Abstract
Inefficient polyglutamylation is a mechanism of resistance to methotrexate (MTX) in childhood T-lineage acute lymphoblastic leukemia (T-ALL) and in acute myeloid leukemia (AML) in comparison with childhood c/preB-ALL. We analyzed the profile of MTX polyglutamylation in childhood c/preB-ALL, T-ALL, and AML (n = 45, 15, and 14, respectively), the activity of the MTX-polyglutamate synthesizing enzyme folylpolyglutamate synthetase (FPGS) (n = 39, 11, and 19, respectively) and of the MTX-polyglutamate breakdown enzyme folylpolyglutamate hydrolase (FPGH) (n = 98, 25, and 34, respectively). MTX-Glu4-6 accumulation after 24 hours exposure to 1 μmol/L [3H]-MTX in vitro was lower in T-ALL (threefold) and AML (fourfold) compared with c/preB-ALL (P ≤ .001). The FPGS activity was twofold lower in T-ALL and AML than in c/preB-ALL samples (P < .01). FPGH activity was not different between c/preB-ALL and T-ALL, but threefold higher in AML (P < .001). FPGS, FPGH, and the ratio FPGS/FPGH were correlated with MTX-Glu4-6 accumulation (r = .49, r = −.34 and r = .61, respectively). Multivariate analysis showed that FPGS, but not FPGH, was an independent contributor for MTX-Glu1-6 accumulation, but not for MTX-Glu4-6 accumulation. In conclusion, low FPGS activity is associated with low accumulation of MTX-Glu4-6 in T-ALL and AML. For the group of AML as compared with the group of ALL, a high FPGH activity can play an additional role.
NOWADAYS, CHILDHOOD acute lymphoblastic leukemia (ALL) has an event-free survival of 70%, which is in striking contrast to that for childhood acute myeloid leukemia (AML), which is approximately 40%.1 Methotrexate (MTX) is an important drug in the treatment of childhood ALL,2 but clinical trials have shown that AML patients have low response rates to MTX, similar to the response rates of clinically resistant relapsed ALL patients (summarized by Bender3). Although in these trials, the dosages of MTX administered were low and the number of patients was limited, MTX is not included in standard therapy protocols for AML.
Little is known about the mechanisms of MTX resistance in pediatric AML. Studies in a limited number of samples from mainly adult AML patients have suggested that intrinsic MTX resistance can be ascribed to lower polyglutamylation capacities of AML cells compared with ALL cells.4-7 Inefficient polyglutamylation will result in a decrease of MTX-polyglutamates, especially MTX-Glu4-6, which are preferentially retained intracellularly and provoke inhibition of thymidylate synthase and enzymes involved in purine nucleotide synthesis.8
For childhood ALL, accumulation of MTX and MTX-polyglutamates was correlated with event-free survival9 and with short-term antileukemic effect.10 Less efficient polyglutamylation of MTX was observed in leukemic blasts from children with T-ALL compared with c/preB-ALL, both in vitro11and in vivo.12 13
The polyglutamylation defect in T-ALL and AML cells was associated with a lower activity of folylpolyglutamate synthetase (FPGS), the enzyme that catalyzes the polyglutamate chain formation, as compared with c/preB-ALL cells.14 The difference in FPGS activity was not associated with a decreased FPGS mRNA expression,15 but with a lower affinity of FPGS for MTX in AML cells.16
Besides decreased synthesis of the glutamate side chain, polyglutamylation defects may also be caused by increased breakdown of polyglutamates by folylpolyglutamate hydrolase (FPGH).17Recently, a number of reports have demonstrated a possible role for FPGH in contributing to MTX resistance in experimental model systems. In human soft tissue sarcoma cell lines, intrinsic MTX resistance resulting from impaired polyglutamylation18 could be explained by a higher FPGH activity compared with MTX responsive cell lines.19 H35 rat hepatoma and human CCRF-CEM leukemia cell lines have recently been reported to acquire MTX resistance by increasing FPGH activity compared with the MTX sensitive parental cell lines.20 21
A role for FPGH in clinical resistance to MTX has not been established, although in a recent report including eight ALL and seven AML samples, Longo et al.22 reported that the ratio FPGS/FPGH was better at predicting the amount of MTX-polyglutamates accumulated to determine either activity alone. In the present study, we examined the FPGS and FPGH activities as well as the polyglutamylation profile in childhood leukemia samples and found evidence that inefficient polyglutamylation was associated with a high FPGH activity in AML, but not in T-ALL.
MATERIALS AND METHODS
Patient specimens.
Bone marrow and/or peripheral blood was obtained with informed consent from 108 children with newly diagnosed common or preB-ALL (c/preB-ALL), 29 children with T-ALL, and 29 children with AML at first diagnosis before start of therapy. Samples from another six AML patients were obtained at time of relapse; these patients had not received MTX previously. Boys represented 56% of the c/preB-ALL patients and 79% of the children with T-ALL. Median age was 51.5 months (range, 15 to 190 months) for c/preB-ALL and 90.5 months for T-ALL (range, 13 to 179 months). Characteristics of the AML patients are presented in Table 1.
Mononuclear cells were isolated by Ficoll density gradient centrifugation as described previously.23 Samples with a leukemic cell percentage below 80% were enriched for blasts by removing nonmalignant cells using monoclonal antibodies (MoAbs) linked to magnetic beads (Dynabeads M-450; Dynal Inc, Oslo, Norway) as previously described.24 The samples were washed twice with RPMI containing 2% fetal calf serum (FCS) and resuspended in culture medium consisting of RPMI 1640 (Dutch modification; GIBCO, Uxbridge, UK) plus 20% FCS, 2 mmol/L L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.125 μl/mL fungizone, 200 μg/mL gentamycin (all obtained from Flow Laboratories, Irvine, UK), 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite (purchased from Sigma, Zwijndrecht, the Netherlands).
Reagents.
Methotrexate was a gift from Pharmachemie (Haarlem, The Netherlands). MTX-Glu2 was purchased from Schircks Company (Jona, Switzerland). [3,5,7-3H]-MTX (20 Ci/mmol) was obtained from Moravek Biochemicals (Brea, CA). [2,3-3H]-L-glutamic acid (19 Ci/mmol) formulated in 0.01 N HCl (NET 395) was provided by New England Nuclear (Boston, MA). Other reagents used were of analytical grade.
MTX polyglutamylation.
Ten million freshly isolated leukemic cells were incubated for 24 hours with 1 μmol/L 3H-MTX (final specific activity 2 Ci/mmol) in 5 mL culture medium. After the cells were harvested and washed three times in phosphate-buffered saline (PBS) by centrifugation (5 minutes at 300g, 4°C), the pellet was resuspended in 1 mL PBS. A sample of 90 μL was counted for radioactivity, 10 μL was used to determine the number of cells (including trypan blue positive cells); the remaining suspension was centrifuged 5 minutes at 12,000gand the pellet was kept at −20°C until extraction.
To extract the polyglutamates, the pellet was resuspended in 150 μL ice-cold PBS and left on ice for 20 minutes after the addition of 50 μL 40% trichloroacetic acid (TCA). After centrifugation, 400 μL Tri-octylamine/1,1,2-tri-chloro-tri-fluoro-ethane (1/4, vol/vol) was added to neutralize the extract. After vortexing and centrifugation, the upper waterlayer was stored at −20°C until HPLC analysis.
MTX-polyglutamates were analyzed using an anion exchange column (Partisphere SAX, Whatman, I.D. 4.6 mm, length 12.5 cm, particle size 5 μm) running 4 minutes with 98% buffer A (60 mmol/L NH4H2PO4, pH 5.5) and 2% buffer B (600 mmol/L NH4H2PO4, pH 5.5), followed by a gradient for 16 minutes to 100% buffer B.25This was subsequently reduced to 2% buffer B over 5 minutes and maintained for another 5 minutes. UV detection was at 309 nm. The data are expressed as pmol MTX-Glun/109cells, which allows comparison with previously described values.7,9,10,12 13
Folylpolyglutamate synthetase.
FPGS activity was assayed as described in detail by Jansen et al.26 Briefly, 15 × 106 freshly isolated cells were washed twice in PBS and suspended in 250 μL extraction buffer (50 mmol/L Tris-HCl, 20 mmol/L KCl, 10 mmol/L MgCl2, and 5 mmol/L dithiotreitol (DTT), pH 7.6). Crude cell extracts were obtained by sonification (three times for 5 seconds at 14 micron, with 10 seconds intervals, at 4°C) followed by centrifugation (15 minutes, 12,000g, at 4°C). The protein content of the supernatant was determined using the Biorad protein assay.27 The FPGS assay mixture contained in a volume of 250 μL: 200 μg protein, 4 mmol/L [3H]-L-glutamic acid ([3H]-Glu; final specific activity: 6.6 Ci/mol) and 250 μmol/L MTX in 100 mmol/L Tris, 10 mmol/L ATP, 20 mmol/L MgCl2, 20 mmol/L KCl, and 10 mmol/L DTT at a pH of 8.85. After 2 hours incubation at 37°C, during which the reaction was proven to be linear, the reaction was stopped by the addition of 1 mL 5 mmol/L ice-cold, nonlabeled L-glutamic acid. MTX-[3H]-Glu2 was separated from unreacted [3H]-glutamic acid by Sep-Pack C18 reverse phase column chromatography (Millipore, Waters Associates, Etten-Leur, The Netherlands). The amount of MTX-3[H]-Glu2was measured by direct β-scintillation counting. Controls without MTX were subtracted to correct for polyglutamylation of endogenous folates present in the samples. HPLC analysis demonstrated that under these conditions, MTX-Glu2 was the only product formed. The FPGS activity is expressed as pmol MTX-Glu2 formed per hour per 106 cells for comparison with polyglutamylation parameters, which are expressed per 109 cells. FPGS activities expressed per milligram of cellular protein are also provided. Viability of the samples before protein extraction was not of influence on the reported FPGS activities (r = −.06,P = .7, n = 62).
Folylpolyglutamate hydrolase.
The FPGH activity was assayed as described by O’Connor et al.28 Briefly, 2 × 106 cells were washed twice in PBS and suspended in 100 μL extraction buffer (100 mmol/L Tris-HCl, pH 6.9). Crude cell extracts were obtained by sonification (three times for 5 seconds at 14 micron, with 10 seconds intervals, at 4°C) followed by centrifugation (15 minutes, 12,000g, at 4°C). The protein content of the supernatant was determined using the Biorad protein assay.27 The FPGH reaction mixture contained 25 μg protein and 20 nmol MTX-Glu2 in 200 μL 100 mmol/L Tris-HCl, pH 6.9 and was incubated for 1 hour in a 37°C waterbath. Under the conditions used, the reaction was linear. The reaction was stopped by heating the samples for 3 minutes at 95°C. After cooling on ice for 15 minutes, the samples were centrifuged (15 minutes, 12,000g, 4°C) and the supernatant was stored at −20°C until HPLC analysis. MTX-Glu2 was separated from the product MTX (ie, MTX-Glu1) by HPLC as described for the polyglutamate chain length analysis. Both freshly obtained and cryopreserved samples were analyzed, since cryopreservation had no effect on FPGH activity as observed by us and by others.29FPGH activity is expressed as nanomoles MTX formed per hour per milligram of protein or as nanomoles MTX formed per hour per 106 cells for comparison with other MTX polyglutamylation parameters. Viability of the samples before protein extraction was not of influence on the reported FPGH activities (r = .09,P = .3, n = 106).
Statistical analysis.
The Mann-Whitney U-test was used to compare c/preB-ALL with T-ALL and AML data. To determine any relation of the MTX-polyglutamylation parameters, the Spearman correlation test was applied. Multivariate statistical comparisons were conducted including phenotype, FPGS, FPGH, and accumulation of MTX-Glu1-6 or accumulation of MTX-Glu4-6. Analyses were two-tailed at the significance level of P < .05.
RESULTS
MTX accumulation and polyglutamylation.
Total MTX accumulation, ie, MTX-Glu1-6, in samples from c/preB-ALL patients (n = 45) ranged from 205 to 4,838 pmol/109 cells as shown in Fig1. The range was more narrow for 15 T-ALL and 14 AML samples tested (maximum accumulation, 1,943 and 2,103 pmol MTX-Glu1-6/109 cells, respectively); no T-ALL or AML sample accumulated more total MTX than the 75th percentile of the c/preB-ALL samples. The median amount of total MTX accumulation was 1.5-fold lower in T-ALL compared with c/preB-ALL samples (888 v1,321 pmol/109 cells, respectively; P = .02), but was not significantly different between AML and c/preB-ALL samples (1,216 v 1,321 pmol/109 cells, respectively).
Accumulation of total MTX, ie, MTX-Glu1-6(pmol/109 cells; left panel) and of long chain polyglutamates, ie, MTX-Glu4-6 (pmol/109 cells; right panel) in c/preB-ALL (n = 45), T-ALL (n = 15), and AML cells (n = 14) after 24 hours in vitro incubation with 1 μmol/L [3H]-MTX. Each patient sample is represented by a dot, lines represent the median values.
Accumulation of total MTX, ie, MTX-Glu1-6(pmol/109 cells; left panel) and of long chain polyglutamates, ie, MTX-Glu4-6 (pmol/109 cells; right panel) in c/preB-ALL (n = 45), T-ALL (n = 15), and AML cells (n = 14) after 24 hours in vitro incubation with 1 μmol/L [3H]-MTX. Each patient sample is represented by a dot, lines represent the median values.
Separation of the accumulated MTX-polyglutamates based on chain length resulted in different patterns for c/preB-ALL, T-ALL, and AML. A spectrum with increasing amounts from unmetabolized MTX to MTX-Glu5 was found for c/preB-ALL samples as shown in Fig2. For T-ALL, similar amounts of MTX-Glu1 to MTX-Glu5 were observed, whereas for AML samples the main metabolites were MTX-Glu1-3 (Table 1).
Distribution of MTX metabolites (MTX-Glu1-6) expressed as mean value pmol MTX-Glun/109 cells (±SE) for 40 c/preB-ALL (□), 13 T-ALL (▨), and 14 AML (▪) patients. Leukemic cells were incubated for 24 hours with 1 μmol/L [3H]-MTX as described in Materials and Methods.
Distribution of MTX metabolites (MTX-Glu1-6) expressed as mean value pmol MTX-Glun/109 cells (±SE) for 40 c/preB-ALL (□), 13 T-ALL (▨), and 14 AML (▪) patients. Leukemic cells were incubated for 24 hours with 1 μmol/L [3H]-MTX as described in Materials and Methods.
The median percentage of MTX present as the pharmacologically more important long-chain polyglutamates MTX-Glu4-6 was 66% for the c/preB-ALL cells (range, 34% to 90%) compared with 42% in the T-ALL samples (range, 19% to 85%; P < .001) and 29% in the AML samples (range, 0% to 62%; P < .001). Consequently, the median absolute amount of MTX-Glu4-6 was 906 pmol/109 cells in c/preB-ALL samples versus 290 pmol/109 T-ALL cells (P = .001) and 225 pmol/109 AML cells (P < .001) (Fig 1).
FPGS and FPGH activity.
Samples of 39 children with c/preB-ALL, 11 with T-ALL, and 19 with AML contained sufficient cells to be assayed for FPGS activity (Table2). The median FPGS activity for c/preB-ALL samples was twofold higher than the median FPGS activity for T-ALL or AML samples (P < .01). When the activity was normalized per milligram of cellular protein, comparable differences were found.
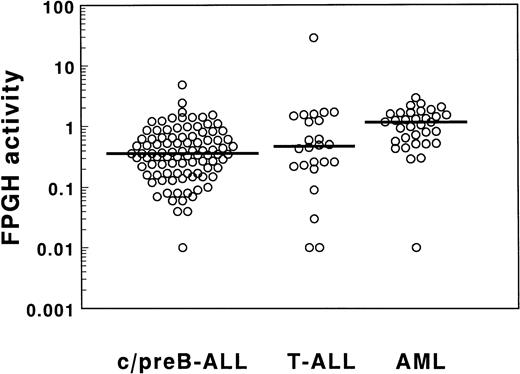
The FPGH activity of ALL samples showed a broad range as depicted in Table 2 and Fig 3. The median FPGH activity was not different between 94 c/preB-ALL and 24 T-ALL samples. A narrow range in FPGH activities was found for the AML cells with the exception of one outlier in which the FPGH activity was below detection limit. The median value was threefold higher for the AML samples compared with the c/preB-ALL samples when expressed per cell (P < .001). The FPGH activity normalized per milligram of cellular protein was 1.4-fold higher in AML cells compared with c/preB-ALL cells (P = .05; Table 2). Differences in FPGH activity between the small groups of AML subtypes (mainly M1, M2, and M4) were not statistically significant (Table 1). FPGH activity was not related to FPGS activity in the total group of 62 acute leukemia samples (r = −.09;P = .5), nor in the separated groups of 36 c/preB-ALL, 9 T-ALL, and 17 AML samples.
FPGH activities in cell extracts of 94 c/preB-ALL, 24 T-ALL, and 33 AML samples determined by incubation with 100 μmol/L MTX-Glu2 as a substrate for FPGH. Data are expressed as nmol MTX formed/h/106 cells.
FPGH activities in cell extracts of 94 c/preB-ALL, 24 T-ALL, and 33 AML samples determined by incubation with 100 μmol/L MTX-Glu2 as a substrate for FPGH. Data are expressed as nmol MTX formed/h/106 cells.
Correlation of FPGS or FPGH with polyglutamylation.
A significant correlation was found between FPGS activity and accumulation of MTX-Glu1-6 in 20 c/preB-ALL samples analyzed for these parameters (r = .62; P = .004). The same was true for FPGS activity and accumulation of MTX-Glu4-6 (r = .49; P = .04) (Table3). Inclusion of these parameters from eight T-ALL and nine AML samples, resulted in similar findings, as shown in Fig4A for 33 samples of which FPGS and MTX-Glu4-6 accumulation data were available.
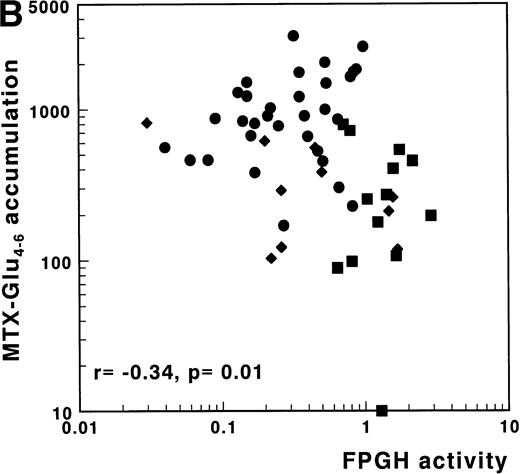
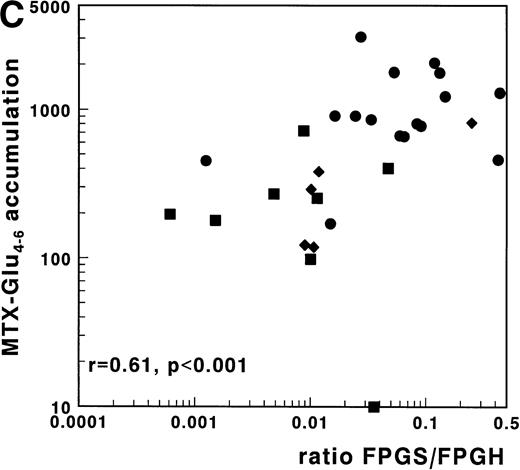
(A) Correlation of FPGS activity (pmol MTX-Glu2 formed/h/106 cells) with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu4-6/109 cells). (B) Correlation of FPGH activity (nmol MTX formed/h/106 cells) with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu4-6/109 cells). (C) Correlation of the ratio FPGS/FPGH activities expressed per 106 cells with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu1-6/109 cells). Spearman rank correlation coefficients are presented for the total group. Statistical parameters for c/preB-ALL, T-ALL, and AML samples are presented separately in Table 3. (•), c/preB-ALL; (⧫), T-ALL; (▪), AML.
(A) Correlation of FPGS activity (pmol MTX-Glu2 formed/h/106 cells) with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu4-6/109 cells). (B) Correlation of FPGH activity (nmol MTX formed/h/106 cells) with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu4-6/109 cells). (C) Correlation of the ratio FPGS/FPGH activities expressed per 106 cells with accumulation of MTX-Glu4-6 (expressed as pmol MTX-Glu1-6/109 cells). Spearman rank correlation coefficients are presented for the total group. Statistical parameters for c/preB-ALL, T-ALL, and AML samples are presented separately in Table 3. (•), c/preB-ALL; (⧫), T-ALL; (▪), AML.
No correlation between FPGH and accumulation of MTX-Glu1-6was observed in the separate subgroups or in the total group of 61 acute leukemia samples. The importance of FPGH activity for the differences in MTX polyglutamylation between T-ALL, AML, and c/preB-ALL, however, was suggested by the significantly inverse relation between FPGH and the accumulation of MTX-Glu4-6(r = −.34, P = .01) (Fig 4B). No correlation was observed between FPGH activity and the accumulation of MTX-Glu4-6 when analyzed within the separate groups of ALL or AML samples (Table 3).
For 29 patients, FPGS and FPGH activities as well as MTX-Glu4-6 accumulation data were available. In this group a strong and significant positive correlation was observed for the FPGS/FPGH ratio with the total MTX accumulation (r = .63;P < .001) and with the accumulation of MTX-Glu4-6 (r = .61; P < .001) as shown in Fig 4C. The correlation of the FPGS/FPGH ratio with accumulation of MTX-Glu4-6 was stronger than that of the FPGS or FPGH activities independently with accumulation of MTX-Glu4-6 in this group of samples (r = .41;P = .03 and r = −.46; P = .01, respectively). Multivariate regression analysis with inclusion of FPGS, FPGH, and phenotype, showed that FPGS was the independent predictive factor for the accumulation of MTX-Glu1-6, but not for the accumulation of MTX-Glu4-6. FPGH did not independently contribute to polyglutamylation.
DISCUSSION
In this study, we show that inefficient polyglutamylation, a potential mechanism for intrinsic MTX resistance in AML cells, can be explained by a lower FPGS activity along with a higher FPGH activity in AML cells compared with c/preB-ALL cells. Also for T-ALL, inefficient polyglutamylation was associated with a lower FPGS activity, but not with a higher FPGH activity. Long-chain polyglutamates are known to be preferentially retained intracellularly.8 Because relative resistance to MTX observed in T-ALL and AML cells compared with c/preB-ALL was associated with a rapid efflux of the drug,4,30 defects in polyglutamylation provide a plausible explanation for this phenomenon. Compared with ALL, shorter chain MTX-polyglutamates have actually been observed in adult AML samples6,7 and in four pediatric AML samples.5Also the lower response rate to MTX in T-ALL might at least partially be explained by defects in polyglutamylation.11-13
In the present study consisting of a large group of pediatric leukemia samples, the distribution pattern of the MTX polyglutamates was different in c/preB-ALL versus T-ALL and AML. Consistent with other studies,12 31 we observed that in c/preB-ALL, MTX was metabolized to long-chain polyglutamates with MTX-Glu5as the main metabolite. For AML, however, MTX-Glu1-3 were observed as the major metabolites. The accumulation of the pharmacologically more important MTX-Glu4-6 was lower in T-ALL (threefold) and AML (fourfold) compared with c/preB-ALL cells, which may contribute to the differential MTX sensitivity of these leukemias.
Inefficient MTX polyglutamylation in T-ALL and AML cells has previously been explained by a lower FPGS activity compared with c/preB-ALL cells.14 In the present study, the same differences between c/preB-ALL, T-ALL, and AML were observed. Since polyglutamylation is a dynamic process of synthesis and breakdown, not only the FPGS activity but also the activity of FPGH, which hydrolyzes the polyglutamates, should be taken into account. Laboratory studies using cell lines have shown that increased FPGH activity conferred a shift in the distribution of the folylpolyglutamates towards the short-chain polyglutamates.32 In addition, MTX resistance has been associated with a high FPGH activity in several human sarcoma cell lines, in a human leukemia cell line and in a rat hepatoma cell line.19-21 Moreover, incubation of leukemic blasts with 2-mercapto-methylglutaric acid (MMGA), an inhibitor of FPGH, resulted in a polyglutamylation pattern for AML cells resembling the pattern observed for c/preB-ALL samples.33
We investigated the activity of FPGH in 151 pediatric acute leukemia samples and found no difference between c/preB-ALL, and T-ALL, but a significant three-fold higher FPGH activity in AML cells compared with c/preB-ALL cells. Since the accumulation of total MTX per 106 cells was similar for AML and c/preB-ALL cells, relatively more FPGH enzyme per picomole accumulated MTX is present in AML cells compared with c/preB-ALL cells. This might partially explain the lower amounts of MTX-Glu4-6 observed in AML compared with c/preB-ALL. Although AML cells are larger than ALL cells, a difference in FPGH activity was also observed when the activity was expressed per milligram of cellular protein. This suggests that a high FPGH activity contributes to intrinsic MTX resistance in childhood AML.
Given the intracellular compartmentation of FPGH, extrapolation of the FPGH activity measured in cell extracts to the in vivo situation, however, should be made cautiously. Studies on tumor cell lines32,34 have shown that FPGH is primarily localized in the lysosomes and therefore lysosomal transport of the polyglutamates is likely to be a limiting factor in the breakdown of MTX-polyglutamates by FPGH.35 In addition, secretion of the enzyme has been observed in tumor cell lines, which could imply that FPGH activity as measured in cell free extracts is an overestimation of the intracellular functional activity.20,28 29
A number of reports demonstrated that the affinity of FPGH for MTX metabolites increased with increasing polyglutamate side chain length.35,36 Therefore, differences in FPGH activity between AML and ALL cells, as reported in this study, might be more pronounced when assayed using MTX-Glu5 as a substrate. However, we37 and others18 36 observed that human FPGH displays an exopeptidase activity, ie, sequentially hydrolyzing the outermost glutamate residue. This results in a complex cleavage pattern with hydrolyzed products subsequently serving as substrates again. For a more straightforward determination of FPGH activity, we used MTX-Glu2 as a substrate, which will be hydrolyzed to MTX.
To investigate the relative contribution of FPGS and FPGH in the process of polyglutamylation, we determined the relation of these enzymes to in situ MTX polyglutamylation irrespectively of the type of leukemia. A significant strong correlation for both FPGS and FPGH activity with the accumulation of MTX-Glu4-6 was found. This relation was stronger when, within the same group of samples, the ratio of FPGS/FPGH was used instead of single enzyme activity, indicating that the relative contribution of both enzymes is of importance as suggested recently in a study with 15 childhood acute leukemia samples.22 No correlation could be observed for FPGH and accumulation of MTX-Glu4-6 within the (sometimes small) separate subgroups. However, for the total population of leukemic samples, a role for FPGH in the process of polyglutamylation seems evident as illustrated by Fig 4A-C.
Multivariate analysis, including FPGS, FPGH, and phenotype, did not demonstrate that either enzyme activity contributed independently to the accumulation of MTX-Glu4-6. However, this study shows that, compared with c/preB-ALL, FPGH is significantly higher in AML while the accumulation of MTX-Glu4-6 is significantly lower in AML. So, for the group of AML compared with the group of ALL, FPGH might be a significant factor while the differences in FPGH activity within one subtype of leukemia do not contribute to polyglutamylation defects. The absence of a causal relation for FPGH on MTX-Glu4-6 might be explained by additional factors contributing to the accumulation of MTX-Glu4-6. These factors may include diminished MTX transport and high levels of DHFR, which may reduce the pool of MTX available for polyglutamylation.38
Interestingly, a multivariate model to predict accumulation of MTX-Glu1-6 showed FPGS as the only independent predictor. Since MTX accumulation has been described to be a prognostic factor within a group of c/preB-ALL patients,9 this finding might provide a molecular tool to predict MTX-based therapy outcome. In this respect, mRNA expression levels of FPGS, as reported to be correlated to functional FPGS activity,13,15 are currently being investigated in our laboratory.39
This report focuses on polyglutamylation defects as a resistance mechanism to MTX, but other factors can also contribute to MTX resistance including (1) defects at the level of membrane transport as has been reported to occur in one third of AML samples,40(2) increased levels of the main target enzyme DHFR, (3) mutations in the DHFR gene leading to decreased affinity for MTX, or (4) presence of functionally active efflux systems (reviewed by Bertino2and Peters and Jansen41). Although the first two mechanisms have been described as playing a role in MTX resistance in childhood ALL (reviewed by Pieters et al,42 Jansen and Pieters,43 Gorlick et al44), polyglutamylation defects seem to be more predominant factors underlying the presumed clinical MTX resistance described for AML cells.5
In conclusion, an important independent role is described for FPGS in predicting the overall MTX accumulation in childhood leukemia subtypes. A high FPGH activity is associated with a low accumulation of MTX-Glu4-6 in childhood AML, but not in T-ALL. These data suggest that inhibitors of FPGH29 or novel antifolates for which FPGH has a decreased affinity compared with MTX might offer new approaches to circumvent MTX resistance and to treat children with AML.
ACKNOWLEDGMENT
The authors thank Dr P.D. Bezemer of the Department of Epidemiology and Biostatistics, Vrije Universiteit, Amsterdam, the Netherlands for helpful discussions during the preparation of this manuscript.
Supported by Grant No. VU 94-679 from the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marianne G. Rots, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit, PO Box 7057, 1007 MB Amsterdam, the Netherlands; e-mail: marianne.rots@azvu.nl.

![Fig. 1. Accumulation of total MTX, ie, MTX-Glu1-6(pmol/109 cells; left panel) and of long chain polyglutamates, ie, MTX-Glu4-6 (pmol/109 cells; right panel) in c/preB-ALL (n = 45), T-ALL (n = 15), and AML cells (n = 14) after 24 hours in vitro incubation with 1 μmol/L [3H]-MTX. Each patient sample is represented by a dot, lines represent the median values.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1677/4/m_blod40516001x.jpeg?Expires=1765444525&Signature=ccItPcLInyeLuUP1eLR79gdpKdWApv-iyQMkjbS3tDy7tcYw8zFBjJ2akxiHQjr522UXfz2759wx9SebkWgOIBNg86QUxx~bF5s8Q~rINKIAPypviKloTEK1XIBIy~-5wctg~GDK9l76PMbiyGy6ttqK-dmhZZOPrwB2RKToFPQ0nQdLWlAf9zfoJ1DMj4oA8usiLY4nvvrw5W4RIRXyCHLt2W~XawYlE30aIPUsHjTO01NCuQBPQbDpgTbEOZkO95oNkNpp6AtEhJfGK9Vt7dIgGV5bdThWlx0y5E8~2Gd0wPdpbtbBeYlPO-G9133v6N~pcI3crWbtifGvrXQ3~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Distribution of MTX metabolites (MTX-Glu1-6) expressed as mean value pmol MTX-Glun/109 cells (±SE) for 40 c/preB-ALL (□), 13 T-ALL (▨), and 14 AML (▪) patients. Leukemic cells were incubated for 24 hours with 1 μmol/L [3H]-MTX as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1677/4/m_blod40516002x.jpeg?Expires=1765444525&Signature=rUXhnVAPPetHO89~UCc-2LbKQ~ZEtSnbxNz03UTjQgZ5AfHmBnlNf2JoORPIXV0jQbAnJ6HzYZMZ8fEyYW8Qx9V7uXjf1dt02DA64UV6sDC7honYC-ORMUK-rH5ECsvzJDlWKmdpvUCU9ULHkQvQsjuZLFROZincWua0Tf7a4GMtjqVnK2OWeGp3mLgx50wb0eZ2y0wgcDujFkgy7Es33JMMagwIYJQDPBzosznP8gB2eN6VuV6Bsy-6KbC6aPzj7k65buc4VcAPUue~MfAINZiz7h~dtyft3iSonZ9jVkNK4ieCIIxupqVP3obVfaNa4Msuru3QtfPpdGOeisHA1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)