Abstract
The expression of different isoforms of nitric oxide synthase (NOS) was investigated in B-cell chronic lymphocytic leukemia (B-CLL) to delineate a possible role for nitric oxide (NO) in the control of apoptosis of the tumoral cells. By reverse transcription-polymerase chain reaction (RT-PCR), all B-CLL cells were found to express spontaneously inducible NOS (iNOS) mRNA, whereas endothelial constitutive NOS (ecNOS) mRNA was undetectable. The iNOS protein was detected by immunofluorescence in the cytoplasm of permeabilized leukemic cells and identified by Western blotting, using different anti-iNOS antibodies, as a protein of 135 kD in B-CLL cytoplasmic extracts. B-CLL cell lysates also displayed basal NOS enzymatic activity, as measured by the conversion of14C-labeled L-arginine into 14C-L-citrulline. Ligation of CD23, expressed on the vast majority of B-CLL cells, resulted in increased iNOS expression and activity. The NO released exerted an anti-apoptotic effect on B-CLL cells that was counteracted by NOS inhibitors and engagement of the APO-1/Fas pathway. Therefore, the existence of a functional iNOS in B-CLL cells will provide further insights into the mechanisms that control proliferation and apoptosis in these tumor cells.
© 1998 by The American Society of Hematology.
B-CELL CHRONIC lymphocytic leukemia (B-CLL) is characterized by a progressive accumulation of monoclonal CD5+ B cells arrested in the G0/G1phase of the cell cycle1,2 and which overexpress the CD23 (FcεR2) antigen.3 Most B-CLL B cells in the circulation are nondividing or slowly dividing and their accumulation has been suggested to result from a decreased rate of cell mortality rather than increased proliferation. However, when B-CLL cells are cultured in vitro, they die rapidly as a consequence of apoptosis in contrast to their prolonged life span in vivo, indicating a lack of essential growth factors in the culture medium. Indeed, the rate of B-CLL cell death in vitro can be modulated by different cytokines, some accelerating the apoptotic process, others slowing or counteracting the effects of the previous one.4 For instance, interleukin-4 (IL-4), which affects only weakly the replication of B-CLL cells, exerts an anti-apoptotic effect.5 Similarly, IL-13, which displays only a subset of IL-4–like activities on B-CLL cells,6 inhibits IL-2–induced proliferation and protects them from apoptosis.7 The spontaneous apoptosis of B-CLL cells is also reversed by interferon-α (IFN-α)8 and IFN-γ,9,10 or by soluble factors released by coculture with bone marrow (BM) stromal cells.11 Other growth factors and cytokines, such as granulocyte colony-stimulating factor12,13 and soluble CD23,14 protect B-CLL cell populations from programmed cell death, either spontaneous or elicited by apoptotic reagents. The effect of IL-10 is controversial because one group reports that it inhibits B-CLL cell proliferation and enhances their differentiation but does not induce apoptosis,15 whereas another group has observed an anti-apoptotic action of IL-10 on B-CLL cells.16
The capacity to stimulate the expression of Bcl-2 or to slow its decrease in B-CLL cells is correlated with the anti-apoptotic effect of IL-4,17-20 IFN-α,21,22 or IFN-γ.19 The basic fibroblast growth factor (bFGF), which delays fludarabine-induced apoptosis, also upregulates the expression of Bcl-2 in B-CLL cells.23 However, Bcl-2 does not seem to be involved in the increased apoptosis of B-CLL cells induced by IL-5, or in its reversal by IL-4.24
Therefore, it has been proposed that a defective apoptosis, caused by Bcl-2 overexpression, may explain why B-CLL cells accumulate in G0.25-27 Although the Bcl-2 gene was first shown to be dysregulated in lymphomas associated with a t(14;18) chromosomal translocation, its overexpression in most B-CLL cells is not associated with a gene rearrangement. The anti-apoptotic activity of Bcl-2 is antagonized by the homologous protein Bax, which accelerates the rate of cell death, and an increased Bcl-2/Bax ratio was observed in B-CLL, particularly in patients unresponsive to chemotherapy.28 The enhanced apoptosis triggered by anti-IgM in B-CLL and its reversal by CD6 ligation are also correlated with modulations in the Bcl-2/Bax ratio.29 Gottardi et al30 have studied the expression of the various members of the Bcl-2 family (Bcl-2, Bcl-xL, Bcl-xS, and Bax) in leukemic CD5+ B cells and found that the pattern of expression of these genes was skewed toward prevention of apoptosis, favoring the accumulation of the leukemic cells.
Nitric oxide (NO) plays a role in the control of apoptosis in various cell types, including tumoral cells. The effect of NO on the apoptotic pathway is complex, probably due to the “double-edged sword” aspect of its action, depending on its concentration and on the redox potential of the neighboring cells where it is released.31NO, described as a potent immunoregulator,32,33 is synthesized from L-arginine by NO synthases (NOS).34Recently, Epstein-Barr virus (EBV)-transformed human B lymphocytes and Burkitt's lymphoma B cells have been reported to express low levels of inducible nitric oxide synthase (iNOS) and the NO produced appeared to inhibit apoptosis and EBV virus reactivation.35 In addition, endothelial constitutive NOS (ecNOS) mRNA and NOS protein have been reported in subpopulations of human normal B lymphocytes.36
These data prompted us to study whether these NOS isoforms were present and functional in B-CLL cells. Inasmuch as most B-CLL cells display membrane CD23,37 and as we have shown previously that CD23 ligation results in iNOS activation in human monocytes,38we decided also to investigate the effects of CD23 engagement on NOS expression by B-CLL cells.
Our present work indicates that whereas ecNOS is undetectable, B-CLL cells spontaneously express a functional iNOS that is upregulated after ligation of membrane CD23 and which has an anti-apoptotic role.
MATERIALS AND METHODS
Patients
Thirty-two patients (13 women and 19 men) with B-CLL were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. Samples were obtained either as frozen peripheral blood mononuclear cells (PBMC) (23 cases) or as aliquots of freshly collected blood (9 cases). No patient had received therapy for 1 month before the test.
Cell Isolation
PBMC were prepared by diluting the blood samples in RPMI 1640 medium (GIBCO, Grand Island, NY) containing 20 U/mL heparin and obtained after a 30-minute centrifugation at 400g on Ficoll Hypaque gradient (Pharmacia, Uppsala, Sweden). The adherent cell population was removed by incubation of PBMC (107 cells/mL) in plastic Petri dishes (Nunc, Roskilde, Denmark) for 90 minutes at 37°C in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS; GIBCO). T lymphocytes were removed by one or two consecutive cycles of rosetting of the nonadherent cells with 2-aminoethyl isothiouronium bromide–treated sheep erythrocytes, followed by centrifugation at 400g for 30 minutes over Ficoll-Paque. The nonrosetting leukemic B cells were collected. This population was found to consist of 95% to 100% B cells, as assessed by immunofluorescence with an anti-CD20 monoclonal antibody (MoAb)-fluorescein isothiocyanate (FITC) (Immunotech/Coulter, Marseille Luminy, France).
Purified B-CLL cells were analyzed for their expression of NOS mRNA and NOS protein either immediately or after overnight incubation. In some instances, B-CLL cells were further cultured at 106cells/mL in plastic Petri dishes in RPMI 1640 medium supplemented with 10% FCS and incubated at 37°C for different times in the presence or absence of anti-CD23 MoAb (135 or MHM-6) or mouse IgG1as isotypic control, then analyzed again for the expression of NOS mRNA and NOS protein.
As controls, B and T lymphocytes were purified from the peripheral blood of normal donors by Ficoll-Hypaque centrifugation, followed by negative selection on magnetic beads coated with anti-CD14 and anti-CD3 MoAbs (B lymphocytes), or anti-CD14 and anti-CD19 MoAbs (T lymphocytes) (Immunotech/Coulter). The purity of the populations was checked by immunofluorescence using anti-CD20 and anti-CD2 MoAbs, respectively, and was found to be ≥95% for both cell types.
Reagents
The two NOS inhibitors NG-monomethyl-L-arginine (L-NMMA) and nitro L-arginine methyl ester (L-NAME) were purchased from Sigma (St Louis, MO). The characterization of the different MoAbs directed against the CD23 antigen (135 and MHM-6, both of IgG1isotype) has been reported previously39; the MOPC-21 MoAb (Sigma) is an isotype-matched (IgG1) control antibody. The phycoerythrin-conjugated anti-CD23 MoAb was from Becton Dickinson (Grenoble, France) and the anti-APO-1/Fas MoAb from Immunotech for the clone CH-11 (IgM) and from Biomol (Plymouth Meeting, PA) for the clone BG27 (IgG2a). 14C-labeled L-arginine was from Amersham (Amersham, UK). Isotype-matched control or fluorescent-labeled MoAbs were from Becton Dickinson. The strong acid cation exchange resin, binding to L-arginine but not to L-citrulline (AG 50W-X8, 100-200 mesh, H form) was from Biorad Laboratories (Richmond, CA). All other reagents and chemicals were from Sigma.
Determination of Nitrite Concentrations
To assess the amount of NO produced, the culture supernatants were assayed for the stable end product of NO oxidation, nitrite, using the Griess reagent as previously described.40 Supernatants were tested as such or after treatment with nitrate reductase to reduce nitrate to nitrite. Briefly, 100 μL of the various supernatants was dispensed in 96-well microplates, followed by the addition of 100 μL of a reactive solution containing 1% sulfanilamide in 30% CH3COOH and with 0.1% N-1-naphthyl ethylenediamine dihydrochloride in 60% CH3COOH (1 vol/1 vol). The standard calibration curve was constructed using sodium nitrite diluted in complete medium. Optical densities (OD) were measured at 540 nm using an ELISA plate reader (Titertek Multiskan; Flow Laboratories, Eflab Oy, Helsinki, Finland).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for the Detection of iNOS and ecNOS mRNA in B-CLL Cells
RNA preparation.
The expression of iNOS and ecNOS mRNA was investigated by RT-PCR, according to previously described techniques.41 Briefly, total RNAs were prepared from 1 to 10 × 106 cells by a modified single-step guanidinium isothiocyanate and phenol/chloroform extraction method,42 using the Trizol reagent (GIBCO) according to the specifications of the manufacturer. The RNA pellets were then washed with 75% ethanol, vacuum-dried, and resuspended in diethylpyrocarbonate-treated water.
cDNA synthesis.
Aliquots of 1 to 2 μg from each RNA sample were mixed with 250 μmol/L of each deoxynucleotide triphosphate (dNTP; Pharmacia), 1 μg random hexamer (PdN6; Boehringer Mannheim, Meylan, France) in a 67-mmol/L Tris-HCl pH 8.8 buffer containing 6.7 mmol/L MgCl2, 16.6 mmol/L (NH4)2SO4, in a final volume of 20 μL. The mixture was incubated at 65°C for 5 minutes. After cooling, 200 U of Moloney murine leukemia virus reverse transcriptase (MMLV-RT: GIBCO-BRL, Cergy-Pontoise, France) and 125 U of human placenta ribonuclease inhibitor (HPRI; Amersham France, Les Ulis) were added for a 30-minute incubation at 42°C. The RT was then heat-inactivated at 65°C for 5 minutes, and the 20-μL cDNA synthesis reaction mixtures were diluted to 500 μL in RNAse-free water for use in PCR amplification.
PCR.
Ten microliters of diluted RT product (cDNA) were dispensed in PCR tubes, then 90 μL of a mixture containing 250 μM of each deoxynucleotide triphosphate, dNTP (Pharmacia), 50 μmol/L of each specific upstream and downstream oligonucleotide primer, reaction buffer consisting of 67 mmol/L Tris-HCl pH 8.8 containing varying concentrations of MgCl2 (1.7 or 2.7 mmol/L), 16.6 mmol/L (NH4)2SO4, and finally 1.25 U of Taq polymerase (Boehringer). Tubes were overloaded with 50 μL of mineral oil (Sigma) and PCR was performed in a thermal cycler (GenAmp 9600; Perkin Elmer, Norwalk, CT). After an initial denaturation step at 94°C for 30 seconds, a step cycle program was set as follows for iNOS and β2-microglobulin: denaturation at 94°C for 50 seconds, annealing at 60°C for 50 seconds, extension at 72°C for 50 seconds, for 35 cycles, followed by a final extension at 72°C for 10 minutes. The same conditions were used for endothelial cNOS PCR, except that 37 cycles were performed. PCR products were visualized on a 2% agarose gel stained with ethidium bromide. PCR products were also run on a 7% acrylamide gel, scanned and analyzed using the “Image” software (NIH, Bethesda, MD). The following intron-spanning oligonucleotides, based on the sequences of the corresponding human genes, were used:
iNOS, first couple of primers: forward: 5′ CGG TGC TGT ATT TCC TTA CGA GGC GAA GAA GG 3′ and reverse: 5′ GGT GCT GCT TGT TAG GAG GTC AAG TAA AGG GC 3′. The iNOS message is represented by a 259-bp band. Alternatively, a second couple of primers was used: forward: 5′ ATG CCA GAT GGC AGC ATC AGA 3′, and reverse 5′ ACT TCC TCC AGG ATG TTG TA 3′. The latter set of primers, which defines a fragment of 371 bp, has been used previously to demonstrate the presence of iNOS mRNA in monocytes stimulated through CD23 ligation43 and the sequence of the amplified fragment was found to be identical to that expected from the sequence of the human iNOS gene.44
ecNOS: forward: 5′ GCA TCA CCT ATG ACA CCC TCA GCG 3′ and reverse: 5′ AGC TCG CTC TCC CTA AGC TGG TAG G 3′. The endothelial cNOS message is represented by a 277-bp band.
β2-microglobulin: forward 5′ CAT CCA GCG TAC TCC AAA GA and reverse: 5′ GAC AAG TCT GAA TGC TCC AC 3′. The β2-microglobulin message is represented by a 165-bp band.
The first set of iNOS specific nucleotides was from Clontech (Palo Alto, CA; human iNOS amplimer set), whereas the second set of iNOS primers, as well as the cNOS and β2-microglobulin primers, were synthesized and purified by Oligo-Express and Genset (Paris, France) or Genosys (Cambridge, UK). The human A549 epithelial cell line45 and U937 promonocytic cell line46were used as positive controls of iNOS and ecNOS expression, respectively.
Detection of iNOS and ecNOS protein by fluorescence-activated cell sorting (FACS) analysis.
The presence of iNOS and ecNOS was analyzed by flow cytometry of permeabilized cells, using the Cytoperm permeabilization and fixation kit (Serotec, Oxford, UK). Briefly, 106 cells in 50 μL of phosphate-buffered saline (PBS) were treated at room temperature for 15 minutes with 100 μL of reagent A. After washing, the cell pellet was resuspended in 100 μL of reagent B and 10 μL of either monoclonal anti-iNOS (macNOS, clone 54; Transduction Laboratories, Lexington, KY), or anti-human endothelial constitutive NOS (anti-ECNOS, clone 3; Transduction Laboratories), or their control isotype (IgG1) from Coulter France (Margency) were added for a 30-minute incubation at room temperature. After new washing, the pellet was resuspended in 50 μL of reagent B and 10 μL of an F(ab′)2 fragment of a goat anti-mouse Ig-DTAF (Immunotech) was added for a 30-minute incubation at room temperature in the dark. Cells were washed and fixed in 2% PBS-bovine serum albumin (PBS-BSA) containing 2% formaldehyde. In some instances, cells were also analyzed for iNOS expression by direct fluorescence, using an FITC-labeled anti-iNOS MoAb (FITC-macNOS, clone 6; Transduction Laboratories) and IgG2a-FITC as control isotype (Becton Dickinson). Cells were analyzed by flow cytometry on a FACScan instrument using the lysis II and Procyt softwares. The two anti-iNOS MoAbs (clones 6 and 54) are directed against a protein fragment corresponding to the amino acids 961-1144 of murine iNOS and recognize the human enzyme.
Immunocytochemistry.
Freshly isolated human B-CLL cells were cytocentrifuged on glass slides and fixed with acetone. The slides were then incubated in a humidified chamber, either with a 1/100 dilution of the mouse anti-macNOS (clone 54) MoAb, or with isotype-matched IgG1 MoAb (Becton Dickinson) as a control. Cells were then incubated with a biotinylated anti-mouse IgG antibody and the labeling was revealed using the Vectastain ABC procedure with avidin coupled to horseradish peroxidase (Vector Laboratories).
Quantification of apoptosis.
Apoptosis experiments were performed selectively with B-CLL cells purified from freshly collected blood samples and only in a few instances with B-CLL cells isolated from frozen PBMC samples but, in the latter cases, dead cells and debris were removed by an additional centrifugation on Ficoll after the separation steps and only populations with a viability ≥95% were used for these studies. Cells undergoing early apoptosis were estimated by FACS detection of phosphatidylserine (PS) expression at the outer leaflet of the plasma membrane using FITC-labeled annexin V (Boehringer Mannheim, Germany), with or without simultaneous labeling with propidium iodide, according to a modification of the technique described by Koopman et al.47 Apoptosis-induced DNA fragmentation was also estimated with the ApoAlert DNA fragmentation detection kit (Clontech, Palo Alto, CA), based on terminal deoxynucleotidyl transferase (TdT)-mediated fluorescein-dUTP nick-end (TUNEL) labeling, according to the manufacturer's specifications.
Determination of NOS enzymatic activity.
[14C] L-arginine conversion into [14C] L-citrulline was evaluated according to a modification of a technique described elsewhere.43 Briefly, 107 cells per experimental point were lysed by 4 cycles of freezing on liquid nitrogen and thawing in a 50 mmol/L Tris-HCl buffer pH 7.5, containing the following protease inhibitors: 1 μg/mL leupeptin, 1 μg/mL pepstatin, 40 μg/mL bestatin, 10 μg/mL chymostatin, 50 μg/mL TLCK (all from Sigma), 1 mmol/L AEBSF (Calbiochem), and 5 mmol/L dithiothreitol (DTT) (Sigma). Cell extracts were then centrifuged at 14,000g for 15 minutes at 4°C to remove debris. Supernatants (crude homogenates) were collected and their protein concentration was determined by the Bradford technique.48Twenty microliters of these samples (in triplicate) were then incubated for 15 minutes at 37°C with 100 μL of a 100 mmol/L HEPES, 1 mmol/L MgCl2, pH 7.5 buffer containing 1 mmol/L CaCl2, 1 μmol/L calmodulin, 300 μmol/L β-NADPH, 50 μmol/L tetrahydrobiopterin (BH4), 4 μmol/L FAD, 4 μmol/L FMN, 1 mmol/L L-citrulline, 20 μmol/L unlabeled L-arginine, and 0.66 μCi/mL of L-[guanido-14C]-arginine (NEN, Dreieich, Germany; specific activity 1.8 GBq/mmol). The reaction was performed with or without 2 mmol/L EGTA, to determine the calcium-dependence of the enzyme. An inhibitor of the NO synthase enzyme (L-NMMA, 1 mmol/L) was also used in each set of conditions to determine the background activity. The reaction was stopped by the addition of 2 mL of a 1 vol/1 vol suspension of AG50W-X8 resin in water (BioRad, Hercules, CA; 100-200 mesh, H form), previously treated with 1N NaOH and extensive washing with deionized water until the effluents were neutral. After vigorous mixing, 4 mL of water was added and, after a new mixing, the supernatants were collected. Aliquots of 500 μL were added to 5 mL of a scintillation cocktail (Optiphase Hisafe 2; Wallac, Milton Keynes, UK) and counted in a scintillation counter (Beckman; LS 5000 CE). As a control, 14C-arginine alone was mixed with the resin and negligible radioactivity was recovered in the supernatant (<0.1%). In contrast, when labeled14C-citrulline (NEN) was added to the resin, it was quantitatively recovered in the effluent fraction.
The enzymatic activity was determined as:
where Δ dpm = dpm (test sample ± EGTA) − dpm (test sample + EGTA+L-NMMA), Total dpm = total radioactivity in dpm,Q = total amount of arginine (unlabeled +14C-labeled) in nanomoles, Vr = volume of the reaction in mL, Vc = counted volume in mL, T = time of reaction in min, Vs = volume of sample in mL, and C = concentration of protein in the cell lysate in milligrams per milliliter.
In some instances, the NOS activity was also measured in intact cells by adding 1 μCi/mL of 14C-labeled L-arginine to cultures of B-CLL cells (2 × 106/mL, 1 mL/well in 24-well culture plate) in complete RPMI-1640 medium supplemented with 5% FCS. B-CLL cells were cultured in medium containing or not various stimuli, and in the presence or absence of 1 mmol/L L-NMMA. Aliquots (0.6 mL) of cell-free culture supernatants were collected after various times of incubation, usually 48 hours, and mixed with 1 mL of AG50W-X8 resin. After addition of 1 mL of water, the tubes were centrifuged for 5 minutes at 2,000 rpm and 500 μL of supernatants was collected to determine the amount of 14C-L-citrulline released. The NOS activity is expressed as picomoles per 106 cells of L-arginine converted into L-citrulline.
Detection of iNOS protein in B-CLL extracts by Western blotting.
Cytosolic extracts from unstimulated or stimulated B-CLL cells were prepared by submitting the leukemic cells to four cycles of freeze-thawing in a homogenization buffer consisting of 50 mmol/L Tris, 1 mmol/L EDTA, pH 7.4, containing a mixture of protease inhibitors (10 μg/mL leupeptin, 100 μg/mL phenylmethyl sulfonyl fluoride (PMSF), 10 μg/mL antipain, 10 μg/mL pepstatin, 100 μg/mL chymotrypsin/trypsin inhibitor, and 10 μg/mL chymostatin), and 5 mmol/L DTT. After adjustment of the concentration, the different samples were submitted to electrophoresis on an 8% acrylamide gel containing 0.1 % sodium dodecyl sulfate (SDS). Rainbow markers (Amersham, Les Ulis, France) were run in parallel to estimate the molecular weights. After electrophoresis, the gel was blotted onto a membrane of nitrocellulose (0.45 μmol/L; Schleicher and Schuel, Dassel, Germany) using a wet transfer technique. The efficiency of the transfer was checked by staining the membrane with Ponceau red. After destaining, the membrane was incubated overnight at 4°C in a blocking buffer of 10 mmol/L Tris, 50 mmol/L NaCl, 2.5 mmol/L EDTA pH 7.5, containing 5% nonfat milk. The membrane was rinsed with a 15 mmol/L Tris, 150 mmol/L NaCl, 0.2% Tween-20 buffer containing 1% BSA, then incubated for 2 hours at room temperature with agitation with a 1/500 dilution of a specific anti-iNOS MoAb (macNOS, IgG1, clone 54; Transduction Laboratories), directed against the carboxy-terminal portion of the murine macrophage iNOS and cross-reacting with human iNOS. After washing, the membrane was incubated for 90 minutes at room temperature with a 1/5,000 dilution of a peroxidase-conjugated rabbit anti-mouse IgG (ECL kit; Amersham). Development was performed by incubating the membrane, after washing, with the ECL reagent substrate. Detection was visualized by autoradiography after 1 minute of contact of the membrane with a Biomax-MR1 film (Eastman Kodak, Rochester, NY). As a positive control for iNOS expression, we used either a lysate of IFN-γ–activated murine macrophage RAW cells, provided with the MoAb, or a lysate obtained from A549 cells.
RESULTS
Detection of iNOS mRNA Expression in Human B-CLL Cells by RT-PCR
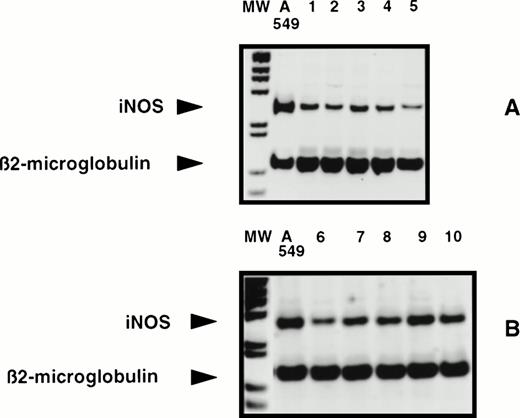
Total RNA was extracted from purified B-CLL leukemic B cells obtained from frozen or freshly collected blood samples. Inasmuch as NOS mRNA in human hematopoietic cells is generally difficult to detect by Northern blot, RT-PCR analysis was performed. When total RNAs were reverse transcribed and amplified in the presence of a couple of amplimers specific for the human iNOS gene, in comparison with the positive A549 control cell line, a 259-bp fragment was regularly observed for all 22 B-CLL cell samples tested by this technique (no differences were observed between fresh and frozen samples), as exemplified in Fig 1. The size of the amplified fragment is in agreement with the expected size deduced from the sequence of the human iNOS gene. Similar results were obtained with the second couple of primers (not shown), which was used previously to show the induction of iNOS in human monocytes following the engagement of CD2343 and whose sequence of the amplified fragment was identical to that of human hepatocyte iNOS.44 No signal was detected in the absence of RNA or of Taq polymerase and, when tested by RT-PCR, the U937 monocytic and RPMI-8226 myeloma cell lines were negative for the presence of iNOS mRNA (not shown). In contrast, endothelial constitutive NOS (ecNOS) mRNA was not detectable in the different B-CLL cells tested, whereas in U937 cells used as a positive control of ecNOS expression a 277-bp fragment was visualized, as previously reported46 (not shown).
Detection of iNOS mRNA in human B-CLL cells by RT-PCR. Total RNAs from 10 different human B-CLL cell samples (1 through 10) and from the A549 cell line, tested in two experiments A and B, were reverse transcribed and the cDNA obtained was submitted to 35 cycles of PCR in the presence of human iNOS specific primers, then analyzed by electrophoresis in a 7% acrylamide gel in the presence of BET, as described in Materials and Methods. MW, 1-kb ladder; A549, positive control; lanes 1 through 10, 10 different B-CLL cell samples (five in each panel A and B).
Detection of iNOS mRNA in human B-CLL cells by RT-PCR. Total RNAs from 10 different human B-CLL cell samples (1 through 10) and from the A549 cell line, tested in two experiments A and B, were reverse transcribed and the cDNA obtained was submitted to 35 cycles of PCR in the presence of human iNOS specific primers, then analyzed by electrophoresis in a 7% acrylamide gel in the presence of BET, as described in Materials and Methods. MW, 1-kb ladder; A549, positive control; lanes 1 through 10, 10 different B-CLL cell samples (five in each panel A and B).
As a control, RT-PCR experiments were also performed with RNA extracted from purified populations of blood derived T and B lymphocytes. Whereas T cells were found to be negative for the expression of iNOS mRNA, an amplification fragment was occasionally observed with B cells but the intensity of the band was faint when compared to that obtained with B-CLL RNA (not shown).
Detection of iNOS Protein in B-CLL Cells
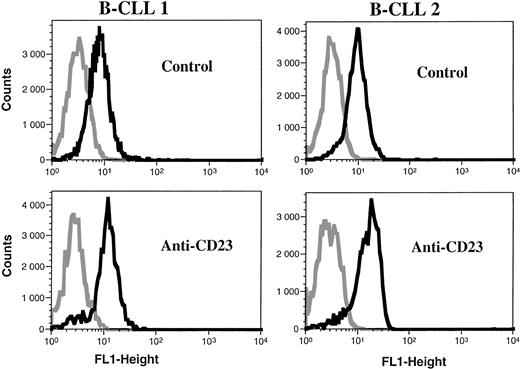
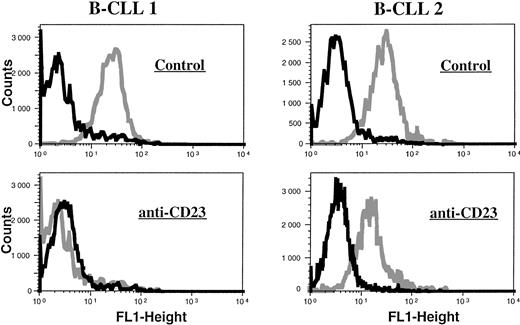
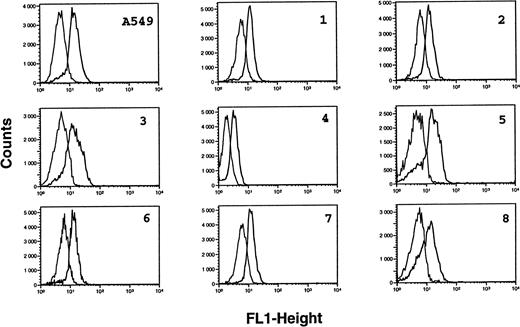
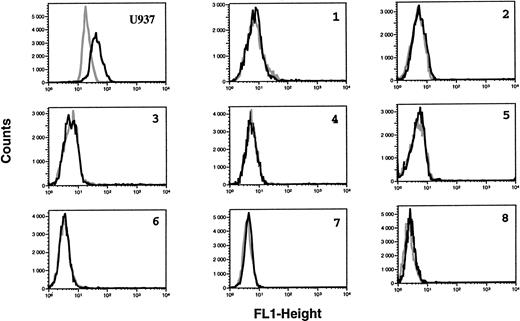
B-CLL cells were analyzed for the intracytoplasmic expression of ecNOS and iNOS proteins by indirect immunofluorescence FACS analysis on permeabilized cells. All B-CLL cells tested were significantly labeled with the anti-mac NOS (clone 54), as shown in Fig 2. The percentage of positive cells was in the range of 13% to 82% (mean, 54%; n = 13). This result was further confirmed by direct immunofluorescence on permeabilized cells using another anti-iNOS MoAb (clone 6) directly conjugated to FITC, and also by immunocytochemistry (not shown). In contrast, B-CLL cells were essentially negative for the expression of ecNOS (range of positive cells: 0% to 12%; mean: 4.3%; n = 13), as depicted in Fig 3.
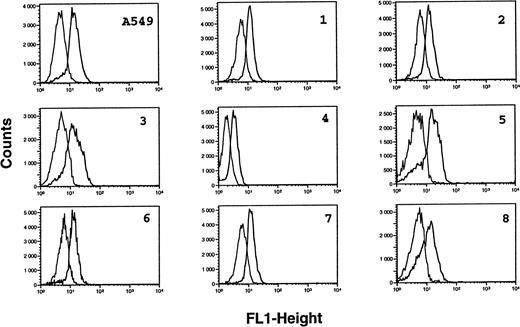
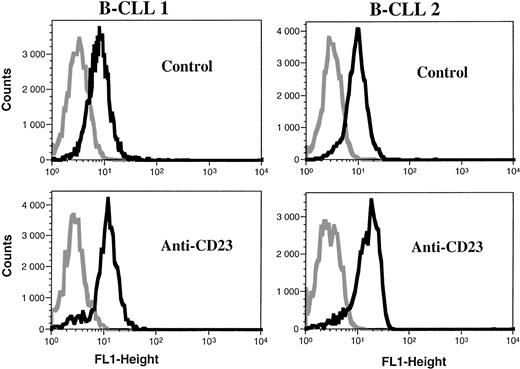
Analysis of iNOS protein expression in B-CLL cells by flow cytometry. B-CLL cells from eight different patients and A549 cells (positive control for iNOS expression) were permeabilized with the Cytoperm kit and labeled with either the monoclonal anti-iNOS (clone 54) or unrelated IgG1 MoAb as an isotype-matched control, then incubated in the presence of a fluoresceinated goat F(ab′)2 anti-mouse Ig antibody. Fixed cells were analyzed by flow cytometry with a FACScan instrument, using the lysis II and Procyt softwares. Gray line, control labeling with IgG1; black line, labeling with anti-iNOS. The statistical significance was P < .001, according to the Kolmogorov-Smirnov test and the data are representative of a total of 13 different B-CLL cell samples tested.
Analysis of iNOS protein expression in B-CLL cells by flow cytometry. B-CLL cells from eight different patients and A549 cells (positive control for iNOS expression) were permeabilized with the Cytoperm kit and labeled with either the monoclonal anti-iNOS (clone 54) or unrelated IgG1 MoAb as an isotype-matched control, then incubated in the presence of a fluoresceinated goat F(ab′)2 anti-mouse Ig antibody. Fixed cells were analyzed by flow cytometry with a FACScan instrument, using the lysis II and Procyt softwares. Gray line, control labeling with IgG1; black line, labeling with anti-iNOS. The statistical significance was P < .001, according to the Kolmogorov-Smirnov test and the data are representative of a total of 13 different B-CLL cell samples tested.
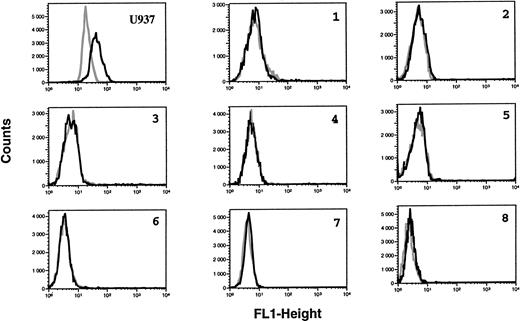
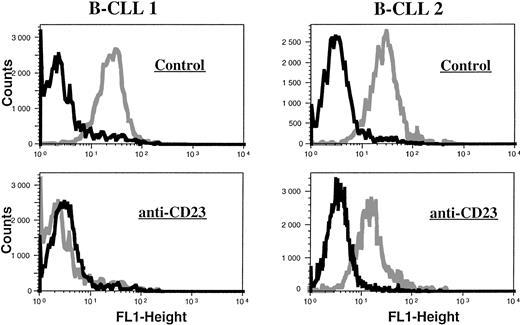
Lack of ecNOS protein expression detectable in B-CLL cells by flow cytometry. B-CLL cells from eight different patients and U937 cells (positive control of ecNOS expression) were permeabilized with the Cytoperm kit and labeled with either the monoclonal anti-ecNOS or unrelated IgG1 MoAb as an isotype-matched control, then incubated in the presence of a fluoresceinated goat F(ab′)2 anti-mouse Ig antibody. Fixed cells were analyzed by flow cytometry with a FACScan instrument, using the lysis II and Procyt softwares. Gray line, control labeling with IgG1; black line, labeling with anti-ecNOS. The data are representative of a total of 13 different B-CLL cell samples tested.
Lack of ecNOS protein expression detectable in B-CLL cells by flow cytometry. B-CLL cells from eight different patients and U937 cells (positive control of ecNOS expression) were permeabilized with the Cytoperm kit and labeled with either the monoclonal anti-ecNOS or unrelated IgG1 MoAb as an isotype-matched control, then incubated in the presence of a fluoresceinated goat F(ab′)2 anti-mouse Ig antibody. Fixed cells were analyzed by flow cytometry with a FACScan instrument, using the lysis II and Procyt softwares. Gray line, control labeling with IgG1; black line, labeling with anti-ecNOS. The data are representative of a total of 13 different B-CLL cell samples tested.
Detection by Western Blotting of the iNOS Protein in B-CLL Cell Lysates
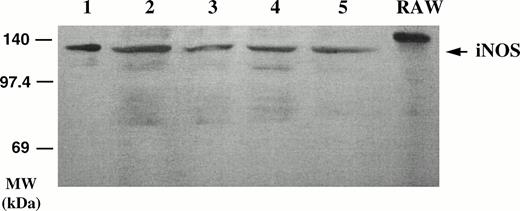
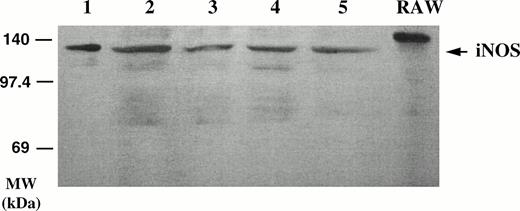
As seen in Fig 4, iNOS was detected by Western blot in the lysates of two different B-CLL cell samples, using the anti-macNOS MoAb (clone 54) for the visualization. The protein detected in the B-CLL lysates had a slightly lower molecular weight than that detected in the lysate of the murine RAW 264.7 macrophage cell line, used as a positive control, but was found to be identical to that detected in lysates of human A549 cells (not shown). Western blotting was repeated with two other MoAbs against the human iNOS (21C10-D10 and 1E8 D8), which were kindly provided by Dr R. Webber (R & D, Minneapolis, MN). A protein of 135 kD was again detected by Western blot in the B-CLL lysates (not shown).
Detection of iNOS protein in B-CLL cell extracts by Western blotting. Homogenates of five different B-CLL cell samples (lanes 1 through 5) were electrophoresed on acrylamide, blotted onto membranes, and probed with a monoclonal anti-iNOS MoAb (clone 54) and revealed by ECL. Right lane, crude extract from IFN-γ–stimulated murine RAW 264.7 macrophage cell line (positive control of iNOS expression).
Detection of iNOS protein in B-CLL cell extracts by Western blotting. Homogenates of five different B-CLL cell samples (lanes 1 through 5) were electrophoresed on acrylamide, blotted onto membranes, and probed with a monoclonal anti-iNOS MoAb (clone 54) and revealed by ECL. Right lane, crude extract from IFN-γ–stimulated murine RAW 264.7 macrophage cell line (positive control of iNOS expression).
Ligation of CD23 on B-CLL Cells Stimulates iNOS Expression
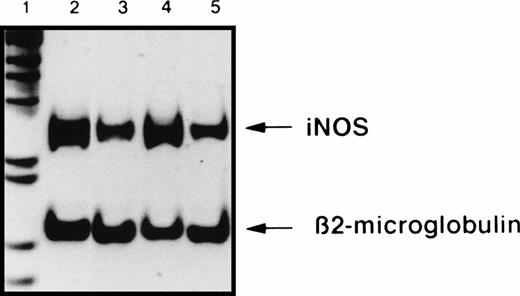
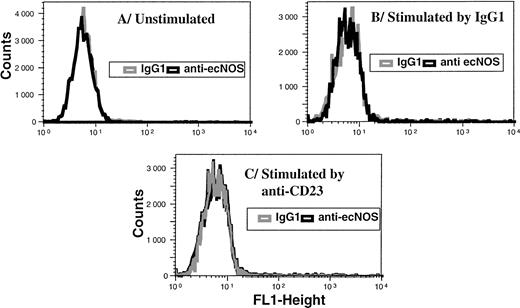
The vast majority of B-CLL cells, unlike their normal counterparts, the mature resting B lymphocytes, spontaneously displayed a high level of membrane CD23 expression. We and others have reported previously that the engagement of CD23, induced in human monocytes by IL-4 pretreatment, resulted in the activation of iNOS in these cells.38,40 43 This prompted us to examine the effect of CD23 ligation on iNOS mRNA expression and protein level in B-CLL cells. Treatment of B-CLL cells with an anti-CD23 MoAb (MoAb 135) stimulated the expression of iNOS mRNA, as detected by RT-PCR (Fig 5). In five different experiments, the level of iNOS mRNA, estimated in comparison with that of β2-microglobulin, was significantly increased following ligation of CD23 (range, 179% to 543%). The expression of the iNOS protein was also increased in B-CLL cells after the engagement of CD23, as seen in Fig 6. In contrast, ligation of CD23 did not induce ecNOS expression in these cells, as shown in Fig 7.
Ligation of CD23 on B-CLL cells increases iNOS mRNA expression. Total RNA was extracted from A549 cells, used as a positive control of iNOS expression (lane 2) and from one B-CLL cell sample, either at time 0 of the start of the culture (lane 3), or after a 16-hour incubation in the presence of 10 μg/mL of anti-CD23 MoAb 135 (lane 4), or of isotype-matched IgG1 (lane 5). The detection by semi-quantitative RT-PCR of iNOS and β2-microglobulin mRNA was performed using the “Image” software for the quantification of the bands after electrophoresis on a 7% acrylamide gel. Lane 1, 1-kb ladder. Results are from one experiment representative of five performed with different B-CLL cell samples. In addition, ligation of CD23 on B-CLL cells did not induce ecNOS mRNA expression (not shown).
Ligation of CD23 on B-CLL cells increases iNOS mRNA expression. Total RNA was extracted from A549 cells, used as a positive control of iNOS expression (lane 2) and from one B-CLL cell sample, either at time 0 of the start of the culture (lane 3), or after a 16-hour incubation in the presence of 10 μg/mL of anti-CD23 MoAb 135 (lane 4), or of isotype-matched IgG1 (lane 5). The detection by semi-quantitative RT-PCR of iNOS and β2-microglobulin mRNA was performed using the “Image” software for the quantification of the bands after electrophoresis on a 7% acrylamide gel. Lane 1, 1-kb ladder. Results are from one experiment representative of five performed with different B-CLL cell samples. In addition, ligation of CD23 on B-CLL cells did not induce ecNOS mRNA expression (not shown).
Ligation of CD23 increases iNOS expression in B-CLL cells. Purified leukemic B cells from two patients were incubated for 48 hours in the presence of control medium or anti-CD23 135 MoAb (10 μg/mL). FACS analysis was then performed by direct immunofluorescence on permeabilized cells with an FITC-labeled anti-iNOS MoAb (clone 6) (black line), using FITC-IgG2a as an isotypic matched control MoAb (gray line).
Ligation of CD23 increases iNOS expression in B-CLL cells. Purified leukemic B cells from two patients were incubated for 48 hours in the presence of control medium or anti-CD23 135 MoAb (10 μg/mL). FACS analysis was then performed by direct immunofluorescence on permeabilized cells with an FITC-labeled anti-iNOS MoAb (clone 6) (black line), using FITC-IgG2a as an isotypic matched control MoAb (gray line).
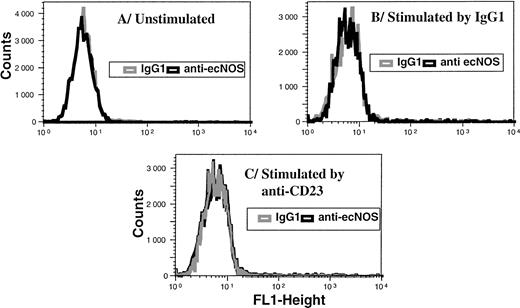
Ligation of CD23 does not elicit ecNOS expression in B-CLL cells. Purified leukemic B cells were incubated for 48 hours in the presence of medium alone (A), mouse IgG1 (10 μg/mL) as a control (B), or anti-CD23 135 MoAb (10 μg/mL) (C). FACS analysis was then performed by indirect immunofluorescence on permeabilized cells as described in Materials and Methods. Gray line, IgG1 isotype control; black line, anti-ecNOS MoAb.
Ligation of CD23 does not elicit ecNOS expression in B-CLL cells. Purified leukemic B cells were incubated for 48 hours in the presence of medium alone (A), mouse IgG1 (10 μg/mL) as a control (B), or anti-CD23 135 MoAb (10 μg/mL) (C). FACS analysis was then performed by indirect immunofluorescence on permeabilized cells as described in Materials and Methods. Gray line, IgG1 isotype control; black line, anti-ecNOS MoAb.
NOS Activity in B-CLL Cell Extracts and Intact Cells
NOS activity was determined in cytosolic extracts of different B-CLL cells, unstimulated or incubated with anti-CD23 MoAb or IgG1 as a control, by measuring the conversion of labeled14C-L-arginine into 14C-L-citrulline. These homogenates were found to display a catalytic activity of about 20 pmol/min/mg, which was increased about three times after stimulation with anti-CD23 (Table 1A). NOS activity was also quantified in intact 14C-L-arginine-loaded B-CLL cells by measuring the release of radiolabeled 14C-L-citrulline in the culture supernatants. A significant basal NOS activity was detected, as estimated by the L-NMMA–inhibitable release of labeled citrulline in supernatants from control B-CLL cultures. Addition of anti-CD23 MoAb resulted in increased activity, whereas isotype-matched IgG1 had little effect (Table 1B). Attempts to detect the release of nitrite/nitrate in the supernatants of B-CLL cells stimulated or not with anti-CD23 MoAb were hampered by large interindividual variations. Nonetheless, when paired samples were compared, L-NAME was found to reduce the basal nitrite release, whereas treatment with anti-CD23 resulted in a significant increase in nitrite production over basal release. The latter increase was largely reduced by L-NAME (Table 2).
Anti-Apoptotic Role of NO in B-CLL Cells
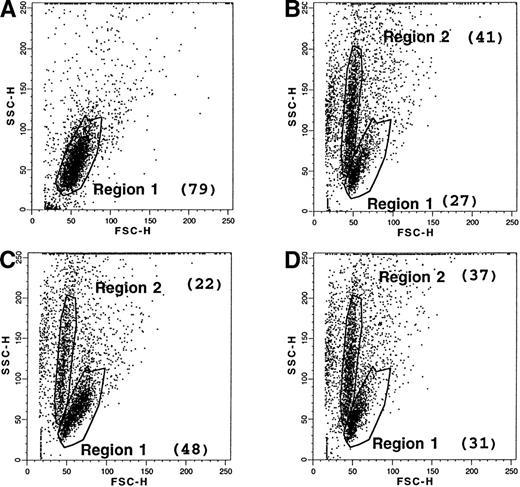
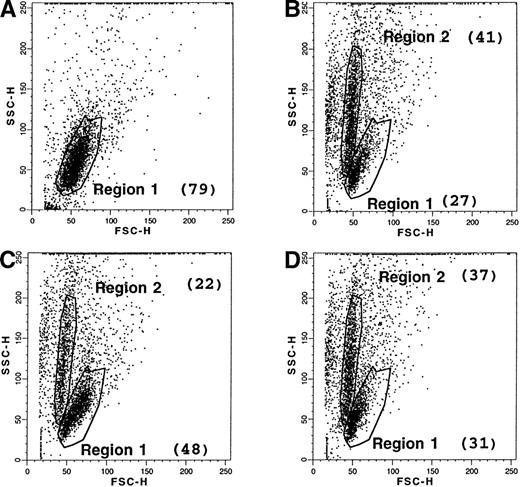
Cells undergoing apoptosis display an early modification in the distribution of their membrane phospholipids, characterized by an exposure of PS at the outer leaflet. The latter can be quantified by the use of FITC-labeled annexin, which has a high affinity for PS. Therefore, freshly collected B-CLL cells were stained with fluorescent annexin, either at day 0 or after 2 days of culture with medium alone or in the presence of anti-CD23 MoAb. When the dot plots (side scatter/forward scatter) of these cells were compared, untreated cells at day 0 displayed a single distribution (region R1: 79% of total cells), as shown in Fig 8, and were negative (<5%) for the labeling with FITC-annexin V. After 2 days of culture in medium alone, a marked change in the dot-plot distribution was apparent. Although some cells retained the same pattern as day 0 cells (region R1: 27% of total cells), a new population was detected, characterized by heterogeneous granulometry (region R2: 41% of total cells). Most of these cells (about 85%) were positively labeled with annexin V, confirming that they were entering apoptosis, whereas the population in the region R1 consisted of “intact” cells, mostly negative for this marker (0% to 11%), as shown in Fig 9. B-CLL cells incubated in the presence of anti-CD23 MoAb also displayed a bimodal dot-plot distribution, but the percentage of the R1 population (48%) versus the R2 population (22%) was much higher than for incubated control cells. Interestingly, the inhibitory effect of CD23 ligation was partially reversed in the presence of L-NMMA, an inhibitor of the NOS pathway. When global B-CLL cell populations were examined for the expression of the annexin V marker, a significant reduction in the positive cells was observed after incubation of these cells with anti-CD23, as shown in the examples presented in Fig 10. The CD23-driven reduction in the percentage of annexin V+ cells was largely reversed by addition of L-NMMA, as shown in Table 3.
Effect of CD23 ligation and L-NMMA on the apoptosis of B-CLL cells. Purified leukemic B cells from a freshly collected B-CLL sample were labeled with FITC-annexin V, either at day 0 (A), or after 2-day culture with medium alone (B), or with anti-CD23 MoAb (clone 135; 10 μg/mL) (C), or with anti-CD23 MoAb (10 μg/mL) and L-NMMA (1 mmol/L) (D). An FITC-labeled goat anti-rabbit Ig F(ab′)2 was used as a control of nonspecific binding. Dot plots (SSC v FSC) and percentage of annexin V+ cells were analyzed by the Procyt software. The data are from one representative experiment out of three.
Effect of CD23 ligation and L-NMMA on the apoptosis of B-CLL cells. Purified leukemic B cells from a freshly collected B-CLL sample were labeled with FITC-annexin V, either at day 0 (A), or after 2-day culture with medium alone (B), or with anti-CD23 MoAb (clone 135; 10 μg/mL) (C), or with anti-CD23 MoAb (10 μg/mL) and L-NMMA (1 mmol/L) (D). An FITC-labeled goat anti-rabbit Ig F(ab′)2 was used as a control of nonspecific binding. Dot plots (SSC v FSC) and percentage of annexin V+ cells were analyzed by the Procyt software. The data are from one representative experiment out of three.
Distribution of annexin V+ cells in B-CLL cells. Purified leukemic B cells from freshly collected blood samples from two patients were incubated for 48 hours with medium alone, then labeled with FITC-annexin V or an FITC-labeled goat F(ab′)2 anti-rabbit Ig as control. Regions 1 and 2 were determined as before by FACS analysis, according to the SSC/FSC distribution pattern, and the percentage of annexin V+cells was estimated for each region, R1 and R2, using the Procyt software.
Distribution of annexin V+ cells in B-CLL cells. Purified leukemic B cells from freshly collected blood samples from two patients were incubated for 48 hours with medium alone, then labeled with FITC-annexin V or an FITC-labeled goat F(ab′)2 anti-rabbit Ig as control. Regions 1 and 2 were determined as before by FACS analysis, according to the SSC/FSC distribution pattern, and the percentage of annexin V+cells was estimated for each region, R1 and R2, using the Procyt software.
CD23 ligation reduces the percentage of annexin V-labeled B-CLL cells. Freshly collected leukemic cells from two patients were incubated for 48 hours in the presence of medium alone (upper histograms) or containing anti-CD23 MoAb (MoAb 135, 10 μg/mL) (lower histograms), then labeled with FITC-annexin V. FACS analysis was performed as previously on the global cell population, using an FITC-labeled goat anti-rabbit Ig F(ab′)2 (black line) as a control of nonspecific labeling for FITC-Annexin V (gray line).
CD23 ligation reduces the percentage of annexin V-labeled B-CLL cells. Freshly collected leukemic cells from two patients were incubated for 48 hours in the presence of medium alone (upper histograms) or containing anti-CD23 MoAb (MoAb 135, 10 μg/mL) (lower histograms), then labeled with FITC-annexin V. FACS analysis was performed as previously on the global cell population, using an FITC-labeled goat anti-rabbit Ig F(ab′)2 (black line) as a control of nonspecific labeling for FITC-Annexin V (gray line).
To determine whether this observation was also true for events occurring later in apoptosis, DNA fragmentation was also quantified in B-CLL cells using the TUNEL method, a fluorescence technique that detects the TdT-catalyzed labeling of 3′ ends with FITC-dUTP. As seen in Table 4, the percentage of B-CLL cells spontaneously undergoing DNA fragmentation after 48 hours of culture was very low, in comparison with that detected by the annexin V technique, but markedly increased in the presence of a nontoxic concentration of the NOS inhibitor L-NAME (1 mmol/L). L-NAME also elicited an augmentation in the percentage of apoptotic cells in B-CLL cells incubated with anti-CD23 MoAb, not active alone, albeit to a lesser degree than in control B-CLL cells (P < .01 between both groups). These data confirm that inhibition of NO production favors the programmed death of B-CLL cells, as measured by their DNA fragmentation, and that CD23 ligation to some extent protects these cells from apoptosis by increasing the amount of NO released.
The role of APO-1/Fas was also investigated. As presented in Table 5, the anti-CD95 (APO-1/Fas) MoAb (clone CH-11) had no effect alone, but it markedly potentiated the DNA fragmentation observed in the presence of L-NAME. This potentiation was not caused by an increase in Fas expression at the membrane of B-CLL cells as assessed by FACS analysis: B-CLL cells incubated for 48 hours in the presence of medium alone displayed weak labeling (between 2% and 33% positive cells for the different B-CLL tested), as estimated by FACS analysis with the anti-Fas MoAb (clone BG27), and neither this expression nor the mean fluorescence intensity was affected in the presence of the NOS inhibitor L-NMMA.
DISCUSSION
Our data indicate that iNOS-specific mRNA can be detected by RT-PCR in purified leukemic B lymphocytes from B-CLL patients. The presence of this mRNA is unlikely to be due to a contamination by other cell types, inasmuch as blood samples were collected from hyperleukocytic patients and similar results were observed when rigorous depletion of residual contaminating cells was performed. In addition, the presence of iNOS in unstimulated human T lymphocytes and monocytes has not been reported. In some instances, an amplification fragment corresponding to the iNOS was observed with RNA extracted from blood-derived B cells, but the intensity of the bands was weak when compared with those of B-CLL cells. Therefore, the presence of iNOS mRNA in resting mature small B lymphocytes cannot be totally excluded, but our previous attempts to detect an NO-dependent cGMP production in normal B lymphocytes or in B lymphocytes triggered by CD23 ligation were unsuccessful.40
iNOS mRNA was detected in B-CLL cells prepared from frozen samples of PBMC as well as in freshly collected and processed blood samples. In contrast, ecNOS mRNA was undetectable. Reiling et al36 have reported the presence of ecNOS mRNA and protein in a fraction of normal B lymphocytes, but the positive population has not been extensively characterized.
iNOS protein was also detected in B-CLL cells by immunofluorescence of permeabilized cells using different anti-iNOS MoAbs directed against the mouse protein (and cross-reacting with the human iNOS) or against human iNOS. The same antibodies, when used in Western blot to probe B-CLL cell extracts, recognized a protein of about 135 kD, in agreement with the molecular weight of the human iNOS.44 iNOS mRNA and protein, without significant catalytic activity, have been detected in some human hematopoietic cells, suggesting that the conformation of iNOS (dimerization) and/or that important cofactors, such as tetrahydrobiopterin, may be required for the protein to achieve its enzymatic function.49 NOS activity could be evidenced both in intact B-CLL cells and lysates by measuring the L-NMMA inhibitable rate of conversion of L-arginine to L-citrulline, indicating that the iNOS protein detected in these cells was functionally active. This activity is relatively low compared with that measured in IFN-γ–activated murine macrophages such as the RAW 264.7 cell line (around 1,000 pmol/mg/min), but is of the same order of magnitude as that detected in human EBV-transformed B lymphocytes and EBV+ Burkitt's lymphoma cell lines.35 Some of the latter cells, albeit expressing low levels of iNOS mRNA, did not display detectable NOS activity, yet were sensitive to the effect of NO, as shown by the reactivation of EBV in the presence of L-NMMA.35 Therefore, although the level of NO produced by B-CLL cells is relatively low, this does not preclude its importance as a modulator of the functions of these cells, notably apoptosis. Indeed, our experiments clearly point to an anti-apoptotic role for NO in B-CLL cells. First, the percentage of cells displaying PS at their outer leaflet is reduced when the CD23 is cross-linked at the membrane, in correlation with increased iNOS expression and activity, and the latter effect is reversed in the presence of an inhibitor of the NOS pathway. Second, while the percentage of cells spontaneously undergoing DNA fragmentation after 48 hours of culture is very low, the DNA fragmentation of B-CLL cells is markedly augmented in the presence of an NOS inhibitor, L-NAME. The cross-linking of CD23 reduces this induction of DNA fragmentation elicited by L-NAME. The discrepancy between the two techniques (annexin V and TUNEL) may be due to the fact that exposure of PS occurs earlier than DNA fragmentation in the apoptotic process, or that the annexin V–based technique is more sensitive, allowing the detection of a higher percentage of cells undergoing programmed cell death. Nevertheless, both techniques show that inhibitors of the NOS pathway exert a marked pro-apoptotic action on B-CLL cells. This would indicate that NO released by the tumoral cells may contribute to their survival in vivo and may explain the observation that mature circulating normal B lymphocytes exhibit a higher cell death rate than B-CLL lymphocytes.50 Incubation of ex vivo cultured mature B cells with NO or NO donors was also shown to delay programmed cell death and to rescue these cells from antigen-induced apoptosis through a cGMP-dependent mechanism and by preventing the decrease in the expression of the protooncogene Bcl-2, both at the mRNA and protein level, suggesting the existence of a pathway linking NO signaling with Bcl-2 expression.51
Recently, members of the CED-3/ICE (IL-1 converting enzyme) family of proteases, or caspases, have been shown to be involved in the apoptosis of B-CLL cells.52 One of their substrates is poly (ADP[adenosine 5′-diphosphate]ribose) polymerase, or PARP, an enzyme involved in DNA repair. Interestingly, we observed that ligation of the CD23 molecule, which is a hallmark of B-CLL cells, resulted in increased iNOS expression and catalytic activity, as previously described for human monocytes induced to express CD23. This would fit with the role ascribed to CD23 in the control of B-CLL cell viability, as reported by Fournier et al.53 Therefore, it is conceivable that in vivo, through interaction with one of its ligands or counterstructures (IgE, CD21, CD11b, and CD11c), stimulation of CD23 expressed on B-CLL cells may lead to activation of the iNOS pathway in these cells, which in turn will counteract the normal apoptotic process and lead to an accumulation of the tumoral cells. It is important to recall that Sarfati et al54 have reported a prognostic role for serum sCD23 level at the time of B-CLL diagnosis, with a less favorable outcome for the patients with a high sCD23 level.
The capacity of APO-1/Fas to trigger an apoptotic signal in B-CLL cells is controversial. The low basal expression of APO-1 in these cells55 can be stimulated by treatment with various reagents and some groups have observed increased apoptosis in these cells after their stimulation with anti-APO1/Fas antibody that was correlated56 or not57 with a decreased Bcl-2 expression. However, another group has reported that Fas+B-CLL cells were resistant to anti–Fas-mediated cytotoxicity and suggested that a combination of high Bcl-2 protein level and resistance to Fas-mediated apoptosis may contribute to the extended in vivo survival of these cells.58 Our data confirm that anti-Fas alone does not elicit apoptosis of B-CLL cells, but can amplify the pro-apoptotic effect of NOS inhibitors. In agreement with previous reports, we found a low expression of the Fas molecule on unstimulated B-CLL cells and this expression was not modified in the presence of L-NMMA. Therefore, the potentiation observed cannot be attributed to an increase in Fas expression in the presence of NOS inhibitors. Mannick et al59 have shown that NOS activity in human leukocytes inhibited Fas-induced apoptosis via a cGMP-independent mechanism and inhibited Fas-induced cleavage of PARP by members of the caspase family of cysteine proteases. These data suggest that Fas activity is under the control of the NO signaling pathway. The suppression of NOS may result, in addition to its own pro-apoptotic effect, in a possibility for the Fas-driven apoptotic pathway to be triggered by engagement of the molecule with specific MoAb, explaining the potentiating effect that we observed.
Recently, it has been shown that theophylline, an inhibitor of cyclic nucleotide phosphodiesterases, synergized with chlorambucil to increase the apoptotic process in B-CLL cells through a mechanism involving cyclic adenosine monophosphate (cAMP).60 61NO stimulates cyclic guanosine monophosphate (cGMP) accumulation through activation of soluble guanylate cyclase. Therefore, an increase in cGMP could prevent cAMP accumulation through its action on the cAMP-specific, cGMP-stimulated phosphodiesterase and thus counteract the apoptotic process. Experiments are in progress to identify the possible targets of NO action in the apoptotic cascade.
ACKNOWLEDGMENT
The help of Nicole Romquin for the RT-PCR techniques, Danny Rouillard for the flow cytometry analysis, and David Marsh for the English style was greatly appreciated.
Supported by INSERM, the Ligue Nationale Contre le Cancer, and by a grant (no. 6957) from the Association pour la Recherche contre le Cancer (ARC). H.Z. was supported by a fellowship from ARC.
Address reprint requests to Jean-Pierre Kolb, PhD, U365 INSERM, Institut Curie, 26 rue d'Ulm, 75231, Paris Cedex 05, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.