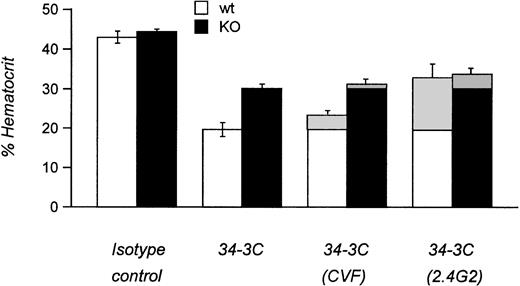
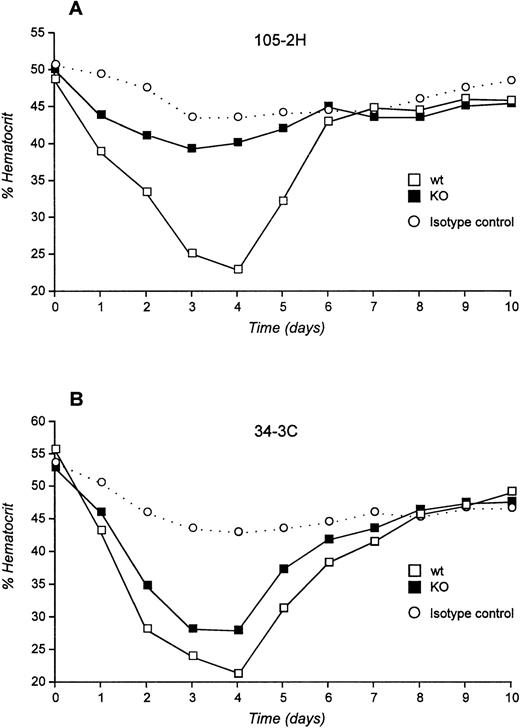
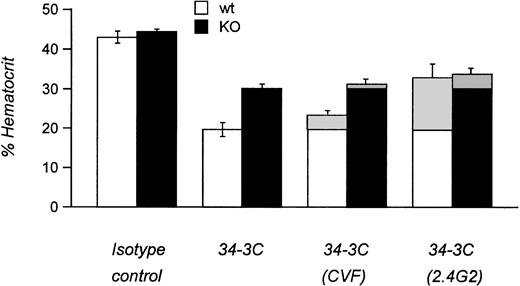
We next examined the pathogenicity of αMRBC by a single IP injection of purified MoAb in FcγRIII KO mice or their wild-type controls. As shown in Fig 2A and B, 450 μg of the IgG1 MoAb 105-2H or 120 μg of the IgG2a MoAb 34-3C was required to develop a strong but transient AIHA with an average hematocrit (Ht) of 23% and 21% at day 4 after injection in wild-type mice, respectively. Under these conditions the decrease in Ht induced by 105-2H and 34-3C recovered to normal levels of about 40% to 50% around day 7. In contrast, FcγRIII-deficient mice were completely resistent to the pathogenic effects of 105-2H with mean Ht levels remaining at ≥40% (Fig 2A). 34-3C induced a less severe but persistent anemia with a decrease in Ht levels to 28% in FcγRIII KO mice (Fig 2B), indicating that, in addition to FcγRIII, either FcγRI and FcγRII, or both, may have a contributory role. However, two observations argue against a significant contribution of FcγRII but rather indicate that FcγRI is responsible for this residual anemic phenotype. First, the prior administration of the FcγRII and FcγRIII blocking antibody 2.4G2 resulted in partial protection from 34-3C-induced AIHA in wild-type controls at similar levels to those in FcγRIII KO mice not receiving 2.4G2 (Fig3). Second, blocking FcγRII in the absence of FcγRIII through 2.4G2 did not further improve the protective effect in FcγRIII KO mice (Fig3). These results are consistent with previous evidence obtained with FcR γ-chain knock-out mice deficient for both FcγRI and FcγRIII but not FcγRII, in which the profound anemia normally induced by 34-3C is completely absent.18
Experimental AIHA induced by MRBC antibodies in FcγRIII wild-type and FcγRIII KO mice. (A and B) Daily hematocrits of FcγRIII wild-type (□) and FcγRIII KO (▪) mice injected with the pathogenic MRBC MoAbs 105-2H (A) and 34-3C (B). No decrease of Ht level was observed for mice injected with nonpathogenic isotype control MoAbs (○). Ht values lower than 40% were considered as anemic. Shown are the mean values obtained from 5 to 10 mice in each group (SD <3%).
Experimental AIHA induced by MRBC antibodies in FcγRIII wild-type and FcγRIII KO mice. (A and B) Daily hematocrits of FcγRIII wild-type (□) and FcγRIII KO (▪) mice injected with the pathogenic MRBC MoAbs 105-2H (A) and 34-3C (B). No decrease of Ht level was observed for mice injected with nonpathogenic isotype control MoAbs (○). Ht values lower than 40% were considered as anemic. Shown are the mean values obtained from 5 to 10 mice in each group (SD <3%).
Experimental AIHA in FcγRIII wild-type and FcγRIII KO mice treated with CVF and 2.4G2. Mean hematocrits of FcγRIII wild-type (□) and FcγRIII KO (▪) mice at day 4 after injection induced by the MRBC antibody 34-3C. Hatched areas indicate the differences in AIHA induction obtained by treatments with cobra venom factor to deplete complement C3 (CVF) or with the anti-FcγR MoAb 2.4G2 to block specifically the two low-affinity receptors FcγRII and FcγRIII (2.4G2). Results are expressed as the percentage of mean hematocrit ± SEM obtained from 5 to 10 mice in each group.
Experimental AIHA in FcγRIII wild-type and FcγRIII KO mice treated with CVF and 2.4G2. Mean hematocrits of FcγRIII wild-type (□) and FcγRIII KO (▪) mice at day 4 after injection induced by the MRBC antibody 34-3C. Hatched areas indicate the differences in AIHA induction obtained by treatments with cobra venom factor to deplete complement C3 (CVF) or with the anti-FcγR MoAb 2.4G2 to block specifically the two low-affinity receptors FcγRII and FcγRIII (2.4G2). Results are expressed as the percentage of mean hematocrit ± SEM obtained from 5 to 10 mice in each group.
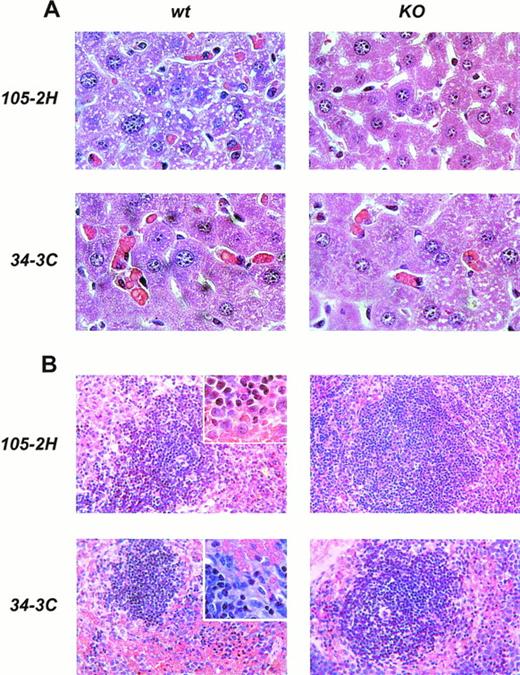
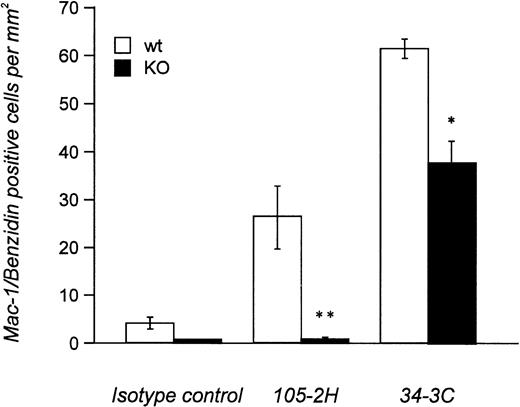
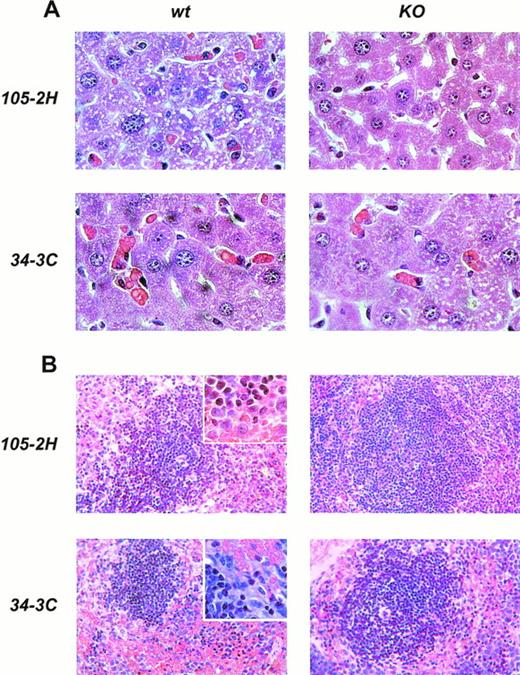
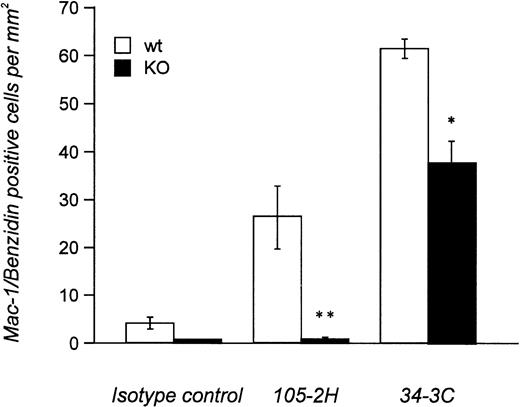
Histopathological examination of mice treated with IgG1 αMRBC 105-2H showed a marked degree of erythrophagocytosis in the liver accompanied by splenic engorgement in wild-type mice but not in FcγRIII-deficient mice, as assessed by conventional eosin/hematoxylin staining (Fig 4). Studies in op/op mice have indicated that in addition to the splenic macrophage, the hepatic Kupffer cell may be an important effector cell to the development of AIHA.18 Thus, we also performed immunohistochemistry with the M1/70 MoAb specific for the Mac-1 antigen on macrophages residing in the spleen and the liver. This allows a more quantitative estimation of FcγR-dependent erythrophagocytosis, especially in the liver. From a total number of 196 ± 23 Mac-1+ cells detected per mm2 liver section at day 2 postinjection, 27 ± 5 (n = 6) were Benzidin-positive containing ingested MRBCs (Fig 5). This pathology, equivalent to 13.8% erythrophagocytosis observed for wild-type controls, was completely absent in FcγRIII KO mice, supporting the notion that AIHA induced by 105-2H is exclusively mediated through FcγRIII. In the case of the IgG2a αMRBC 34-3C, a reduced pathology was seen in FcγRIII-deficient mice (38 ± 4 Mac-1/Benzidin-positive cells, n = 5) compared with wild-type mice (62 ± 2 Mac-1/Benzidin-positive cells, n = 5). The reduction in erythrophagocytosis by around 40% in FcγRIII KO mice is consistent with the idea that FcγRIII contributes significantly but not exclusively to the histopathological manifestations typical for AIHA induced by 34-3C. The contribution of both FcγRI and FcγRIII may also account for the stronger pathogenicity observed in general with 34-3C (IgG2a) compared with 105-2H (IgG1).19
Histopathology from anemic FcγRIII wild-type and FcγRIII KO mice. Representative histological appearance of liver (A) and spleen (B) from FcγRIII wild-type and FcγRIII KO mice on day 2 after AIHA-induction by the injection of the pathogenic IgG1 105-2H and IgG2a 34-3C MoAbs (hematoxylin and eosin stained; inset: higher magnification).
Histopathology from anemic FcγRIII wild-type and FcγRIII KO mice. Representative histological appearance of liver (A) and spleen (B) from FcγRIII wild-type and FcγRIII KO mice on day 2 after AIHA-induction by the injection of the pathogenic IgG1 105-2H and IgG2a 34-3C MoAbs (hematoxylin and eosin stained; inset: higher magnification).
Quantitative analysis of in vivo-erythrophagocytosis in the liver from anemic FcγRIII wild-type and FcγRIII KO mice. Liver sections from FcγRIII wild-type (□) and FcγRIII KO (▪) mice on day 2 after injection induced by either 105-2H or 34-3C MRBCs were processed for Mac-1 immunostaining of macrophages counterstained with benzidin for the detection of erythrocytes. The amount of Mac-1+ liver macrophages containing ingested erythrocytes per mm2 was assessed microscopically. Results are expressed as the mean values ± SEM obtained from three to five mice in each group. Significance is determined by Student’s t-test (*P < .05; **P < .001).
Quantitative analysis of in vivo-erythrophagocytosis in the liver from anemic FcγRIII wild-type and FcγRIII KO mice. Liver sections from FcγRIII wild-type (□) and FcγRIII KO (▪) mice on day 2 after injection induced by either 105-2H or 34-3C MRBCs were processed for Mac-1 immunostaining of macrophages counterstained with benzidin for the detection of erythrocytes. The amount of Mac-1+ liver macrophages containing ingested erythrocytes per mm2 was assessed microscopically. Results are expressed as the mean values ± SEM obtained from three to five mice in each group. Significance is determined by Student’s t-test (*P < .05; **P < .001).
The role of complement in AIHA induced by 105-2H and 34-3C has been analyzed by depleting complement C3 with cobra venom factor (CVF). Similar to several other reports,24 CVF-treated wild-type and FcγRIII KO mice were not significantly protected from anemia (Fig3 and data not shown), indicating only a minor role, if any, of complement receptor-mediated erythrophagocytosis in this model of AIHA.
Concluding remarks.
In the present study we identified FcγRIII to be involved in the disease process of murine AIHA. Loss of FcγRIII in mice resulted in complete protection from disease development induced by the cytotoxic IgG1 autoantibody 105-2H. The lack of IgG1-mediated erythrophagocytosis in vitro and in vivo in FcγRIII KO mice coincides with a strong reduction in anemia. This result provides direct in vivo evidence that the interaction between IgG1 and FcγRIII contributes significantly to the development of experimental AIHA.
In case of 34-3C, partial protection from anemia occurred in FcγRIII-deficient mice, indicating that FcγRIII is normally involved in experimental AIHA caused by this IgG2a autoantibody. It further suggests that IgG2a may also act via FcγR other than FcγRIII, the most likely being the high-affinity receptor FcγRI. This was supported by functional blocking studies using the anti-FcγRII/III 2.4G2 antibody indicating a minor, if any, role of FcγRII on macrophages. In accordance, the predominance of FcγRI and not FcγRII has been recently shown in the phagocytosis of IgG2a-coated MRBCs.25 Because the 105-2H and 34-3C MoAbs reacted with the same autoantigenic epitope on MRBCs their relative affinities for FcγRIII (IgG1 = IgG2a) and FcγRI (IgG2a > > > IgG1)5 6 are thus of prime importance for the differences in induction of anemia.
Previous studies on the pathogenesis of murine idiopathic thrombocytopenic purpura (ITP) suggested a role for FcγR,26 supported by findings that disease development induced by the cytotoxic anti-platelet 6A6 MoAb is abolished in FcR γ-chain–deficient mice.18 Because the 6A6 autoantibody was of the IgG1 subclass, we suggest that the pathology of ITP induced by this antibody may be predominantly mediated by FcγRIII. Confirmatory studies in FcγRIII-deficient mice would further strengthen this hypothesis. Our observation, at least, that FcγRIII-deficient mice have higher platelet counts (1,640 ± 220 × 103/μL, n = 6) than normal mice (1,160 ± 120 × 103/μL, n = 9) suggests a role for FcγRIII also in the clearance of IgG-coated platelets. The findings that individual FcγR interact differently with IgG isotypes in mediating autoimmune injury might be relevant for the potential use of these receptors as therapeutic targets in the treatment of AIHA in humans. Clinical studies in which AIHA have been treated in similar ways are rather limited. Thus, current approaches on targeting FcγR binding sites7 in combination with humanized FcγR mouse models27-29 will be useful to establish the significance of either FcγRIII or FcγRI blockade as therapeutic modalities for human AIHA.
ACKNOWLEDGMENT
We thank Margot Zielinska for the isotype-matched control antibodies (5E5, W6/32) and Frank Heusohn for support in histology data processing.
Supported by the Deutsche Forschungsgemeinschaft Grants No. Ge892/2-2 and SFB 265/A09 to J.E.G. and R.E.S., and by a grant from the Swiss National Foundation for Scientific Research to S.I.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to J. Engelbert Gessner, PhD, Department of Clinical Immunology, Hannover Medical School, Carl-Neuberg Str 1, 30625 Hannover, Germany; e-mail: Gessner.Johannes@MH-Hannover.DE.