Abstract
Tolerance to a vascularized allograft can be induced in adult animals by pregraft donor-specific blood transfusion (DST). Mechanisms underlying this effect appear to depend on unresponsiveness of alloreactive T-helper cells. In this study, we examined the roles of DST and cellular components of the allograft that are important in inducing T-cell unresponsiveness in a rat model. DST alone did not tolerize alloreactive recipient T-helper cells, but the combination of DST and heart allograft induced profound inhibition of the antidonor proliferative response in spleen but not in lymph node cells. When heart allografts were depleted of passenger leukocytes by pretreating the donor with cyclophosphamide or by parking the graft for 2 months in a tolerant recipient, tolerance induction in DST-treated recipients was abrogated. Tolerance could then be restored in a majority of DST-treated recipients of passenger leukocytes depleted grafts by injecting them at the time of grafting with donor, but not third-party, dendritic cells. This indicates that graft passenger leukocytes, most likely dendritic cells, are required for DST-induced allograft tolerance.
IT HAS LONG BEEN established that pretransplant donor-specific blood transfusions (DST) result in the enhancement or tolerance of vascularized allografts in rodents.1-3 In addition to DST (reviewed in Anderson and Brennan4), donor unrelated pregraft blood transfusions (BT) have also been shown to either enhance kidney allograft survival or to reduce the incidence of acute rejection in humans.5-7Results obtained in experimental animal models suggest that the effects of BT may be related to either fortuitous exposure to graft donor-specific antigens or to T-cell cross-reactivity,2,8suggesting that the immunomodulatory effect of DST and random BT may share common mechanisms. At present, these mechanisms remain poorly understood; however, studies in rodents have provided important information. Unexpectedly, donor-specific cytotoxic T lymphocytes (CTL) can be found in nonrejected cardiac or renal allografts from DST-treated rats, indicating the absence of clonal deletion of alloreactive CD8+ T cells.9-11 Moreover, the fact that tolerance can be abrogated by the administration of exogenous interleukin-2 (IL-2) or interferon-γ (IFN-γ) after grafting suggests that alloreactive CD4+ T cells are also present, but somehow silenced.11 12
T-helper (Th) cells play a pivotal role in acute allograft rejection, and DST-induced allograft tolerance is likely due to a dramatic downregulation of Th function as shown in rodent models.11-13 Possible mechanisms underlying this defect are not clear: anergy has been proposed,12 as has an immune deviation towards a Th2-type reaction.14 However, we have recently shown in a rat model that both Th1- and Th2-related cytokines were downregulated in heart allografts from DST-treated animals, as compared with untreated recipients.13
It is still unclear whether the DST by itself is tolerogenic. Although the presence of donor-specific suppressor T cells in DST-treated rats has been reported in vitro,15 such cells could be detected in our model in DST-treated animals only after the transplantation.16 Moreover, results obtained in human have shown that fully major histocompatibility complex (MHC)-incompatible BT resulted in an increase in both donor-specific T-helper and CTL precursors,17 18 indicating that allogenic BTs sensitize rather than tolerize. Based on these results, we sought to define which kind of stimulus within the allograft is responsible for turning off the T-helper response in DST-treated recipients. We report that graft passenger leukocytes, known to be highly immunogenic, are necessary for tolerance induction in DST-treated rats. This effect can be substituted by purified donor dendritic cells (DCs), indicating that these antigen-presenting cells can govern the cellular events leading to both rejection and tolerance, depending on the context in which they are involved.
MATERIALS AND METHODS
Animals and transplantation.
Eight- to 12-week-old male Lewis.1W (RT1.u), Lewis.1A (RT1.a), and Lewis (RT1.l) rats were obtained from the Zentral Institut Für Versuchstierzucht (Hannover, Germany) or kindly provided by E. Günther (Georg-August Universität Göttingen, Göttingen, Germany). Heterotopic heart grafts were performed using the Ono and Lindsey technique,19 and function was monitored daily by palpation through the abdominal wall. Some DST-treated recipients received a heart allograft that was parked for 2 months in another tolerant DST-treated recipient. The grafts were harvested from the tolerant recipient’s abdominal location and regrafted using the Ono and Lindsey technique19 into fresh DST-treated recipients. Skin grafts were performed using standard procedures.20
Donor and recipient pretreatments.
Allograft recipients received either no pretreatment or DST. Blood (1 mL), collected from a Lewis.1W donor by cardiac puncture into a syringe containing heparin (final concentration, 20 U/mL), was immediately injected intravenously (IV) into Lewis.1A recipients 14 and 7 days before the transplantation. This regimen induces long-term allograft tolerance (see Fig 7 for allograft survivals), which is donor MHC specific, because third-party blood transfusions do not prolong graft survival3 and because long-term tolerant recipients accept donor-type, but not third-party MHC expressing skin grafts (Fig 1). Where indicated, donor rats received, 5 days before graft harvesting, a single intraperitoneal dose (300 mg/kg) of cyclophosphamide (Sigma, St Louis, MO) to deplete heart graft leukocytes.21
DST-induced allograft tolerance is donor-specific. Long-term (>100 days) tolerant heart allograft recipients were challenged with donor-type (Lewis.1W) and third-party-type (Lewis. RT1.l) and recipient-MHC type-expressing skin grafts. Note the rejection of third-party but not of donor-type skin graft.
DST-induced allograft tolerance is donor-specific. Long-term (>100 days) tolerant heart allograft recipients were challenged with donor-type (Lewis.1W) and third-party-type (Lewis. RT1.l) and recipient-MHC type-expressing skin grafts. Note the rejection of third-party but not of donor-type skin graft.
Preparation of DCs.
Highly enriched (75% to 85%) suspensions of mature spleen DCs were prepared as we previously described.22 Bone marrow-derived DCs (>85% pure as assessed by class II staining and morphology) were prepared as described by Talmor et al.23 Briefly, femoral bone marrow cells were cultured in 24-well plates in complete medium containing mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; 1/1,000 dilution of surpernatant from murine GM-CSF–transfected COS cells) and rat IL-4 (1/1,000 dilution of supernatant from rat IL-4–transfected COS cells). Cells were fed on days 2, 4, 6, and 8 and transferred to 100-mm culture dishes on day 9 to induce maturation and were used on day 11. After three washes, cells were resuspended in RPMI at 5 × 105/mL and kept on ice until injected.
Proliferation assays.
A standard one-way mixed lymphocyte culture (MLC) was performed. Spleen and LN cell suspensions were prepared as previously described24 from DST-treated but ungrafted, as well as from grafted DST-treated or untreated rats 5 days after the transplantation. In some experiments, CD4+ and CD8+ cells were depleted from the responding cell population using anti-CD4 or anti-CD8 monoclonal antibodies (MoAbs) followed by antimouse IgG-conjugated Dynabeads (Dynal, Oslo, Norway) as previously described.22Irradiated donor-type Lewis.1W (RT1.u), third-party Lewis (RT1.l), or Buffalo (RT1.b) splenocytes served as stimulator cells. Responder and stimulator cells (2 × 105 cells/well) were plated in 96-well round-bottom plates in triplicate in a final volume of 200 μL of complete medium and cultured at 37°C in 5% CO2. Proliferation of the responder population was assessed from day 1 to 7 by measuring the incorporation of [3H]TdR (0.5 μCi per well; Amersham, Les Ulis, France) during the last 8 hours of culture. Cells were then harvested on glass fiber filters and [3H]TdR incorporation was measured by standard scintillation procedures (Packard Instruments, Meriden, CT).
Immunohistology.
Heart tissue samples removed from untreated or cyclophosphamide-treated LEW.1W donors were embedded in Tissue Tek (OCT Compound; Bayer Diagnostics, Puteau, France), snap-frozen in liquid nitrogen, and stored at −70°C. Five-micrometer cryostat sections were cut, air-dried, and fixed in acetone for 10 minutes at room temperature. Sections were then labeled using a three-step indirect immunoperoxidase technique using either OX1+OX30 (CD45, all leukocytes) or OX6 (MHC class II antigen) MoAbs. Nonspecific staining was controlled for by omission of the first antibody. The number of positively stained cells was counted on each slide using a grid mounted in the ocular eyepiece.
Statistical analysis.
Graft survivals were compared using the Kaplan Meier method.
RESULTS AND DISCUSSION
DST does not tolerize T-helper cells in the absence of an allograft.
Previous studies have shown that DST, with or without a subsequent allograft in adult rat, did not tolerize CTL but instead prime them to donor alloantigens.25 To analyze CD4+ T-cell function in DST-treated rats, we performed MLCs with spleen and lymph node (LN) responder cells from transfused animals killed 14 days after the first DST (the time at which the heart transplantation is normally performed in this model). After mixing with stimulator cells, Lewis.1W (RT1.u), or third-party Buffalo (RT1.b) irradiated splenocytes, proliferative responses were assessed daily from day 1 to 7 of culture. As shown in Fig 1, both spleen and LN cells mounted a potent proliferative response against both donor and third-party alloantigens. The kinetic and level of proliferation of LN cells against donor and third party stimulators were not significantly modified by DST (Fig 2). In contrast, splenocyte proliferation appeared greater for DST-treated than for naive animals from day 3 to 7 of culture (Fig 2).
DST does not tolerize Th cells to donor alloantigen. Spleen and LN cells were prepared from untreated naive or DST-treated (1 mL on days −14 and −7) rats 14 days after the first transfusion. Cells (2 × 105) were plated in triplicate with an equal number of donor-specific Lew.1W (RT1.u) (A) or third-party Buffalo (RT1.b) (B) irradiated spleen cells in 96-well round-bottom plates and cultured for 1 to 7 days. Thymidine incorporation during the last 8 hours of each of these cultures was assessed. Representative data of three independent experiments are shown.
DST does not tolerize Th cells to donor alloantigen. Spleen and LN cells were prepared from untreated naive or DST-treated (1 mL on days −14 and −7) rats 14 days after the first transfusion. Cells (2 × 105) were plated in triplicate with an equal number of donor-specific Lew.1W (RT1.u) (A) or third-party Buffalo (RT1.b) (B) irradiated spleen cells in 96-well round-bottom plates and cultured for 1 to 7 days. Thymidine incorporation during the last 8 hours of each of these cultures was assessed. Representative data of three independent experiments are shown.
Some investigators have proposed that DST and random BT effects are mediated by an anergy of donor-reactive T cells after encountering alloantigens on resting donor B cells.26,27 However, our results and those of others suggest that this may not always be the case. Indeed, allogenic DST primes the recipient CTLs against donor alloantigens,10,25 and the data presented here suggest that donor-reactive Th cells are not anergized by DST (Fig 2). In humans, MHC-mismatched BT resulted in an increase of donor-specific T-helper cell precursors. Moreover, it has been shown that resting B cells can induce tolerance to minor histocompatibility antigen, but not to MHC antigen-mismatched allografts.28
Both DST and heart allograft are required to tolerize splenic Th cells.
We previously showed that expression of both Th-1– and Th-2–type cytokine mRNA was strongly diminished in heart grafts from DST-treated rats as compared with allografts in naive recipients.13 This suggests that Th function is depressed. To further assess donor-reactive Th function in these animals, we performed MLC with spleen and LN cells from untreated and DST-treated allograft recipients killed 5 days after transplantation. The results of the antidonor proliferative response are described in Fig 3. Both spleen and LN cells from untreated animals exhibited a strong proliferative response against irradiated donor-type spleen cells. Depleting CD4+ but not CD8+ cells from the responding population abolished this response indicating that, as previously described,29proliferation in MLC is mediated by T-helper cell ( Fig4). In DST-treated animals, LN cells proliferated in the presence of donor antigen, whereas the response of spleen cells was dramatically reduced. Although these cells proliferated normally in response to Concanavalin A (Fig 5), their response against third party stimulator cells from Buffalo (RT1.b) (Fig 5) or Lewis (RT1.l) (data not shown) rats were also reduced, indicating a nonspecific in vitro suppression. However, it is clear that in vivo the effect of DST is donor-specific, because third-party pregraft BT did not enhance Lewis.1W heart graft survival in Lewis.1A recipients3 and because long-term tolerant animals accept donor-type but not third-party-type skin grafts (Fig 1). It was important to exclude that this difference between pregraft and postgraft profiles could reflect kinetic variations in the pattern of the proliferative response in DST-treated rats. To answer this question, we performed the same experiments as described in the Fig 2with DST-treated ungrafted animals killed 12 days after the second transfusion (equivalent to day +5 after transplantation) or with DST-treated animals that received on day 0 a third DST instead of an allograft. As expected, in both cases the proliferative response was similar to that described in Fig 2 (data not shown). Finally, these results led us to conclude that both DST and allograft are required to tolerize splenic alloreactive T-helper cells. These results are consistent with our previous preliminary report showing that DST and heart allograft, but not DST alone, induce suppressor T cells in recipient spleen.16
Both DST and heart allograft are required to tolerize recipient splenic Th cells. Spleen and LN cells were prepared from DST-treated and untreated heart graft recipient rats killed 5 days after the transplantation. Proliferation against irradiated spleen from LEW.1W (RT1.u) or third-party Buffalo (RT1.b) and Lewis (RT1.l) (data not shown) was assessed every day as described in Fig 1. Representative data of five independent experiments are shown.
Both DST and heart allograft are required to tolerize recipient splenic Th cells. Spleen and LN cells were prepared from DST-treated and untreated heart graft recipient rats killed 5 days after the transplantation. Proliferation against irradiated spleen from LEW.1W (RT1.u) or third-party Buffalo (RT1.b) and Lewis (RT1.l) (data not shown) was assessed every day as described in Fig 1. Representative data of five independent experiments are shown.
The proliferative response during MLC is mediated by CD4+ cells. Spleen cells were prepared from DST-treated and untreated heart graft recipients sacrificed 5 days after the transplantation. Total, CD4-depleted, or CD8-depleted (Dynal) cells (2 × 105) were plated in triplicate with an equal number of Lew.1W (RT1.u) irradiated spleen cells in 96-well round-bottom plates and cultured for 5 days. Thymidine incorporation was assessed during the last 8 hours. Representative data of three independent experiments are shown.
The proliferative response during MLC is mediated by CD4+ cells. Spleen cells were prepared from DST-treated and untreated heart graft recipients sacrificed 5 days after the transplantation. Total, CD4-depleted, or CD8-depleted (Dynal) cells (2 × 105) were plated in triplicate with an equal number of Lew.1W (RT1.u) irradiated spleen cells in 96-well round-bottom plates and cultured for 5 days. Thymidine incorporation was assessed during the last 8 hours. Representative data of three independent experiments are shown.
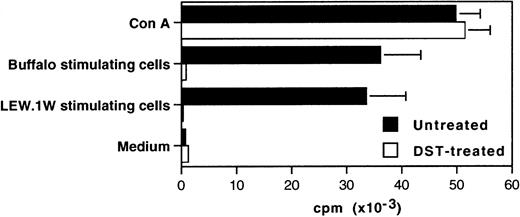
The suppression of the proliferative response against alloantigens in spleen cells from DST-treated and grafted animals is not donor-specific. Spleen cells were prepared from DST-treated and untreated heart graft recipients killed 5 days after the transplantation. Cells (2 × 105) were plated in triplicate alone with an equal number of Lew.1W (RT1.u) or third-party Buffalo (RT1.b) irradiated spleen cells or in the presence of 5 μg/mL of Concanavalin A (Con A) in 96-well round-bottom plates and cultured for 5 days. Thymidine incorporation was assessed during the last 8 hours. Representative data of five independent experiments are shown.
The suppression of the proliferative response against alloantigens in spleen cells from DST-treated and grafted animals is not donor-specific. Spleen cells were prepared from DST-treated and untreated heart graft recipients killed 5 days after the transplantation. Cells (2 × 105) were plated in triplicate alone with an equal number of Lew.1W (RT1.u) or third-party Buffalo (RT1.b) irradiated spleen cells or in the presence of 5 μg/mL of Concanavalin A (Con A) in 96-well round-bottom plates and cultured for 5 days. Thymidine incorporation was assessed during the last 8 hours. Representative data of five independent experiments are shown.
The disappearance of the proliferative response in splenocytes from DST-treated rats after the transplantation is unlikely to be due to trapping of alloreactive CD4+ T cells in heart graft, because this response can be restored in vitro with a combination of IL-2 (50 U/mL) and anti–transforming growth factor-β (TGF-β) MoAb.29a Different T-cell response patterns between spleen and LN compartments have been previously shown in other models25,30 and could be related to differential migratory properties of naive and activated T cells. Also, this discrepancy might be due to differential access of alloantigen in these two compartments. Indeed, it has been shown that, after IV infusion, allogenic leukocytes are almost exclusively eliminated in spleen in normal euthymic rats,31 and, moreover, after vascularized allografts in rodents, graft interstitial DCs rapidly migrate into recipient spleen and then associate with CD4+ T cells.32 Thus, it is possible that alloantigens do not gain access to LN compartments after BT and allograftment, and therefore LN T cells in vivo might be naive for these antigens. A role for spleen as a critical site for the induction of tolerance by DST is further supported by previous studies showing that splenectomy abrogates the induction of unresponsiveness after IV alloantigen injection.33 34
Graft passenger leukocytes are required for induction of tolerance in DST-treated rats.
Having demonstrated the requirement for the heart allograft to induce unresponsiveness of splenocytes from DST-treated rats to donor alloantigens, we then sought to determine which cell type within the graft could be responsible for turning off the Th response. It is well known that organ allografts contain two distinct cell subsets controlling tissue immunogenicity. Resident parenchymatous cells of the heart themselves are thought to be antigenic but poorly immunogenic, mainly because of their lack of expression of costimulatory molecules. In contrast, bone marrow-derived passenger leukocytes and more precisely interstitial DCs are potent stimulators of allogenic responses both in vitro and in vivo and are suggested to account for the majority of the allograft immunogenicity.35 Indeed, depletion of passenger leukocytes from allografts before transplantation has been shown to enhance allograft survival in some rodent combinations.21,36-38 Thus, to differentiate the role of passenger leukocytes and heart graft tissue in the tolerizing effect of the graft in DST-treated rats, we pretreated heart donors with a single dose of cyclophosphamide (300 mg/kg) 5 days before organ harvesting. As shown in Fig 6, this treatment led to a greater than 95% reduction in the number of interstitial CD45 and class II-positive cells in the donor hearts at the time of transplantation. Previous studies have shown that these cyclophosphamide-sensitive cells expressing class II molecules are exclusively interstitial DCs.39,40 Interstitial DC-depleted hearts were acutely rejected upon grafting into DST-treated recipients, with a mean survival time (MST) ± SD of 11.7 ± 4.3 days (not significant [NS] as compared with untreated recipients of normal heart grafts, MST = 11.9± 3.4 days; Fig 7). Moreover, depletion of passenger leukocytes did not significantly prolong allograft survival in untreated recipients (8.4 ± 1.5 days). This result suggest that the mechanism of acute rejection occurring in these animals mostly depends on the presentation of graft derived alloantigen by host antigen-presenting cells (APC; indirect pathway of allorecognition) instead of alloantigen recognition on donor APC (direct pathway). Heart grafts from cyclophosphamide-treated donors into syngenic recipients survived indefinitely (n = 5), indicating that abrogation of graft’s ability to induce tolerance in DST-treated recipients was not simply due to myocardial toxicity of cyclophosphamide. Finally, 3 DST-treated recipients received heart allografts that were parked in tolerant DST-treated recipients for 2 months, a period by which donor interstitial DCs are replaced by recipient interstitial DCs.41 Rejection occurred in these animals in 10, 12, and 22 days. Therefore, we conclude that graft interstitial DCs are required for the induction of tolerance in DST-treated rats.
The effect of pretreatment of the donor with cyclophosphamide on leukocyte content in heart. Cryostat section of heart tissue samples from untreated (▪) or cyclophosphamide-treated (□) donor rats were labeled with OX1+OX30 (CD45) and OX6 (class II MHC) MoAbs using a three-step immunoperoxidase technique. Positive cells were counted using a grid in ocular eyepiece and each bar represents the mean ± SD of 4 animals in each group.
The effect of pretreatment of the donor with cyclophosphamide on leukocyte content in heart. Cryostat section of heart tissue samples from untreated (▪) or cyclophosphamide-treated (□) donor rats were labeled with OX1+OX30 (CD45) and OX6 (class II MHC) MoAbs using a three-step immunoperoxidase technique. Positive cells were counted using a grid in ocular eyepiece and each bar represents the mean ± SD of 4 animals in each group.
Allograft survivals. Donors were pretreated with a single intraperitoneal injection of cyclophosphamide (cyp) at 300 mg/kg on day −5. Recipients were pretreated with two DSTs (1 mL of fresh blood) on days −14 and −7. Recipients were posttreated where indicated by IV injection of 2.5 × 105 donor or third-party splenic or bone marrow-derived DCs in 100 μL RPMI at the end of the surgical procedure.
Allograft survivals. Donors were pretreated with a single intraperitoneal injection of cyclophosphamide (cyp) at 300 mg/kg on day −5. Recipients were pretreated with two DSTs (1 mL of fresh blood) on days −14 and −7. Recipients were posttreated where indicated by IV injection of 2.5 × 105 donor or third-party splenic or bone marrow-derived DCs in 100 μL RPMI at the end of the surgical procedure.
In a previous study, Heineman et al,42 using rats from a different genetic background than the one used in this study, showed that donor irradiation before graft harvesting also abrogated the induction of allograft tolerance in a DST-treated recipient, indicating that this effect is not restricted to particular strains. Although cyclophosphamide could theoretically interfere with activity of DST-induced regulatory cells, it is unlikely that sufficient amount of this drug could be carried over into the recipient with the graft, because hearts were extensively washed before engraftment and because the heart does not efficiently accumulate cyclophosphamide.43
The injection of donor-type, but not third-party, DCs can restore tolerance to a passenger leukocyte-depleted heart allograft in a majority of DST-treated animals.
To further assess the role of donor interstitial DCs in inducing tolerance in DST-treated rats, we injected DST-treated recipients of an interstitial DCs-depleted heart allograft with donor and third-party (Lewis, RT1.l) DCs at the time of transplantation. Because it is difficult to obtain a sufficient number of donor DCs from heart, they were prepared from spleen or bone marrow. The results in Fig 7 show that the IV injection of 2.5 × 105 (the average number of interstitial DCs in a rat heart; see McKenzie et al21) donor spleen or bone marrow-derived DCs restored indefinite allograft survival in 56% (5/9) and 63% (5/8), respectively, of DST-treated recipients of interstitial DCs-depleted heart graft (P < .001 as compared with noninjected recipients). In contrast, the injection of a similar number of third-party (RT1.l) DCs did not significantly prolong allograft survival (Fig 7). Increasing the number of DC injected to 1 × 106 cell per recipient did not further increase the percentage of surviving allografts.
Previous studies in rats have shown that interstitial DCs play a critical role in inducing acute allograft rejection by directly stimulating recipient CD4+ and CD8+ T cells (direct pathway of allorecognition).35,41,44 We showed here that the direct pathway of allorecognition could also elicit tolerance in CD4+ T cells from DST-treated rats. Because, in the absence of graft interstitial DCs, the hearts are rejected in these animals, this rejection is probably due to indirect recognition of donor alloantigens. Interestingly, interstitial DCs have been shown to disappear more rapidly from allografts in DST-treated than in untreated recipients.9 However, it is not known whether this results from in situ cell death or migration. Based on the present results, we hypothesize that heart interstitial DCs rapidly migrate to the spleen of a DST-treated host. This migration would result in an inactivation rather than a stimulation of recipient splenic CD4+ T cells. The absence of suppression in the lymph nodes compared with spleen could be explained by the inability of DCs to access to the lymph nodes from the blood.45 We recently showed that the bystander suppression of allogenic proliferative response observed in spleen from DST-treated and grafted rats is related to TGF-β.29a Moreover, TGF-β1 was overexpressed in nonrejected allografts from DST-treated recipient, and allograft tolerance was abrogated by anti–TGF-β MoAb treatment. This strongly suggests that induction of allograft tolerance in this model is dependent on TGF-β–producing cells. It is possible that donor Ags present on graft interstitial DCs are required for activation of, and TGF-β production by, these putative regulatory cells.
Hart and Fabre46 have showed that the presence of interstitial DCs in kidney graft was also required for tolerance to be induced in passively enhanced recipient rats. They suggested that the mechanism of induction of passive enhancement was related to an interaction of enhancing antibodies with donor interstitial DCs.46 We have previously reported, in our model, that a high level of anti-class II IgG2a could be detected at the time of transplantation in the DST-treated rat.47 It is likely that these antibodies interact with graft interstitial DCs during the first 2 to 3 days after grafting, because they are the only cells expressing class II antigen in normal rat heart.40However, the fact that interstitial DC-depleted allografts were rejected in unmodified recipients indicates that anti-class II antibodies do not simply act through opsonisation and destruction of interstitial DCs. Nevertheless, anti-class II Ab could modify interstitial DCs function and mask class II molecules that would prevent CD4+ T-cell activation. On the other hand, rapid opsonisation of interstitial DCs could favor their processing by recipient spleen APC and then would allow indirect alloantigen presentation to T cells in a tolerogenic fashion.
In addition to their major role in initiating primary T-cell response both in vitro and in vivo,48 DCs could also exert an inhibitory action on the immune response. The strongest support for this theory is evidenced by the critical role of DCs in the negative selection process occurring in thymus. In the periphery, DCs may also be involved in maintaining tolerance to peripheral tissue antigen.49 Süss and Shortman50 have shown that a subset of CD8+ splenic DCs in mice have the capacity to delete CD4+ T cells that interact with them, by FasL/Fas-mediated apoptosis, suggesting that this DC subset could have a regulatory function. In addition, we recently showed that rat spleen DCs exhibit a Ca2+-dependent NK-like cytotoxic function.22 Bone marrow-derived DC progenitors in mouse have been shown to induce T-cell hyporeponsiveness in vitro and to prolong heart allograft survival when injected 7 days before grafting.51 Finally, donor DCs are thought to play an important role in establishing spontaneous tolerance to liver allografts in rodent.51 Together with our present study, these data suggest that, in addition to their role in triggering allograft rejection, DCs might also be involved in allograft tolerance under certain circumstances.
In conclusion, we have shown that the presence of interstitial DCs is required for tolerance to be induced in heart allograft recipient rats pretreated by DST. Thus, direct recognition of alloantigen does not always evoke acute allograft rejection but could also, in DST-primed recipients, tolerize T-helper cells. Our results suggest that the pregraft immunologic status of allograft recipients and, in particular, whether they have received pregraft BTs should be carefully considered in studies analyzing the effect of organ DC depletion on allograft survival in humans.
ACKNOWLEDGMENT
The authors thank Dr Ralph Steinman and Brian Wong for critically reading the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Régis Josien, MD, PhD, INSERM UNIT 437, Immeuble Jean Monet, 30, bd Jean Monet, 44035 Nantes Cédex 01, France; e-mail: rjosien@nantes.inserm.fr.