Abstract
Using multicolor flow-cytometry we have examined 19 patients with paroxysmal nocturnal hemoglobinuria (PNH) (18 with active disease and 1 spontaneous remitter) to determine absolute numbers of lymphocyte subsets and the proportion of glycosylphosphatidylinositol (GPI)-deficient clones amongst these subpopulations. Lymphocyte subsets were abnormal in all patients; the most frequent findings were low absolute numbers of natural killer (NK) cells (median, 0.08 × 109/L; normal range, 0.2 to 0.4 × 109/L) and low absolute numbers of B cells (median, 0.05 × 109/L; normal range, 0.06 to 0.65 × 109/L). GPI-deficient B, T, and NK cells were identified in 88%, 84%, and 89% of patients, respectively. The proportion of GPI-deficient cells within individual lymphoid lineages was highly variable, though in most patients the percentage of GPI-deficient NK cells was considerably higher than B or T cells. These observations can be explained when mechanisms of normal lymphopoiesis are considered. Despite these quantitative and qualitative abnormalities, no patients suffered an excessive number or severity of infections. The detection of PNH clones amongst all lymphocyte lineages may provide important information regarding the natural history of the disease and additional insights into kinetics of adult lymphopoiesis.
© 1998 by The American Society of Hematology.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired hematopoietic stem cell disorder in which a somatic mutation of the X-linked PIG-A gene results in a partial or absolute deficiency of all proteins linked to the cell membrane by the glycosylphosphatidylinositol (GPI) anchor.1-6 Most, if not all, patients with PNH have evidence of underlying bone marrow failure. In addition, up to 50% of patients with aplastic anemia go on to develop a PNH clone. Thus, there is a very close association between aplastic anemia and PNH. The successful use of immunosuppressive therapy such as antithymocyte globulin (ATG) for the treatment and control of aplastic anemia provides strong evidence that there may be an immunological component to the disease, and it appears that this promotes the development of PNH clones.7,8 The most profound clinical feature of PNH is a hemolytic anemia due to a deficiency from the red blood cell (RBC) membrane of the complement regulatory molecules CD55 (decay accelerating factor [DAF]) and CD59 (membrane inhibitor of reactive lysis [MIRL]). This pathobiological property renders erythrocytes abnormally sensitive to the lytic action of complement and was exploited as the basis for the acidified serum lysis (Ham) test for the diagnosis of PNH.9-12 This test has now largely been superseded by flow cytometry as the standard diagnostic test for PNH.13 14 The use of flow cytometry and specific combinations of monoclonal antibodies has enhanced the ability to detect small PNH clones within multiple hematopoietic cell lineages. Consequently, this has led to reclassifying PNH into two types: (1) hemolytic PNH, characterized by overt episodes of hemolytic anemia, and (2) hypoplastic PNH in which clones of GPI-deficient hematopoietic cells are detectable with no overt hemolysis but may have other symptoms such as aplastic anemia, leukopenia, or thrombocytopenia.
In PNH the presence of GPI-deficient clones within the platelet, monocyte, and granulocyte components of the myeloid lineage is well documented, and a small number of investigators have also unequivocally described PNH clones within peripheral blood lymphocyte populations.13,15-20 Indeed, GPI-deficient T-lymphocyte populations have been described in two patients in long-term clinical remission for PNH with no detectable granulocyte or red cell clones.21,22 Evidence for the presence of cells derived from the PNH clone within the natural killer (NK) cell lineage is less clear, mainly due to the fact that there is no single pan–NK-cell antigen analogous to CD3 and CD19 that defines T- and B-lymphocyte populations, respectively. The majority of NK cells can be identified phenotypically on a multiparameter basis by concomitant expression of CD16 and CD56 membrane determinants with a lack of CD3 or by a CD3−CD7+ composite phenotype.23 24 To identify precisely PNH clones within the NK-cell population, three-color flow cytometry is a minimum requirement.
Lymphopenia is a well-recognized feature of PNH, although few authors have examined lymphocyte subset distributions and absolute numbers in any detail. In this present study, multicolor flow cytometry is used to show that lymphocyte subset abnormalities are a common feature of PNH and to present unequivocal evidence that all lymphoid lineages may contain distinct PNH clones.
MATERIALS AND METHODS
Patients studied.
After obtaining informed consent, peripheral blood samples were collected from 19 adult patients with PNH (including 1 patient who showed spontaneous remission 15 years ago) and 5 healthy adult normal controls. Patients who showed clearly demonstrable evidence of macroscopic hemoglobinuria were classified as hemolytic, and those without were classified as hypoplastic. The patient group comprized 8 males and 11 females, with an age range of 22 to 66 years. Full blood counts were performed on a Sysmex K1000 cell counter (Sysmex, Milton Keynes, UK). The initial diagnosis of PNH was made by flow cytometry, showing the absence of GPI-linked proteins from red cell and/or granulocyte surface membranes as described elsewhere or by a positive Ham test.20 Lymphocyte subpopulation numbers were determined on all patient samples by flow cytometry. GPI-linked antigen expression by lymphocytes was studied on all patient samples by a whole blood lysis technique and also by lymphocytes isolated by density sedimentation technique.
Lymphocyte subsets.
An initial whole blood lysis screen was performed to assess the proportions and absolute numbers of major lymphocyte populations (B, T, and NK cells), T-cell subsets, and activated T cells. Briefly, 30-μL volumes of the following combinations of monoclonal antibodies (fluorescein isothiocyanate [FITC]/phycoerythrin [PE] or FITC/PE/Cy5) were pipetted into five 12 × 75-mm polystyrene tubes (Becton Dickinson, San Jose, CA): CD45/CD14; control; CD4/CD8/CD3; CD3/HLA-DR/CD19; and CD16/CD56/CD3. One hundred microliter volumes of EDTA anticoagulated whole blood were then added, mixed, and incubated in the dark at ambient temperature for 20 minutes and punctuated by gentle mixing every 5 minutes. A 2-mL volume of a 1/10 dilution of fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson) was added to each tube, gently mixed, and incubated for a further 10 minutes in the dark. The tubes were then centrifuged at 300g for 90 seconds, the supernatant discarded, and the white cell pellet resuspended in a 2-mL volume of FACSFlow (Becton Dickinson) supplemented with 0.5% bovine serum albumin (BSA; Sigma Chemicals, St Louis, MO). The cells were again pelleted and washed with a further 2-mL volume of FACSFlow/BSA. Following this final wash stage, the cells were resuspended in a 300-μL volume of a 1/10 dilution of CellFIX (Becton Dickinson) and incubated for a minimum of 5 minutes before flow cytometry analysis. The cells were then analyzed using a FACScan flow cytometer (Becton Dickinson) and Lysis II software (Becton Dickinson) as previously described collecting a minimum of 10,000 gated lymphocyte events.24 Control studies comprised a CD45/CD14 Leucogate (Becton Dickinson)–stained sample, and cells stained with irrelevant isotype matched negative control antibodies. The sources and specificities of monoclonal antibodies used for immunophenotyping are summarized in Table1. Absolute numbers of lymphocyte subsets were calculated using the Sysmex lymphocyte count multiplied by the percentage of lymphocytes expressing individual CD determinants divided by 100.
Separation of mononuclear cells.
Mononuclear cells were fractionated from 10-mL volumes of EDTA anticoagulated blood by conventional density sedimentation with Lymphoprep (Nycomed, Oslo, Norway). Cells collected from the plasma/lymphoprep interface were washed twice with 10-mL volumes of FACSFlow/BSA, and the white blood cell count was adjusted to 50 × 109/L for subsequent immunophenotyping studies.
Immunophenotyping and flow cytometry of mononuclear cells.
Ten-microliter volumes of cells were stained in U-shaped 96-well microtiter plates with the following two- and three-color combinations of monoclonal antibodies (FITC/Cy5 and FITC/PE/Cy5): CD59/CD3, CD59/CD4, CD59/CD8, CD59/CD19, CD59/CD7/CD3, and CD16/CD7/CD3). The cell/antibody combinations were mixed, incubated at room temperature for 30 minutes, and then centrifuged at 400g for 15 seconds to pellet the cells. After removal of excess antibody and washing twice with 150-μL volumes of FACSFlow/BSA, the cells were resuspended to 300 μL in FACSFlow before flow cytometry studies. Cells were then analyzed by flow cytometry using a FACScan flow cytometer and Lysis II software. A minimum of 10,000 gated lymphocytes were collected on the basis of low side scatter characteristics and either CD3, CD4, CD8, or CD19 positivity.
Detection of the GPI-linked antigen CD48 expression by lymphocyte subpopulations using whole blood lysis.
One hundred–microliter volumes of whole blood were stained as described above with the following combinations of monoclonal antibodies (PE/Cy5 or FITC/PE/Cy5): CD48/CD3, CD48/CD4, CD48/CD8, CD48/CD19, CD48/CD7/CD3, and CD16/CD7/CD3. The cells were analyzed by flow cytometry using a FACScan flow cytometer and Lysis II software. A minimum of 5,000 gated events were collected on specific lymphocyte populations identified by low side scatter characteristics and either CD3, CD4, CD8, or CD19 positivity (summarized in Fig 1). Data were stored as list mode files for subsequent analysis.
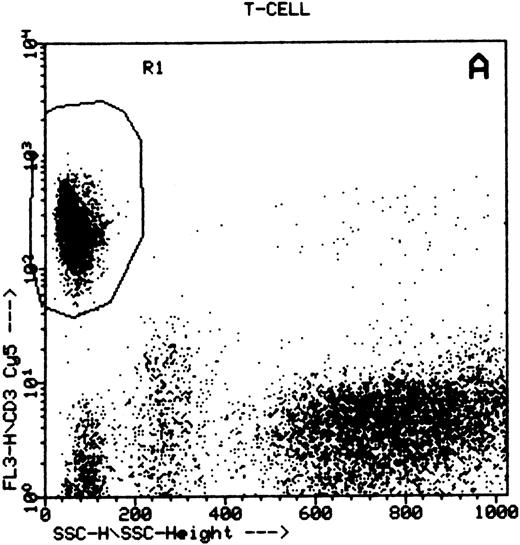
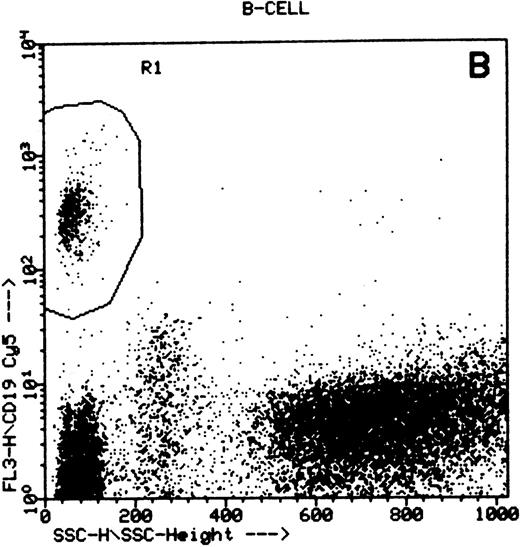
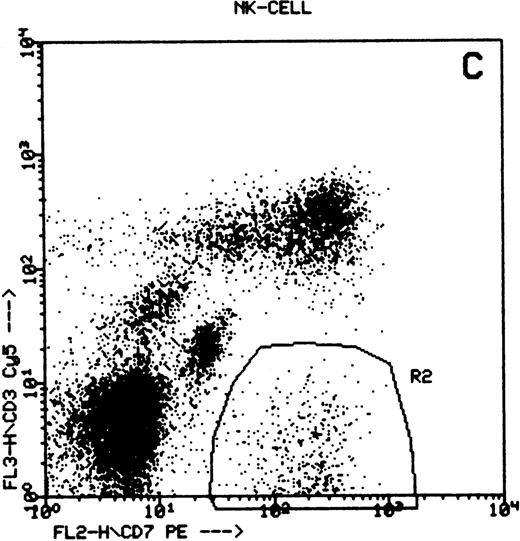
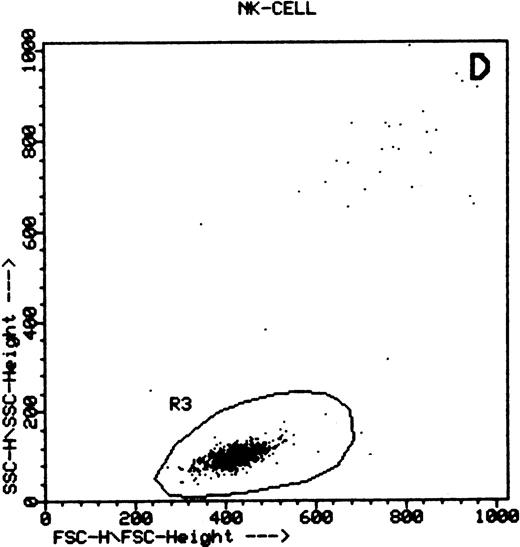
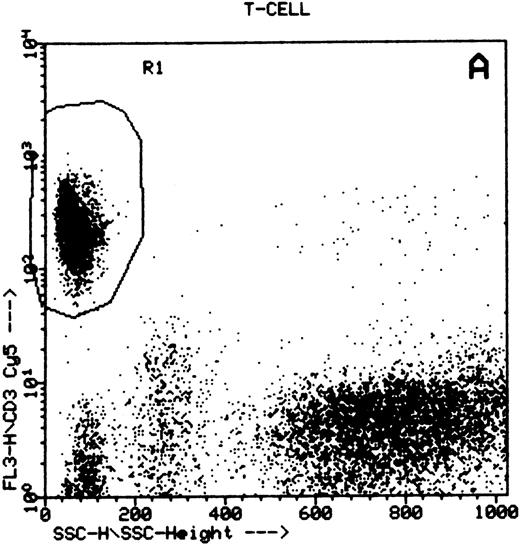
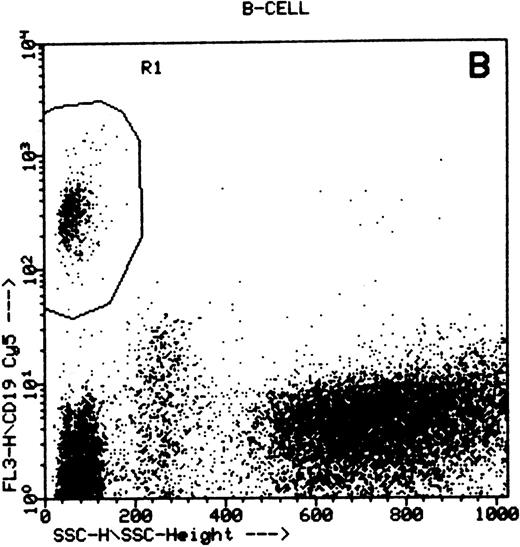
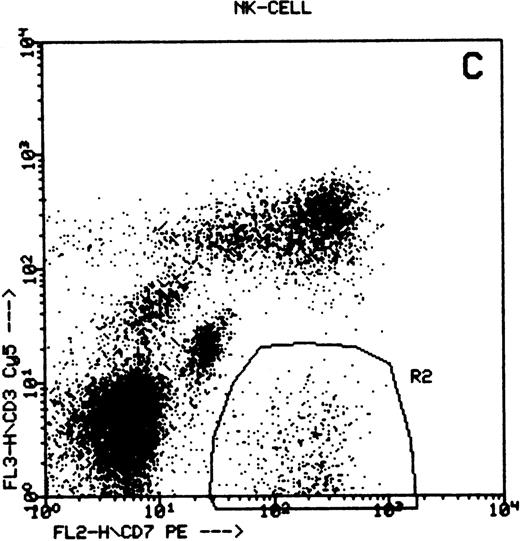
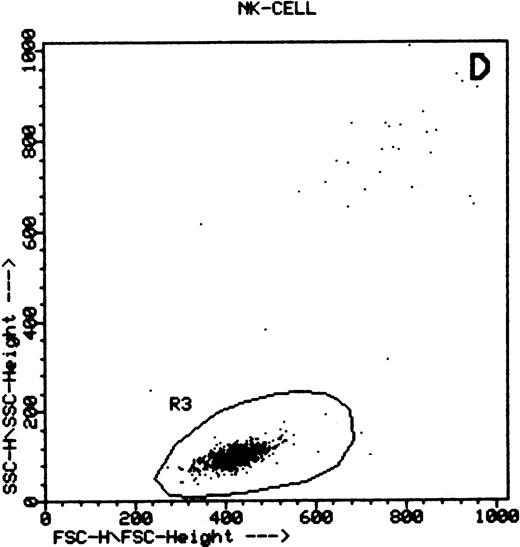
Summary of flow cytometry gating procedures for the study of GPI-linked antigens (CD48 or CD59) by peripheral blood T cells, B cells, and NK cells. Plot (A) shows a region (R1) drawn around CD3+ cells with low side scatter characteristics; plot (B) shows a region (R1) drawn around CD19+ cells with low side scatter characteristics; plots (C and D) show the gating procedure for NK cells. An initial region or anchor gate (R2) is set around CD7+CD3− cells. This region is then applied to an FSC versus SSC plot and a second region (R3) drawn around the lymphocytes with low FSC and SSC characteristics. Only events (cells) meeting these two criteria are collected and analyzed.
Summary of flow cytometry gating procedures for the study of GPI-linked antigens (CD48 or CD59) by peripheral blood T cells, B cells, and NK cells. Plot (A) shows a region (R1) drawn around CD3+ cells with low side scatter characteristics; plot (B) shows a region (R1) drawn around CD19+ cells with low side scatter characteristics; plots (C and D) show the gating procedure for NK cells. An initial region or anchor gate (R2) is set around CD7+CD3− cells. This region is then applied to an FSC versus SSC plot and a second region (R3) drawn around the lymphocytes with low FSC and SSC characteristics. Only events (cells) meeting these two criteria are collected and analyzed.
Detection of GPI antigen expression by NK cells.
NK cells were identified by a multiple gating procedure using combined fluorescence and light scattering characteristics (Fig 1). An initial gate (R1) was drawn around CD7+CD3− cells on a FL2 versus FL3 plot. This region was then applied to a FSC/SSC plot and a second gate (R2) drawn around the lymphocyte population (low FSC and SSC characteristics) to exclude contaminating granulocytes (high FSC/SSC). A minimum of 5,000 events were acquired that met these combined gating criteria. An FL1 histogram of CD48 expression (whole blood lysis) or CD59 (separated cells) was then examined to determine GPI-linked expression by NK cells. Purity of gating was assessed using a CD16/CD7/CD3 combination of monoclonal antibodies and the same gating criteria. In all cases CD16 positivity (a pan NK cell marker) exceeded 90%.
The sources and specificities of all monoclonal antibodies used for immunophenotyping are summarized in Table 1.
RESULTS
The clinical, hematological, and laboratory features of the 19 patients examined in the study are summarized in Table 2. Nine patients had hypoplastic PNH, eight hemolytic PNH, and a single patient, who originally presented with hemolytic PNH, was nonhemolytic with only 0.5% GPI− RBCs and a normal hemoglobin. A single patient showed spontaneous remission of PNH 15 years ago. Blood count parameters showed a neutropenia (<2.0 × 109/L) in 9 of 19 patients, anemia in 13 of 19 patients, and thrombocytopenia in 8 of 19 patients (<150 × 109/L). A lymphopenia was shown in 12 of 19 patients (<1.5 × 109/L). With the exception of case PNH030, GPI-deficient cells were found in both the RBCs and neutrophils in all remaining cases. Flow cytometry studies showed that in the majority of these patients (16 of 18) the granulocyte PNH clone was larger than the RBC PNH clone. However, there was wide variation in size of granulocyte clone, ranging from 1.4% to 99.5%. Similar wide variation (0.5% to 79.5%) was evident for the RBC PNH clone, further complicated by the presence of partially deficient (type II) RBCs in seven patients.
Lymphocyte subset analysis and absolute numbers.
The absolute numbers of CD3+ (T cells) CD3+CD4+ (T-helper), CD3+CD8+ (T-cytotoxic) CD19+ (B cells), and CD3−CD16+ NK cells were determined for each patient. The results (Table 3) showed a wide variety of abnormalities with no single patient having a normal distribution of lymphocyte subsets. Absolute numbers of T cells were generally normal, though three patients did show a T lymphopenia. A mild CD4 lymphopenia was a common finding (10 of 19), with a CD8 lymphopenia in 5 of 19 and a mild CD8 lymphocytosis in two cases. Absolute numbers of NK cells were markedly reduced in 17 of 19 (89%) of patients. B-cell numbers were low in 11 of 19 cases (58%) and borderline low in an additional 5 (26%) patients. A significant increase in activated T cells (>20% expression of HLA-DR) was shown in three patients.
Expression of GPI-linked proteins by T lymphocytes in patients with PNH.
Of the 19 patients studied, GPI-deficient populations of T cells were found in 15. The percentage of cells affected ranged from 0.2% to 21.6% (Fig 2 and Table 4). One case showed a population of partial GPI-deficient cells comprising 8.1% of total T cells. Further studies of CD4+ and CD8+ T-cell components for individual patients revealed similar proportions of PNH clones, and statistical analysis (paired t-test) showed no significant difference (t = 2.48; degrees of freedom [df] = 12; P = .028). Representative histograms of CD48 and CD59 staining by T lymphocytes from PNH patients and normal controls are shown in Fig 3. No GPI− lymphocytes were found in any of the normal controls studied.
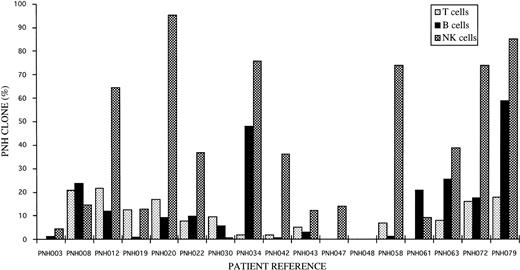
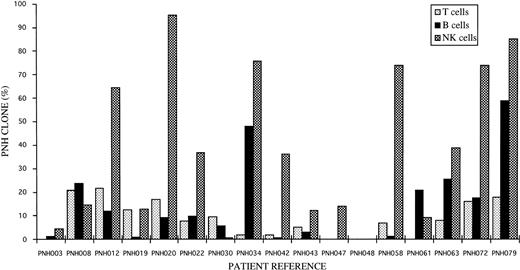
Distribution of GPI− (PNH) clones amongst T, B, and NK lymphocytes for individual patients studied.
Distribution of GPI− (PNH) clones amongst T, B, and NK lymphocytes for individual patients studied.
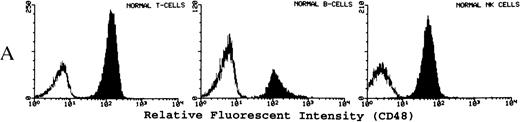
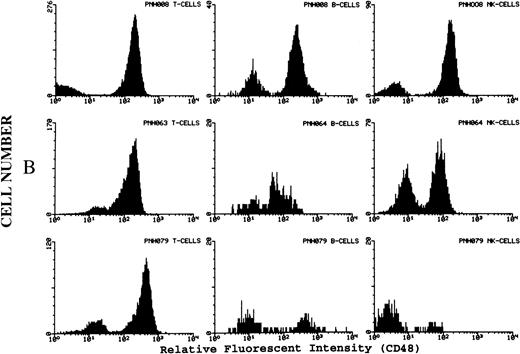
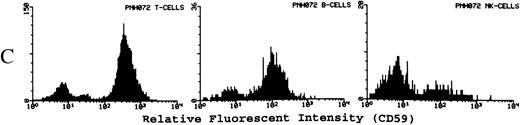
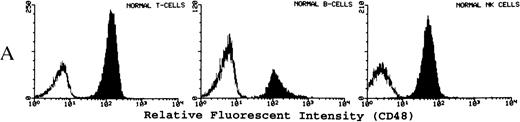
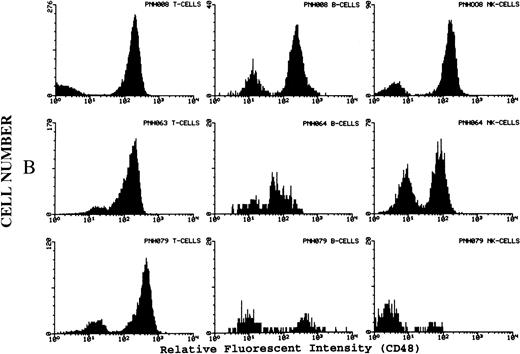
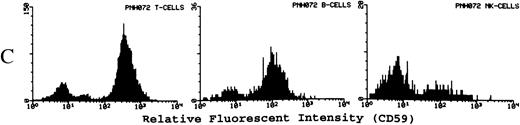
(A) Representative fluorescence histogram profiles of the expression of the GPI-anchored membrane protein CD48 (solid peaks) by normal peripheral blood T cells, B cells, and NK cells. Negative control antibody staining is shown by the clear histogram peaks. No GPI-deficient cells were detected amongst normal lymphocytes. (B) Representative staining profiles of CD48 expression by T cells, B cells, and NK cells from three patients with PNH. Individual histograms show clear bimodal distributions, with GPI-deficient and GPI+ populations readily discernible in all cases. Two particular features are noteworthy: (1) The lymphocytes from case PNH063 show a partial GPI-deficiency. This is similar to their partially deficient (type II) RBCs. (2) In two of the three patients the proportion of GPI-deficent NK cells is much higher than the proportions of B and T cells. (C) For comparison, typical CD59 histogram profiles of lymphocyte subpopulations for case PNH072 are shown. Again, the proportion of NK cells that are GPI-deficient exceeds that of B and T cells.
(A) Representative fluorescence histogram profiles of the expression of the GPI-anchored membrane protein CD48 (solid peaks) by normal peripheral blood T cells, B cells, and NK cells. Negative control antibody staining is shown by the clear histogram peaks. No GPI-deficient cells were detected amongst normal lymphocytes. (B) Representative staining profiles of CD48 expression by T cells, B cells, and NK cells from three patients with PNH. Individual histograms show clear bimodal distributions, with GPI-deficient and GPI+ populations readily discernible in all cases. Two particular features are noteworthy: (1) The lymphocytes from case PNH063 show a partial GPI-deficiency. This is similar to their partially deficient (type II) RBCs. (2) In two of the three patients the proportion of GPI-deficent NK cells is much higher than the proportions of B and T cells. (C) For comparison, typical CD59 histogram profiles of lymphocyte subpopulations for case PNH072 are shown. Again, the proportion of NK cells that are GPI-deficient exceeds that of B and T cells.
Expression of GPI-linked proteins by B lymphocytes in patients with PNH.
Studies of B lymphocyte populations identified by flow cytometry characteristics of low SSC, and CD19 positivity revealed GPI− populations in 16 patients, with 2 showing partially deficient populations. The percentage of GPI-deficient cells ranged from 0.8% to as high as 58.9% (Fig 2 and Table 4). No GPI-deficient B cells were found in the normal controls studied. Representative patient and control histograms of CD48 and CD59 expression are shown in Fig 3.
Expression of GPI-linked proteins by NK cells.
Using a novel gating strategy to identify NK cells, GPI− populations were found in 16 of the 17 cases studied. The results ranged from 0.1% to 95% GPI-deficient cells (Fig2 and Table 4). The two patients that also showed partially GPI-deficient B-cell and/or T-cell populations also showed similar partially deficient patterns of expression for NK cells. No GPI− populations were found amongst normal donor NK cells studied by the same methods. Representative patient and control histograms of CD48 and CD59 expression are shown in Fig 3. For individual patients the percentage of GPI-deficient NK cells was generally much higher than the percentage of GPI-deficient T cells and B cells (Fig 2 and Table 4).
DISCUSSION
The investigation of peripheral blood lymphocyte subsets by flow cytometry provides important information regarding the status of the peripheral cellular immune system. What it does not provide is any information regarding kinetics of cell production and destruction. For normal healthy individuals, absolute numbers of lymphocyte subpopulations and levels of activation antigen expression fall within tightly defined references ranges, and values outside these are found in a range of disorders including infection, inflammatory conditions, auto-immune disease, and malignancy. Results from this study clearly show that lymphocyte subset abnormalities are a common feature of patients with PNH. Low absolute numbers of B cells and NK cells were the most frequent abnormalities detected (95% of patients), along with a mild reduction in CD4+ T cells in 53% of patients. There is little published data on lymphocyte subsets in PNH, though low levels of circulating B cells and NK cells have been described previously.25,26 These specific lymphocyte subset abnormalities are not exclusive to PNH because similar findings have been reported in patients with aplastic anemia.27-29 There is no clear explanation for these abnormalities, though they may simply reflect the general reduction in normal hematopoietic activity associated with PNH. However, they highlight that cells of both innate (NK cell) and specific (T and B cell) immune systems show quantitative abnormalities. Whether these reductions are accompanied by any functional impairment, clinical evidence of cellular or humoral immunodeficiency, and subsequent increased susceptibility to infection is unclear. However, none of the patients has suffered an excessive number or severity of infections.
The second part of this study examined whether the PNH abnormality was detectable in peripheral blood lymphocytes. Although previous studies have unequivocally shown GPI-deficient B and T cells, the extent of involvement of the NK cell lineage in vivo is less certain.25 30 By applying a more precise gating strategy to identify NK cells than has been previously used, we have clearly shown the presence of GPI-deficient NK cells, and more significantly that they comprise a significant proportion of total NK cells. Similarly, a novel and precise multiparameter gating strategy was used to examine expression of two GPI-linked antigens (CD48 and CD59) on peripheral blood B cells, T cells, and T-cell subpopulations. Results showed that in a high proportion of PNH patients all lymphoid cells comprised a chimera of normal and PNH clones. The proportion of GPI-deficient cells within individual lymphocyte lineages was highly variable, though in 81% of patients investigated with active disease, the percentage of GPI-deficient NK cells was considerably higher than the percentage of GPI-deficient B cells and T cells. The remaining cases showed either NK cells alone affected or higher proportions of GPI-deficient T and B cells than NK cells. The patient who had undergone spontaneous remission of PNH had no detectable granulocyte or RBC PNH clones, but significant proportions of GPI-deficient T and B cells were detected. In addition, partially deficient lymphocytes were detected in only two of the seven patients with type II (partially deficient) RBCs.
To offer a potential explanation for these findings it is firstly necessary to review current concepts of lymphopoiesis and contributory factors affecting development and production of lymphocytes. The evidence for a common lymphoid progenitor cell is very strong, as mice deficient for the stem cell transcription factor Ikaros produce cells of the myeloid lineage but no T, B, or NK cells.31Cell-culture studies suggest that B progenitors diverge from the developmental pathway earlier than T and NK cells, further differentiation of the lymphoid precursor cell produces a bipotential T/NK precursor.32 At this point, commitment to T-cell or NK-cell lineage either requires migration to the thymus or remaining in the bone marrow, respectively, though a murine thymic progenitor cell can generate T, B, and NK cells (reviewed in detail by Spits et al).33 More recent studies in mice have identified a common lymphoid progenitor cell in the bone marrow that has no self-renewal capacity but can generate T, B, and NK cells.34 A critical component of B and T lymphoid development is generation of antigen receptor diversity together with removal of potentially harmful autoreactive cells created by this process. For B cells the removal of autoreactive cells occurs in bone marrow, and up to 90% of newly produced IgM+ B cells survive for only a short period of time. Similarly, there is considerable intrathymic T-cell death with only small numbers of naive T cells surviving the selection process. NK precursors are thought to differentiate in the bone marrow relatively unhindered into mature NK cells and develop their limited receptor diversity by an alternative, less understood mechanism.35 Therefore, for individual PNH patients in whom the majority of hematopoiesis is derived from stem cells with a PIG-A mutation, the probability of a PNH lymphoid progenitor producing a mature, naive GPI-deficient B cell or T cell is inherently low due to high levels of ineffective lymphopoiesis and negative selection processes. For NK cells the opposite is true, as their precursors and differentiation pathways are not subject to the same levels of scrutiny as B and T cells. However, if a naive GPI-deficient B or T cell survives the differentiation and selection process, it will have the capacity to remain in the peripheral lymphoid system for many years. This latter point in particular is supported by the results from patient PNH030 and other reports of patients in long-term clinical remission for PNH with GPI-deficient T-and B-lymphocyte populations but no detectable granulocyte or RBC clones.21 22
As many patients often have prolonged periods of illness before the onset of the characteristic pathology of PNH, it is not possible to predict the exact time point at which the PIG-A mutation occurs or when the progeny of the GPI-deficient stem cell begins to become detectable. We are currently undertaking serial studies on patients to develop a model of cell kinetics in PNH and to analyze whether these can be used to predict the clinical course of the disease. We propose that when PNH stem cells become the predominant source of hematopoietic elements, the myeloid/erythroid series is always the first to be affected, and this leads to the characteristic pathology of severe hemolytic anemia and/or aplasia. Then, depending on the degree of lymphopoietic activity, GPI-deficient lymphocytes emerge, beginning with NK cells and followed eventually by B and T cells. Similarly, if recovery from PNH occurs with restoration of hematopoiesis, the myeloid series will be the first to recover, followed by NK cells. If GPI-deficient T- and B-cell populations were present, then because of their long life span, they would remain detectable for many years. However what is not known is what effect immunosuppressive therapy and/or deficiency of GPI antigens from the lymphocyte membrane has on lymphopoiesis.
To test this model, we are undertaking long-term studies of these patients and also studying cases in which clinical remission of PNH has occurred. It may be that sequential studies of these patients, and particularly the composition of lymphocytes, may provide a better understanding of the natural history and kinetics of the disease. An additional dividend is the probability that the results will also provide important insights into in vivo adult lymphopoiesis.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support of Cymbus Biosciences for generous donation of monoclonal antibodies used in this study. We also thank Drs J. Yin, R.G. Hughes, M. Layton, M. Laffan, L.A. Parapia, M. Hill, D. Bareford, and M. Hamilton for providing patient samples.
Address reprint request to Stephen J. Richards, PhD, Haematological Malignancy Diagnostic Service, The Algernon Firth Building, Leeds General Infirmary, Leeds, LS1 3EX, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.