Abstract
Monoclonal antibodies (MoAbs) that selectively identify Muc-1 core protein (MoAbs DF3-P, VU-4H5) determinants were used to identify the Muc-1 glycoform present on 7 multiple myeloma (MM) cell lines, 5 MM patient plasma cells, 12 MM patient B cells, as well as 32 non-MM cell lines and normal hematopoietic cells. Flow cytometry studies demonstrated that all MM cell lines, MM patient plasma cells, and MM patient B cells expressed Muc-1 core protein epitopes. Circulating B cells from 4 normal donors also expressed Muc-1 core protein. In contrast, Muc-1 core protein was absent on 28 of 32 non-MM neoplastic cell lines, 17 of which expressed Muc-1. Splenic and tonsillar B cells, CD34+ stem cells, resting T cells, and bone marrow plasma cells obtained from normal donors both lacked Muc-1 glycoforms. We next studied the effects of estrogen, progesterone, and glucocorticoid receptor agonists and antagonists on Muc-1 expression, because consensus sequences for the response elements of these steroids are present on the Muc-1 gene promoter. These studies showed that dexamethasone (Dex) induced Muc-1 expression on MM cell lines, as determined by both flow cytometry and Western blot analyses. Dex also induced upregulation of Muc-1 on prostate and ovarian cancer cell lines. Time and dose-response studies demonstrated that Dex induced maximal cell surface Muc-1 expression by 24 hours at concentrations of 10−8 mol/L. Dex induced Muc-1 upregulation could be blocked with a 10-fold excess of the glucocorticoid receptor antagonist RU486, confirming that Dex was acting via the glucocorticoid receptor. No changes in Muc-1 expression were observed on MM cells treated with estrogen and progesterone receptor agonists and antagonists or with RU486. These studies provide the framework for targeting Muc-1 core protein in vaccination and serotherapy trials in MM. In addition, the finding that Muc-1 expression on MM cells can be augmented by Dex at pharmacologically achievable levels suggests their potential utility in enhancing treatments targeting Muc-1 in MM.
ALTHOUGH MULTIPLE myeloma (MM) is sensitive to chemotherapy with a median survival 3 to 4 years, most patients are not cured and eventually succumb to MM. However, MM cells express both patient-specific (ie, idiotype) and myeloma-associated antigens that are ideal targets for immunotherapy. One such MM-associated antigen is the core protein of MUC-1 (polymorphic epithelial mucin, epithelial membrane antigen, DF3 antigen).1 Muc-1 is normally present on the luminal surface of secretory glands as a highly glycosylated transmembrane protein. Its extracellular domain is made up of 20 amino acid tandem repeats, with a variable number (range, 21 to 125) of copies within one Muc-1 molecule.2 The variable number of tandem repeat (VNTR) domain of Muc-1 is immunogenic: most of the 56 monoclonal antibodies (MoAbs) submitted to the TD-4 workshop bound to epitopes within the VNTR domain.3,4 The specificity of antibody binding to the VNTR domain depends largely on the extent of glycosylation of Muc-1.5-7 In contrast to normal glandular tissues, adenocarcinomas of the breast, ovary, pancreas, and lung express Muc-1 in an aberrant, hypoglycosylated form.1 Hypoglycosylated variants of Muc-1 appear to arise from mutations in glycosylation enzymes, resulting in the addition of shorter and less branched carbohydrate chains to the Muc-1 core protein backbone.8These differences in the glycosylation patterns of Muc-1 have permitted the identification of MoAbs that distinguish its various glycoforms.3-7
Muc-1 is defined as an epithelial antigen. It is also expressed on MM cell lines, fresh patient MM cells, and plasmacytomas,9-13as well as other hematological malignancies, including Ki-1–positive B-cell lymphomas, T-cell lymphomas, Hodgkin’s disease, and malignant histiocytosis.9,10,14-16 The function of Muc-1 is not fully understood. Potential roles as an immunostimulatory12,17,18 or as an immunoinhibitory19,20 molecule have been proposed. Furthermore, Muc-1 binds to intercellular adhesion molecule-1 (ICAM-1), suggesting a role in cellular migration.21 Moreover, previous studies have identified consensus sequences for steroid response elements for estrogen, progesterone, and glucocorticoids on the Muc-1 promoter.22,23 Although a regulatory role for ovarian steroids on MUC-1 expression in mouse24and baboon25 uterus has been reported, no studies have yet examined the effect of steroids on MUC-1 expression on human cells.
In the present studies, we characterized the glycoform expression of Muc-1 on freshly obtained MM patient plasma cells and MM cell lines, as well as on normal donor plasma cells. Moreover, we examined Muc-1 glycoform expression on MM cells, as well as on hematologic and carcinoma cell lines. Because the MM clone extends from pre-B cells,26 sIgM+ preswitched B cells,27,28 to late stage B cells,29 30 we also studied Muc-1 glycoform expression on B cells from MM patients as well as normal donors. Given the steroid response elements on the Muc-1 promoter, we also examined the effect of treatment with estrogen, progesterone, and glucocorticoid receptor agonists and antagonists on Muc-1 expression on the cell surface of human MM cells. These studies demonstrate that MM plasma cells and B cells express Muc-1 core protein determinants and that cell surface expression of Muc-1 can be upregulated by dexamethasone. Our data therefore suggest the potential utility of incorporating glucocorticoids into immunotherapeutic treatment strategies targeting Muc-1 in MM.
MATERIALS AND METHODS
Cell lines and culture conditions.
ARH-77, CESS, CMK, K562, MCF.7, RPMI 8226, U266, and ZR-75 cell lines were obtained from the American Type Culture Collection (Rockville, MD). OCI My-5 cells31 were kindly provided by Dr H.A. Messner (Ontario Cancer Institute, Toronto, Ontario, Canada); interleukin-6 (IL-6)–transfected S6B45 MM cells32 were kindly provided by Dr T. Kishimoto (Osaka University, Osaka, Japan). Jurkat, B-15, NALM 6, HZ, DHL6, DHL16, and RL cell lines were provided by Dr Lee Nadler (Dana-Farber Cancer Institute, Boston, MA). L5174T, SW480, HUTU80, HS294T, and AC5 cell lines were obtained from Dr Selwyn Broitman (Boston University Medical School, Boston, MA). AGSB gastric, T.Tu esophageal, and CAPAN-2 pancreatic cancer cells were provided by Drs Robert Hennihan, Anil Rustgi, and Andrew Warshaw, respectively (Massachusetts General Hospital, Boston, MA). 36M, CAOV-3, and OVC3 ovarian cancer cells were obtained from Dr Stephen Cannistra (Dana-Farber Cancer Institute). Lastly, DU145 and PC3 prostate cancer cells were kindly provided by Dr Arthur Pardee (Dana-Farber Cancer Institute). All MM (except as noted below), acute lymphocytic, non-Hodgkin’s lymphoma (NHL), lymphoblastic, leukemic, megakaryocytic, prostatic, and 786-0 renal cancer cell lines were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY). OCI-My5 MM cells were cultured in Iscove’s Modified Dulbecco’s Medium (GIBCO). Breast, colon, duodenal, esophageal, gastric, melanoma, ovarian, A458, and ACHN renal cancer cell lines were cultured in Dulbecco’s Modified Eagle’s Medium. CAPAN-2 pancreatic and Caki-1 renal cancer cells were cultured in McCoy’s 5A medium. All media contained 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and was supplemented with L-glutamine, penicillin, and streptomycin (GIBCO).
Patients.
Peripheral blood (PB) and/or bone marrow (BM) was obtained from 17 patients with MM after informed consent was obtained. MM patients included those at diagnosis, during intermittent chemotherapy, in stable phase, and in relapse. Peripheral blood was obtained from 6 healthy normal volunteer donors, and BM was obtained from 4 donors with nonmalignant disease, termed normal for the purpose of this study.
Antibodies.
DF3 and DF3-P MoAbs were the kind gift of Dr Donald Kufe (Dana-Farber Cancer Institute). The antigenic determinants for these antibodies have been previously characterized.7 VU-3C6 and VU-4H5 MoAbs were purified at our institution from hybridomas kindly provided by Dr J. Hilgers (Vrije Universiteit, Amsterdam, The Netherlands); the epitopes targeted by these antibodies have been extensively characterized.3,4 DF3 and VU-3C6 MoAbs bind to both Muc-1 glycosylated and core protein epitopes, whereas DF3-P and VU-4H5 MoAbs specifically bind to core protein epitopes within the VNTR domain of Muc-1.3,4 B4-FITC (CD19) MoAb was obtained from Coulter (Hialeah, FL). FMC63 (CD19) MoAb33 was conjugated to fluorescein isothiocyanate (FITC). Anti-CD38 (Leu17-phycoerythrin [PE]) MoAb was purchased from Becton Dickinson (San Jose, CA). FMC44 (CD45RA) MoAb34 was conjugated to FITC or PE and antihuman Ig (goat antihuman Ig [H+L]-FITC), and goat antimouse Ig-PE were purchased from Southern Biotechnology (Birmingham, AL).
Phenotypic analyses of cell lines and immunofluorescence.
MM and other neoplastic cell lines were characterized by flow cytometry for cell surface Muc-1 expression. Cells (106) were incubated with either anti–Muc-1 or control MoAbs (5.0 μg) for 45 minutes. Cells were then washed with phosphate-buffered saline (PBS) and incubated for 45 minutes with 2.0 μg of goat antimouse fluorescein-conjugated MoAb (Coulter). Cells were next washed and fixed with 1% formaldehyde PBS. Staining intensities were defined by comparing differences in log fluorescence for anti–Muc-1 MoAbs versus isotype control MoAbs, using the following criteria: (0) no difference; (+) <1 log fold difference ; (++) ≥1 but < 2 log difference; and (+++) ≥2 log difference.
Phenotypic analysis of patient cells and immunofluorescence.
PB or BM aspirates were collected into heparinized vacutainer tubes and centrifuged over a ficoll-hypaque gradient (Pharmacia, Uppsala, Sweden). To identify plasma cells from patients or normal donors, BM mononuclear cells (MC) were stained in three-color immunofluorescence using CD38-FITC and CD45RA-PE together with Muc-1 MoAbs or isotype-matched control MoAbs (indirect immunofluorescence). Files were gated for CD38hi45RA− cells and the staining for Muc-1 epitopes plotted as a histogram as compared with an identically gated sample stained with an isotype-matched control MoAb. For PBMC samples, cells were stained in two-color immunofluorescence using CD19-FITC and Muc-1 or isotype-matched control MoAb (indirect immunofluorescence). The number of CD19+ B cells in MM patients and normal donors is identical when detected using either FMC63 or with B4 MoAb. Files were gated for CD19 cells and side scatter, and the staining for Muc-1 epitopes was plotted as a histogram as compared with identically gated isotype-matched control samples. For MM PBMC, CD19+ B cells as defined here have been previously shown to express CD19 and IgH transcripts with an IgH VDJ sequence identical to that of autologous BM plasma cells.33,35,36Normal T cells were isolated from PBMC using the E-Rosette technique.37 The purity of isolated T cells was confirmed by flow cytometric analysis for CD3 expression. CD34+ cells were isolated from the PB of healthy donors using Ceprate CD34 columns (Cell Pro, Bothell, WA).38
Preparation of steroid agonists and antagonists.
17β estradiol (E2), tamoxifen (TAM), progesterone (PRG), RU486, and water-soluble dexamethasone (DEX) were obtained from Sigma Chemical (St Louis, MO). ICI 182,780 (ICI) was the kind gift from Zeneca Pharmaceuticals (Wilmington, DE). Stock solutions (10−2 mol/L in absolute alcohol) of E2, TAM, PRG, and RU486 were stored at −20°C. Water-soluble Dex was solubilized in PBS at a 10−2 mol/L stock solution and stored at 4°C. Cells were cultured for 24 to 72 hours with varying concentrations of steroids, which were freshly prepared for each experiment.
Immunoblotting assays for MUC-1.
Cell extracts from MM cell lines (RPMI 8226 and S6B45), breast cancer cell lines (MCF.7 and ZR-75), and prostatic cancer cells (DU145) were examined by Western blot analysis. Cells were lysed by incubation for 30 minutes in ice-cold lysis buffer (50 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 0.1% Nonidet P-40, 1 mmol/L EDTA, 5 μg/mL phenylmethyl sulfonyl fluoride [PMSF], 1 mmol/L Na3VO4, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 50 mmol/L NaF). Aliquots of lysates were electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was then transferred onto nitrocellulose membranes, and nonspecific binding was blocked by overnight incubation with 5% skim milk. Subsequently, the membranes were washed with TBS-Tween and incubated with either VU-3C6, VU-4H5, DF3, or DF3-P MoAbs (5 μg; diluted 1:200). DMA1 anti–α-tubulin MoAb (1 μg; diluted 1:1,000) was used as a control antibody. An enhanced chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL) was used to visualize bound antibodies.
RESULTS
Expression of Muc-1 core protein on MM and other neoplastic cell lines.
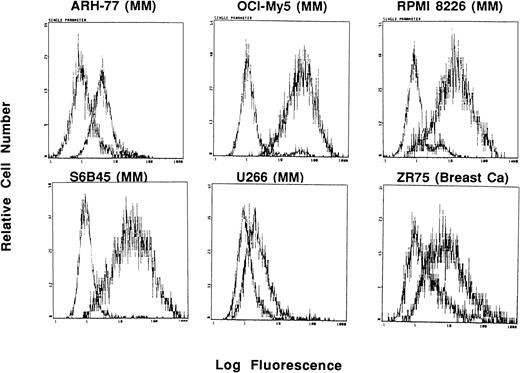
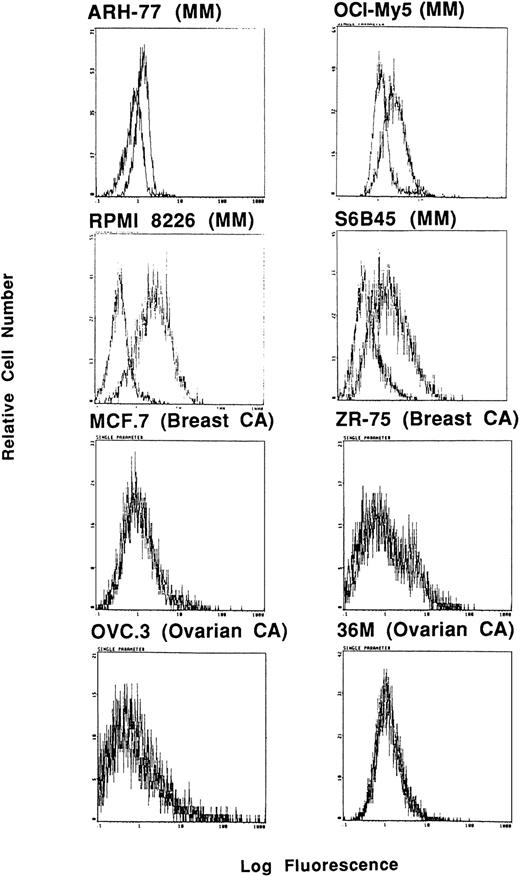
In these studies, Muc-1 expression on 7 human MM cell lines, along with 32 other neoplastic cell lines, was defined by staining with MoAbs that bind Muc-1 glycosylated and core protein epitopes (VU-3C6, DF3) as well as MoAbs binding specifically to Muc-1 core protein (VU-4H5, DF3-P) in flow cytometric analysis. These studies demonstrated that 7 of 7 MM lines (ARH-77, HS Sultan, IM9, OCI My-5, RPMI 8226, S6B45, and U266) expressed Muc-1 on their cell surface, as evidenced by reactivity with the VU-3C6 MoAb. The intensities of Muc-1 expression using the VU-3C6 MoAb were as follows: low (U266), medium (ARH-77, HS Sultan, IM9), and high (OCI My-5, RPMI 8226, S6B45) (Fig 1and Table 1). These results paralleled those obtained with the DF3 MoAb, which identified Muc-1 on 6 of 7 MM lines (Table 1). In particular, DF3 staining was absent on HS Sultan cells, but was present on the remainder of MM cell lines with the following staining patterns: low (ARH-77, IM9) and high (OCI My-5, RPMI 8226, S6B45, U266) (Table 1). Muc-1 was also identified by either VU-3C6 or DF3 MoAbs on 17 of 32 non-MM neoplastic cell lines: Jurkat ALL; HZ NHL; MCF.7 and ZR-75 breast; CESS B-cell lymphoblasts; SW480 colon; T.Tu esophageal; CMK megakaryocytic; 36M, CAOV-3 and OVC3 ovarian; Capan-2 pancreatic; DU145 and PC3 prostatic; as well as A498, ACHN, and Caki-1 renal cancer cells. The intensities of Muc-1 staining among non-MM, Muc-1–positive cells was strongest on the breast, esophageal, ovarian, pancreatic, prostatic, and renal cancer cell lines (Table 1).
Expression of Muc-1 on human MM and breast cancer cell lines. Human MM (ARH-77, OCI My-5, RPMI 8226, S6B45 and U266) and breast cancer (ZR-75) cell lines were examined by flow cytometry for cell surface expression of Muc-1 glycosylated protein by staining with the VU-3C6 MoAb relative to an isotype control MoAb.
Expression of Muc-1 on human MM and breast cancer cell lines. Human MM (ARH-77, OCI My-5, RPMI 8226, S6B45 and U266) and breast cancer (ZR-75) cell lines were examined by flow cytometry for cell surface expression of Muc-1 glycosylated protein by staining with the VU-3C6 MoAb relative to an isotype control MoAb.
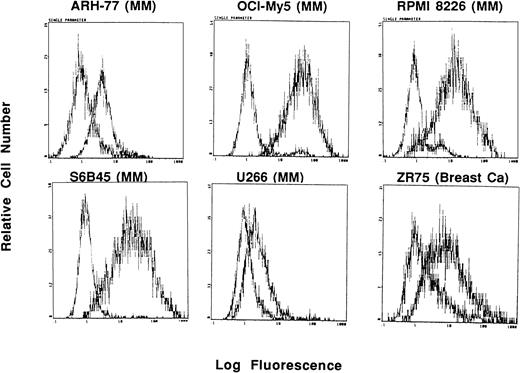
Interestingly, expression of Muc-1 core protein, determined by reactivity with the VU-4H5 and DF3 MoAbs, was more selective for MM cell lines (Fig 2 and Table 1). Using the VU-4H5 MoAb, Muc-1 core protein was detected on the cell surface of 7 of 7 MM cell lines with the following staining intensities: low (ARH-77, HS Sultan, OCI My-5, U266) and medium (IM9, RPMI 8226, S6B45) (Table 1). In addition, Muc-1 core protein was identified on 4 of 7 MM cell lines using the DF3-P MoAb with the following patterns of reactivity: low (U266, OCI My-5, S6B45) and medium (RPMI 8226) (Table1). In contrast to MM cells, 28 of 32 (88%) of non-MM neoplastic cell lines, including MCF.7 and 2R-75 breast as well as OVC.3 and 36M ovarian cell lines, lacked Muc-1 core protein, as determined by reactivity with the VU-4H5 or DF3-P MoAbs. Three of 4 non-MM cell lines that expressed Muc-1 core protein were of B-cell lineage, akin to the origin of MM cells, whereas the fourth cell line was CAPAN-2, which is a pancreatic cancer cell line. Expression of Muc-1 core protein in these 4 non-MM cell lines was of low intensity. As can be seen in Table1, expression of Muc-1 core protein was absent on breast, esophageal, ovarian, prostatic, and renal cancer cell lines, all of which expressed Muc-1 by use of the VU-3C6 and DF3 MoAbs.
Expression of Muc-1 core protein on human MM, breast cancer, and ovarian cancer cell lines. Human MM (ARH-77, OCI My-5, RPMI 8226, and S6B45), breast cancer (MCF.7 and ZR-75), and ovarian cancer (OVC.3 and 36M) cell lines were examined by flow cytometry for cell surface expression of Muc-1 core protein by staining with the VU-4H5 MoAb relative to an isotype control MoAb.
Expression of Muc-1 core protein on human MM, breast cancer, and ovarian cancer cell lines. Human MM (ARH-77, OCI My-5, RPMI 8226, and S6B45), breast cancer (MCF.7 and ZR-75), and ovarian cancer (OVC.3 and 36M) cell lines were examined by flow cytometry for cell surface expression of Muc-1 core protein by staining with the VU-4H5 MoAb relative to an isotype control MoAb.
Expression of Muc-1 core protein on plasma cells from MM patients and normal donors.
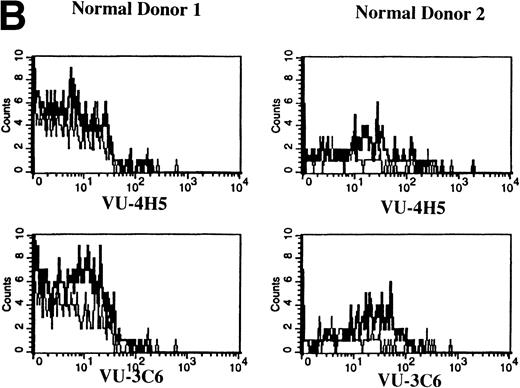
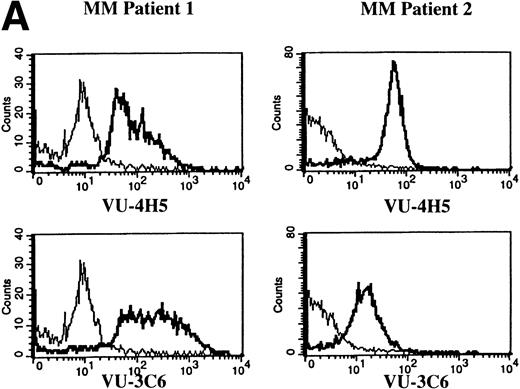
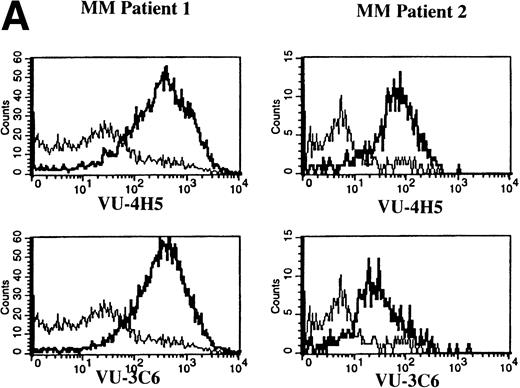
To assess Muc-1 expression on freshly isolated plasma cells from MM patients, BMMC from 5 MM patients were stained in multicolor immunofluorescence using CD38, CD45RA, and MoAbs that detect both Muc-1 glycosylated and core protein epitopes (VU-3C6) or specifically identify core protein (VU-4H5). Of CD38hi45RA− plasma cells from 5 of 5 MM patients, 55% ± 12% (range, 13% to 78%) express Muc-1 core protein (Fig 3 and Table 2). By use of the VU-3C6 MoAb, Muc-1 epitopes were identified on 38% ± 13% (range, 8% to 84%) of MM plasma cells from these 5 MM patients. In contrast, for 4 of 4 donors, normal plasma cells had very low or no expression of any Muc-1 glycoform using either MoAb (Fig 3 and Table 2).
Expression of Muc-1 core protein on MM patient and normal donor plasma cells. BM plasma cells from 2 representative MM patients (A) and 2 representative normal donors (B) are shown. Files were gated for CD38hi45RA− plasma cells (15% to 87% and 1% to 5% were present in MM patients and normal donors, respectively). Muc-1 on plasma cells was plotted as a histogram, with bold lines indicating staining with the VU-4H5 or VU-3C6 anti–Muc-1 MoAbs and the thin lines representing staining by an identically gated isotype-matched control MoAb.
Expression of Muc-1 core protein on MM patient and normal donor plasma cells. BM plasma cells from 2 representative MM patients (A) and 2 representative normal donors (B) are shown. Files were gated for CD38hi45RA− plasma cells (15% to 87% and 1% to 5% were present in MM patients and normal donors, respectively). Muc-1 on plasma cells was plotted as a histogram, with bold lines indicating staining with the VU-4H5 or VU-3C6 anti–Muc-1 MoAbs and the thin lines representing staining by an identically gated isotype-matched control MoAb.
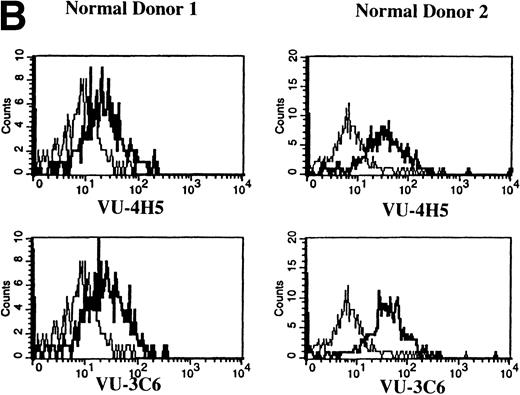
Expression of Muc-1 core protein on B cells from MM patients and normal donors.
Previous work has shown that circulating B cells in MM patients are part of the malignant clone as defined by their expression of clonotypic IgH transcripts.33,35,36 PB B cells from 12 of 12 MM patients were analyzed for expression of Muc-1 epitopes (Fig 4A and Table 2). B cells were defined as CD19+ cells, including those cells with a high side scatter (SSchi).27,36 The SSchi set of MM B cells has been shown to be almost exclusively clonotypic, whereas the SSclow set includes both clonotypic and polyclonal B cells.33 35 The majority of CD19+B cells in MM patients (85% ± 3%) expressed Muc-1 core protein (Fig 4A and Table 2). Both the SSchi and SSclowsets of MM B cells were predominantly Muc-1+ (not shown). Polyclonal B cells (49% ± 10%) from 4 of 4 normal donors, defined as CD19+SSclow PBMC, also expressed Muc-1 core protein (Fig 4B and Table 2). In contrast to normal PB B cells, splenic and tonsillar B cells from 3 of 3 normal donors lacked Muc-1, by virtue of lack of reactivity with the VU-4H5 and VU-3C6 MoAbs (data not shown).
Expression of Muc-1 core protein on MM patient and normal donor B cells. Peripheral blood B cells from 2 representative MM patients (A) and 2 representative normal donors (B) are shown, with Muc-1 staining plotted as a histogram. The bold line shows Muc-1 staining on B cells, and the thin line shows isotype-matched control staining on an identically gated aliquot of cells. Shown are B cells (26% to 32% and 4% to 7% for MM patients and normal donors, respectively), which include SSchi gated PB B cells for MM patients, because these are clonotypic, having IgH and CD19 transcripts.32,34,35 In contrast, SSchi PB B cells are excluded from the B-cell gate in normal donors, because these are monocytes.35
Expression of Muc-1 core protein on MM patient and normal donor B cells. Peripheral blood B cells from 2 representative MM patients (A) and 2 representative normal donors (B) are shown, with Muc-1 staining plotted as a histogram. The bold line shows Muc-1 staining on B cells, and the thin line shows isotype-matched control staining on an identically gated aliquot of cells. Shown are B cells (26% to 32% and 4% to 7% for MM patients and normal donors, respectively), which include SSchi gated PB B cells for MM patients, because these are clonotypic, having IgH and CD19 transcripts.32,34,35 In contrast, SSchi PB B cells are excluded from the B-cell gate in normal donors, because these are monocytes.35
Expression of Muc-1 on normal CD34+ hematopoietic progenitor cells and T cells.
We next examined whether Muc-1 was expressed on CD34+hematopoietic progenitor cells and on resting T cells obtained from 3 normal donors using the VU-4H5 and VU-3C6 MoAbs. Neither PB-derived CD34+ hematopoietic progenitor cells nor resting T cells from normal donors express Muc-1 (data not shown).
Effects of estrogen, progesterone, and glucocorticoid receptor agonists and antagonists action on Muc-1 expression.
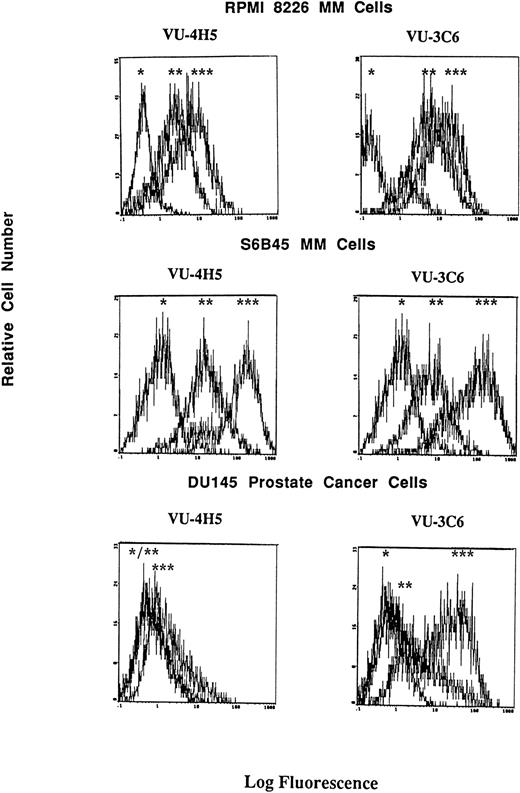
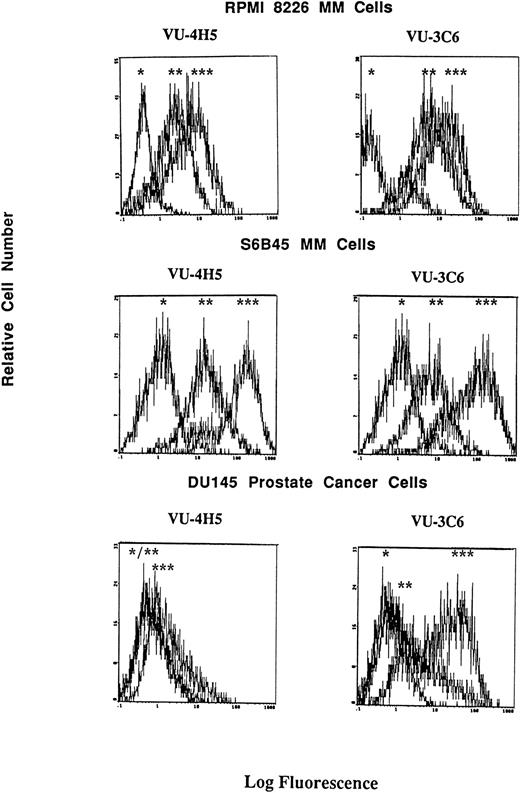
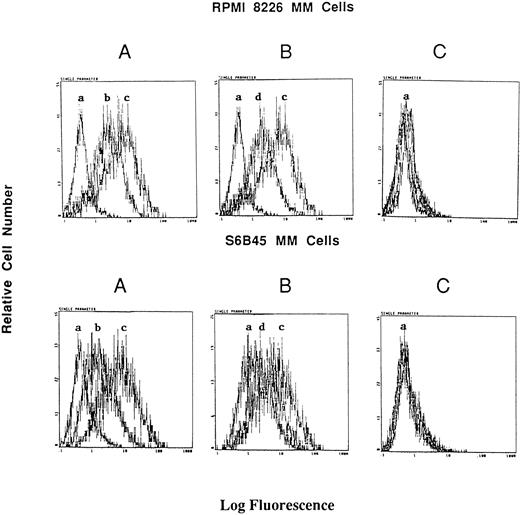
Although consensus sequences of steroid response elements for estrogen, progesterone, and glucocorticoid receptors have been identified on the promoter of the Muc-1 gene,21 26 no functional analyses of these steroid response elements has been reported in human cells. We therefore treated S6B45, RPMI 8226, and OCI My-5 MM cells; MCF.7 and ZR-75 breast cancer cells; CAOV-3 and OVC-3 ovarian cancer cells; and DU145 prostate cancer cells with agonists and antagonists for estrogen (17β estradiol, diethylstilbestrol, tamoxifen, and ICI 182,780), progesterone (progesterone, RU486), and/or glucocorticoid (Dex, RU486) receptors and assayed for related changes in Muc-1 cell surface expression. Dex induced upregulation of Muc-1, as evidenced by reactivity with the VU-4H5, DF3-P, VU-3C6, and DF3 MoAbs, using flow cytometry and/or Western blot analyses of S6B45 and RPMI 8226 (Figs 5 and 6), of OCI My-5 MM cells, of CAOV-3 and OVC-3 ovarian cancer cells, and of DU145 prostate cancer cells (Figs 5 and 6). Dex induction of Muc-1 expression was most pronounced (≥1 log fold increase in fluorescence intensity) on S6B45 and RPMI 8226 MM cells and on DU145 prostatic cancer cells (Fig 5). Dose-response studies using Dex (10−12 to 10−6 mol/L) showed maximal induction of cell surface Muc-1 expression on S6B45 MM (Fig 7) and DU145 prostatic cancer cells at 10−8 mol/L. Dex induction of Muc-1 expression on S6B45, RPMI 8226, and OCI My-5 MM cells, as well as on DU145 prostate cancer cells was also evaluated at several intervals of Dex treatment (24, 48, and 72 hours) and shown to be maximal at 24 hours (data not shown). As can be seen in Fig 8, Dex induction of Muc-1 expression on two MM cell lines (S6B45 and RPMI 8226) was blocked by 10-fold excess of the glucocorticoid receptor antagonist RU486, consistent with a glucocorticoid receptor-mediated mechanism of Muc-1 induction by Dex.
Effect of Dex on cell surface Muc-1 expression. RPMI 8226 MM cells, S6B45 MM cells, and DU145 prostate cancer cells were cultured in media alone or with Dex (10−8 mol/L) for 24 hours. Muc-1 expression was assessed by flow cytometry by staining with the VU-4H5 and VU-3C6 MoAbs relative to isotype control MoAbs. Peaks shown are denoted as follows: (*) staining with isotype control MoAb; (**) staining of cells cultured in media alone using either the VU-4H5 or the VU-3C6 MoAbs; (***) staining of cells cultured with Dex using either the VU-4H5 or the VU-3C6 MoAbs. No change in isotype control MoAb staining was seen with Dex stimulation.
Effect of Dex on cell surface Muc-1 expression. RPMI 8226 MM cells, S6B45 MM cells, and DU145 prostate cancer cells were cultured in media alone or with Dex (10−8 mol/L) for 24 hours. Muc-1 expression was assessed by flow cytometry by staining with the VU-4H5 and VU-3C6 MoAbs relative to isotype control MoAbs. Peaks shown are denoted as follows: (*) staining with isotype control MoAb; (**) staining of cells cultured in media alone using either the VU-4H5 or the VU-3C6 MoAbs; (***) staining of cells cultured with Dex using either the VU-4H5 or the VU-3C6 MoAbs. No change in isotype control MoAb staining was seen with Dex stimulation.
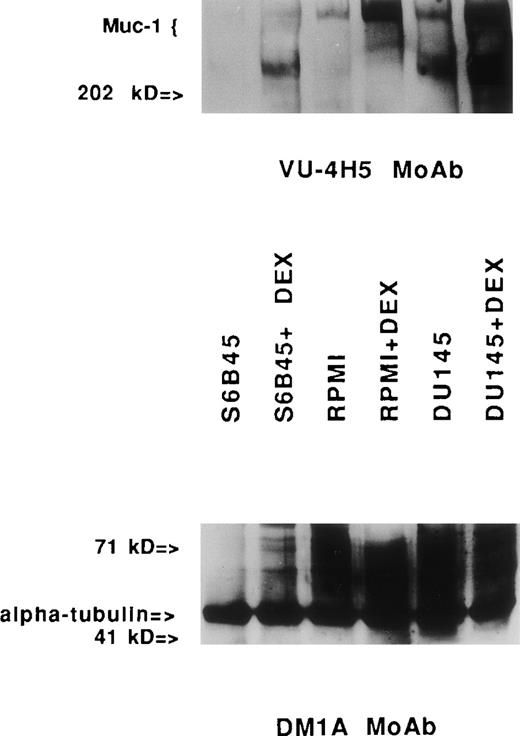
Immunoblotting assays for Muc-1 expression in human MM and prostate cancer cell lines stimulated with Dex. Cell lysates obtained from human MM (S6B45 and RPMI 8226) and prostate cancer (DU145) cells cultured with media alone or with Dex (10−8mol/L) for 24 hours were examined by Western blot analysis using the VU-4H5 MoAb. Dual bands seen in the lysates from S6B45 and DU145 cells show coallelic expression of Muc-1.1 The DMA1 MoAb to -tubulin was used as a control antibody.
Immunoblotting assays for Muc-1 expression in human MM and prostate cancer cell lines stimulated with Dex. Cell lysates obtained from human MM (S6B45 and RPMI 8226) and prostate cancer (DU145) cells cultured with media alone or with Dex (10−8mol/L) for 24 hours were examined by Western blot analysis using the VU-4H5 MoAb. Dual bands seen in the lysates from S6B45 and DU145 cells show coallelic expression of Muc-1.1 The DMA1 MoAb to -tubulin was used as a control antibody.
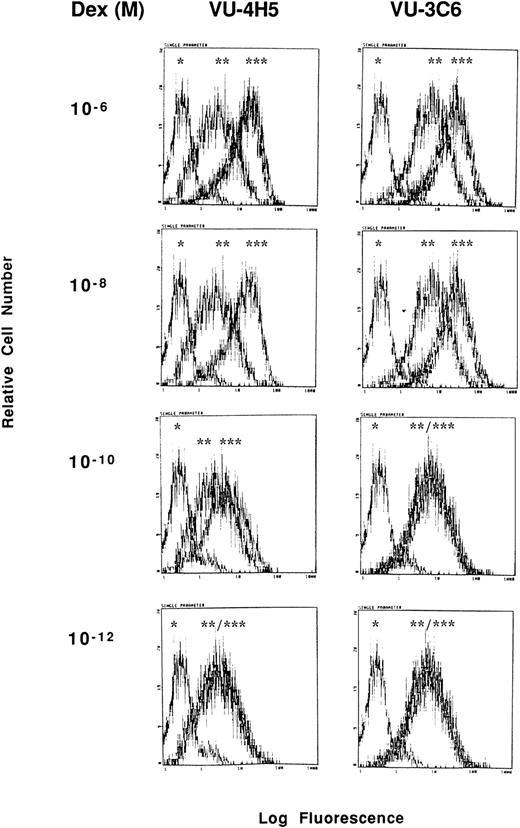
Dose-response relationship for Dex induction of Muc-1 expression on S6B45 MM cells. S6B45 MM cells were cultured for 24 hours with Dex (10−12 to 10−6 mol/L) and analyzed by flow cytometry for Muc-1 expression using VU-4H5, VU-3C6 and isotype control MoAbs. *Cells stained with isotype control. **Cells cultured in media alone and stained with either the VU-4H5 or the VU-3C6 MoAbs. ***Cells cultured with Dex and stained with either the VU-4H5 or the VU-3C6 MoAbs.
Dose-response relationship for Dex induction of Muc-1 expression on S6B45 MM cells. S6B45 MM cells were cultured for 24 hours with Dex (10−12 to 10−6 mol/L) and analyzed by flow cytometry for Muc-1 expression using VU-4H5, VU-3C6 and isotype control MoAbs. *Cells stained with isotype control. **Cells cultured in media alone and stained with either the VU-4H5 or the VU-3C6 MoAbs. ***Cells cultured with Dex and stained with either the VU-4H5 or the VU-3C6 MoAbs.
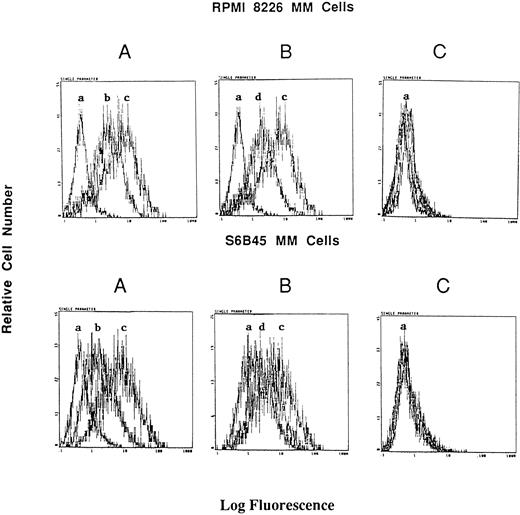
Effect of RU486 on Dex induction of Muc-1 expression on MM cells. S6B45 and RPMI 8226 MM cells were cultured in media alone or with Dex (10−8 mol/L) in the presence or absence of the glucocorticoid receptor antagonist RU486. (A) Changes in Muc-1 expression after culturing with Dex. (B) Changes in Muc-1 expression after culturing with both Dex and RU486. (C) Changes in isotype control MoAb staining in media alone, Dex alone, and Dex plus RU486 cultures. (a) Cells stained with isotype control MoAb. (b) Cells cultured with media alone and stained with the VU-4H5 MoAb. (c) Cells cultured with Dex and stained with the VU-4H5 MoAb. (d) Cells cultured with both Dex and RU486 and stained with the VU-4H5 MoAb.
Effect of RU486 on Dex induction of Muc-1 expression on MM cells. S6B45 and RPMI 8226 MM cells were cultured in media alone or with Dex (10−8 mol/L) in the presence or absence of the glucocorticoid receptor antagonist RU486. (A) Changes in Muc-1 expression after culturing with Dex. (B) Changes in Muc-1 expression after culturing with both Dex and RU486. (C) Changes in isotype control MoAb staining in media alone, Dex alone, and Dex plus RU486 cultures. (a) Cells stained with isotype control MoAb. (b) Cells cultured with media alone and stained with the VU-4H5 MoAb. (c) Cells cultured with Dex and stained with the VU-4H5 MoAb. (d) Cells cultured with both Dex and RU486 and stained with the VU-4H5 MoAb.
No changes in Muc-1 expression were observed on MCF.7 and ZR-75 breast cancer cell lines treated with Dex, as assessed by both FACS and Western blot analyses (data not shown). In addition, treatment of S6B45, RPMI 8226, and OCI My-5 MM cells; MCF.7 and 2R75 breast cancer cells; and DU145 prostate cancer cells with estrogen and progesterone receptor agonists and antagonists, or alone with the glucocorticoid receptor antagonist RU486, did not alter Muc-1 cell surface expression (data not shown).
DISCUSSION
The use of immunotherapy for the treatment of MM has previously targeted cell surface antigens on MM cells such as CD16, CD38, CD54, and HM1.24.39-45 Unfortunately, these antigens are present (CD16, CD38, CD54) or have not been fully examined (HM1.24) on normal tissues. In these studies, we have identified Muc-1 core protein as a selective target for MM-directed immunotherapy. Muc-1 is a suitable target for immunotherapy, because epitopes that give rise to antibody and cytotoxic T-lymphocyte reactivity may be repeated 21 to 125 times within one molecule of Muc-1 due to genetic polymorphisms.1Both antibody46,47 and cytotoxic T-cell responses13,17,18 specific to Muc-1 have been observed in patients with Muc-1–bearing malignancies, and these responses can be elicited by vaccination with Muc-1 conjugated to mannan or keyhole limpet hemocyanin (KLH).48,49 The latter studies are of a phase I nature and show that Muc-1 vaccinations appear to be well tolerated.49 Although no clinical response data have so far been generated from those cancer patients immunized with Muc-1 vaccines, considerable preclinical data using tumor rejection models have shown that Muc-1–directed vaccinations are biologically active. Specifically, vaccinations against Muc-1 using Muc-1 cDNA,50 recombinant vaccinia virus containing Muc-1 gene sequences,51 Muc-1-KLH conjugated protein,52 or fusion cells made up of dendritic cells and Muc-1–bearing tumors53 can prevent establishment and dissemination of inoculated Muc-1–bearing tumors, as well as induce rejection of established Muc-1–positive tumors in mice. Muc-1 may also serve as a target for serotherapy.54,55 Athymic mice xenografted with Muc-1–bearing ovarian tumors show preferential tumor uptake of I125–conjugated DF3 MoAb.54 Moreover, the anti–Muc-1 MoAb HMFG1 conjugated to yttrium-90 has been used in phase I/II clinical trials involving ovarian cancer patients, with benefit suggested when used in an adjuvant setting in patients with no evident disease after surgery.55
The presence of Muc-1 on the cell surface of MM cells has previously been identified by several investigators.9-13 However, these studies did not identify the Muc-1 glycoform present on MM cells. Because glycosylation patterns of Muc-1 vary among normal and malignant tissues, knowledge of the Muc-1 glycoform present on MM cells may be important in identifying selective epitopes to serve as targets for immunotherapy. In these studies, we have demonstrated that Muc-1 core protein is more selectively expressed on MM cell lines, on patient MM plasma cells and B cells, and on certain (ie, circulating) normal donor B cells. In contrast, Muc-1 core protein is absent on 28 of 32 (88%) non-MM neoplastic cell lines, including breast, esophageal, prostate, ovarian, and renal cancer cell lines that express Muc-1 in a glycosylated form. Moreover, a survey of CD34+ cells, resting T cells, splenic and tonsillar B cells, and plasma cells from normal donors did not show cell surface expression of Muc-1 core protein. Lastly, by immunohistochemistry normal human small intestine (VU-4H5), colon (VU-4H5), and breast (DF3-P, VU-4H5) tissues do not show reactivity when stained with the anti–Muc-1 core protein MoAbs used in these studies.7,56 Taken together, the above-described studies demonstrate that MM plasma and B cells, as well as circulating normal B cells, selectively express Muc-1 core protein on their cell surface. The finding that circulating MM B cells, in addition to plasma cells, express Muc-1 core protein is of particular relevance for Muc-1–directed immunotherapy strategies for MM, because MM B cells may be both clonotypic and act as a reservoir of chemotherapy resistance.26-30 35
The significance of Muc-1 expression on MM plasma and B cells, along with circulating normal B cells, remains unclear at this time. A role in the migration of Muc-1–bearing malignant cells has previously been suggested, because Muc-1 binds to ICAM-1.21 Such binding may make it possible for Muc-1–bearing MM plasma cells and B cells to adhere to endothelium and bone marrow stroma expressing ICAM-121,57 or possibly another yet to be defined ligand(s). MM cells are known to make cytokines, including IL-1β and tumor necrosis factor-α (TNF-α),58,59which are potent inducers of ICAM-1 expression60,61 and may stimulate ICAM-1 on endothelial and bone marrow stromal cells. Moreover, Kaposi’s sarcoma-associated herpesvirus (KSHV), which may play a role in the pathogenesis of MM by infecting BM stromal cells,62 may lead to cytokine elaboration and induction of ICAM-1 expression on stromal cells, analogous to that seen in Kaposi’s sarcoma.61,63 In turn, binding of MM cells via Muc-1 to ICAM-1 on endothelial and BM stroma cells may lead to the release of IL-6, an important growth factor for MM cells.64 In addition, a role for Muc-1 in mediating evasion from immune systems has been suggested by several studies, wherein Muc-1 may either act through stearic hindrance to evade contact with immune cells or induce anergy through a yet to be defined mechanism.1,19 20 In such circumstances, malignant plasma cells that express Muc-1 would be able to escape immunosurveillance, unlike normal plasma cells that, in these studies, lacked Muc-1 expression.
Because Muc-1 may have a role in immunoregulation, as well as cell migration and adhesion, we were interested in identifying compounds that modulate Muc-1 expression. In particular, the presence of consensus sequences for several steroid response elements (estrogen, progesterone, and glucocorticoids)22,23 on the Muc-1 promoter suggested that steroids might alter Muc-1 expression. Although no functional analyses for these steroid response elements has been reported in human cells, in vitro studies involving mice and baboons suggest that estrogen and progesterone modulate uterine Muc-1 expression.24,25 In our study, Muc-1 expression was upregulated after Dex treatment of MM cell lines, with maximal stimulation occurred at 10−8 mol/L Dex, doses that correspond to serum levels of Dex that are readily achievable clinically.65 Muc-1 expression was also upregulated on DU145 prostatic cancer cells, as well as on CAOV-3 and OVC-3 ovarian cancer cells, demonstrating that Dex-induced upregulation of Muc-1 is not restricted to MM cells. However, no change in Muc-1 expression was seen on two breast cancer cell lines (MCF.7 and ZR-75) exposed to Dex, showing selectivity of induction of Muc-1 by Dex. In contrast to murine and baboon uterine Muc-1 studies,24 25 no changes in Muc-1 expression were triggered in MM, breast, and prostatic cancer cells treated with estrogen and progesterone receptor agonists and antagonists. These different sequelae may reflect species or tissue specificity for Muc-1 regulation by estrogen or progesterone. In addition, these animal studies involved in vivo treatment for longer periods of time (up to 12 days) with estrogen and progesterone, whereas our studies with cell lines involved in vitro exposure to steroids for up to 72 hours. Hence, we cannot exclude the possibility that longer intervals of exposure to estrogen and progesterone might influence Muc-1 expression on MM cells.
In summary, our studies form the basis for targeting Muc-1 core protein in vaccination and serotherapy trials for MM. The finding that Muc-1 expression can be augmented by Dex at pharmacologically achievable levels suggests the potential utility of incorporating glucocorticoids into immunotherapeutic strategies targeting Muc-1 in MM.
Supported by National Institutes of Health grant CA78378, the Lauri Strauss Leukemia Foundation and the International Myeloma Foundation, a Young Investigator Award from the American Society of Clinical Oncology (S.P.T.), and a grant from the Alberta Cancer Board Research Initiative Program.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth C. Anderson, MD, Division of Adult Oncology, Dana Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.