Abstract
Current in vitro culture systems allow the generation of human dendritic cells (DCs), but the output of mature cells remains modest. This contrasts with the extensive amplification of hematopoietic progenitors achieved when culturing CD34+ cells with FLT3-ligand and thrombopoietin. To test whether such cultures contained DC precursors, CD34+ cord blood cells were incubated with the above cytokines, inducing on the mean a 250-fold and a 16,600-fold increase in total cell number after 4 and 8 weeks, respectively. The addition of stem cell factor induced a further fivefold increase in proliferation. The majority of the cells produced were CD34−CD1a− CD14+(p14+) and CD34−CD1a−CD14−(p14−) and did not display the morphology, surface markers, or allostimulatory capacity of DC. When cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), both subsets differentiated without further proliferation into immature (CD1a+, CD14−, CD83−) macropinocytic DC. Mature (CD1a+, CD14−, CD83+) DCs with high allostimulatory activity were generated if such cultures were supplemented with tumor necrosis factor- (TNF). In addition, p14− cells generated CD14+ cells with GM-CSF and TNF, which in turn, differentiated into DC when exposed to GM-CSF and IL-4. Similar results were obtained with frozen DC precursors and also when using pooled human serum AB+ instead of bovine serum, emphasizing that this system using CD34+ cells may improve future prospects for immunotherapy.
DENDRITIC CELLS (DCs) are the most potent antigen (Ag) presenting cells and appear to be the only cell type capable of initiating a primary T-cell–dependent immune response.1 DCs originate from the bone marrow and their precursors migrate via the blood stream to almost all organs of the body. There, they actively sample their environment by internalizing the surrounding antigens.2,3 When a danger signal such as bacterial infections, heat shock, hypoxia, or trauma4ensues, the DCs metabolism, originally oriented toward high Ag uptake and processing/low Ag presentation, shifts toward a low to nil Ag uptake/high Ag presentation.5,6 This is normally coupled to an upregulation of costimulating surface molecules such as CD40, CD80, and CD86 and is also associated with the migration of DCs from the nonlymphoid tissues to the lymphoid organs where they present Ag to T cells. However, if appropriate costimulative molecules are not expressed on DCs surface at the time of Ag presentation, T-cell anergy may be induced.7-9
Until recently, progress in the understanding of DCs biology has been relatively slow and difficult because of the paucity of these cells in blood and other tissues.1 A major step forward has been made by the establishment of in vitro culture systems, which permits the induction of DCs from precursors. Original systems using adult peripheral CD14+ cells stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin-4 (IL-4), or systems using umbilical cord blood (CB) CD34+ cells stimulated with GM-CSF plus tumor necrosis factor-α (TNF) have been described by Sallusto and Lanzavecchia10 and Santiago-Schwarz et al,11 respectively. These systems have been further modified by others to increase the diversity and the yield12-18 of the cells produced. Recently, the role of FLT3-ligand (FLT3-L) as a potent factor of DC induction has been outlined in vitro and in vivo.19-22 It is now evident that DC will be used as “natural adjuvant” in immunotherapy,23,24 and preliminary results in human clinics are very encouraging.25-27
Despite this progress, the total number of DC available for immunotherapy remains limited. This contrasts with the extensive amplification of myeloid, megakaryocytic, or erythroid precursors obtained after culture of CD34+ cells with the combination of early acting hematopoietic growth factors.28-32 However, the nature and differentiation potential of DC precursors in such systems has not been investigated. To explore this, we produced large amounts of hematopoietic progenitors by culturing CD34+cells purified from CB with FLT3-L, thrombopoietin (TPO), and stem cell factor (SCF). The cells generated in these cultures were then exposed to factors such as GM-CSF, IL-4, and TNF, which are known to be involved in DC differentiation. The combination of these two culture types allowed the production of a large number of DC from a limited number of CD34+ progenitors via alternative differentiation pathways.
MATERIALS AND METHODS
Cytokines
All cytokines were recombinant human material. GM-CSF (Leucomax) (Essex Chimie & Sandoz, Basel, Switzerland) was used at 20 ng/mL. Other cytokines purchased from Peprotech EC (London, UK), and used at the following concentrations: FLT3-L at 25 ng/mL, TPO 10 U/mL, SCF 20 ng/mL, IL-4 20 ng/mL (100 U/mL), and TNF 40 ng/mL (200 U/mL).
Antibodies/Immunoreactants
Specific fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies (MoAbs).
Anti-CD14 (mIgG2a, clone UCHM1) was from Ancell Corp (Bayport, MN); anti-CD1a (mIgG1, clone OKT6) from Ortho Diagnostic Systems Inc (Raritan, NJ); anti–HLA-DR (mIgG2a, clone L243) from Becton Dickinson (Mountain View, CA); anti-CD40 (mIgG1, clone 5C3) and anti-CD80 (mIgM, clone BB1) from Pharmingen (San Diego, CA); anti-CD33 (mIgG1, clone WM-54), anti-CD38 (mIgG1, clone AT13/5), anti-CD8 (mIgG1, clone DK25), anti-CD19 (mIgG1, clone HD37), and anti-CD45RO (IgG2a clone UCHL1) from Dako A/S (Glostrup, Denmark), anti-CD45RA (mIgG1, clone 2H4) from Immunotech (Marseille, France).
Specific phycoerythrin (PE)-labeled MoAbs.
Anti-CD34 (mIgG1, clone 8G12), anti-CD14 (mIgG2b, MoP9), and anti-CD3 (mIgG1, clone SK7) were from Becton Dickinson; anti-CD1a (mIgG1, clone BL6) and anti-CD83 (mIgG2b, clone HB15a) from Immunotech, anti-CD86 (mIgG2b, clone IT2.2) from Pharmingen; anti-CD4 (mIgG1, clone MT310) from Dako.
Monoclonal isotype controls.
FITC- and PE-labeled mIgG1 was from Dako; FITC-labeled IgG2a from Ancell, FITC-labeled mIgM from Pharmingen, and PE-labeled mIgG2b from Immunotech.
Other reactants.
Polyclonal mouse IgG reagent grade was from Sigma Chemical Co (St Louis, MO); FITC-labeled Dextran (molecular weight [MW], 40 kD) from Molecular Probes Inc (Eugene, OR), and anti-CD34 mIgG coated M450 Dynabeads from Dynal A/S (Oslo, Norway).
Purification of CD34+ Cells
CB samples were obtained according to institutional guidelines. CB mononuclear cells (MNC) were recovered after Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation. CD34+ cells were purified using anti-CD34 M450 Dynabeads as described by the manufacturer. In brief, CB MNC were incubated with anti-CD34 beads in a ratio of four cells for one bead for 30 minutes at 4°C under gentle agitation. CD34− cells were removed from the beads by four successive washes with a cold isotonic saline solution complemented with fetal calf serum (FCS) (3%) and sodium citrate dihydrate (0.6%) by using a Dynal specific magnet. CD34+ cells were recovered from the beads after a 45-minute incubation at room temperature with the “Detach-a-bead” included in the kit. Cells were immediately washed and analyzed by flow cytometry. CD34+ cells represented 83% ± 11% (mean ± SD of seven experiments) of the total cell number recovered.
Cell Cultures
Primary culture with FLT3-L, TPO, and with or without SCF.
Five times 104 CD34+ cells were seeded and cultured as described31 in 24-well plates in 1 mL of Iscove’s Modified Dulbecco’s medium (IMDM) supplemented with 10% of FCS and antibiotics (all from GIBCO-BRL, Life Technologies LTD, Paisley, UK), and 1 × 10−5 mol/L dithiothreitol (DTT; Fluka Biochemika, Buchs, Switzerland) with FLT3-L plus TPO with or without SCF. On a weekly basis, global cell count and CD34+ frequency were determined, cellular density was adjusted to 1 to 2 × 105 cells/mL, and cultures were maintained in this way for up to 8 weeks. After 14 days of culture, cells were either maintained in culture in the original conditions or induced at various times to differentiate into DC. Alternatively, cells were cryopreserved in IMDM 20% FCS and 10% dimethyl sulfoxide (Merck, Darmstadt, Germany) for later induction into DC.
Induction of DC.
Fresh or frozen cells recovered after 2 to 8 weeks of primary culture, were extensively washed, counted, and seeded at 1 to 2 × 105/mL in 24-well plates containing 1 mL of IMDM supplemented with 10% FCS. In some instances, FCS was replaced by pooled human serum AB+ (Blood Transfusion Center, Annemasse, France). Cells were induced with different cocktails of GM-CSF, IL-4, TNF, and FLT3-L and analyzed at different time points, as described in Results.
Sorting of DC Precursors
Five to 10 × 106 cells from primary cultures were labeled with anti-CD34 and anti-CD1a PE-conjugated antibodies, and anti-CD14 FITC-conjugated antibody. Cells were sorted on a FACStar Plus cell sorter (Becton Dickinson, operated by D. Wohlwend, University Medical Center, Geneva Switzerland) as CD34−CD1a−CD14+(p14+), and CD34−CD1a−CD14−(p14−) before induction into DC. Cells were systematically reanalyzed after sorting. p14+ were 95% ± 1.9%, and p14− were 97% ± 1.8% pure (mean ± SD of five experiments).
Flow Cytometric Analysis
Cultured cells were washed, suspended at 3 × 104 to 1 × 105 in 50 μL of cold isotonic saline containing 3% FCS and 200 μg/mL of mouse IgG, and incubated for 10 minutes on ice. Specific labeled MoAbs or appropriate isotypic controls were added, and cells were further incubated on ice for 30 minutes. Cells were washed once and incubated in 300 μL of saline-3% FCS containing 10 μg/mL of 7-amino-actinomycin D (7AAD) (Sigma). Cells were analyzed within 20 minutes on a FACScalibur cell analyzer (Becton Dickinson) using FL-1 and FL-2 for specific immunolabeling, and FL-3 for 7AAD staining, which identified living cells as 7AAD low, whereas dead and apoptotic cells were 7AAD high and medium, respectively.33 Cell debris were eliminated from the analysis using a gate on forward and side scatters. Data were analyzed using WINMDI software by J. Trotter, at Scripps Institute (La Jolla, CA).
FITC-dextran labeling.
Cells were incubated with FITC-dextran (0.1 mg/mL), either at 4°C (internalization control) or at 37°C for 1 hour.34 Cells were then washed twice with a cold buffer consisting of phosphate-buffered saline, 0.1% sodium azide, and 10 mg/mL bovine serum albumin, and stained with PE-labeled anti-CD1a antibody for 30 minutes on ice.
Allogenic Mixed Leukocyte Reaction (MLR)
Allogenic T cells were obtained from peripheral blood of healthy adults after Ficoll-Paque gradient (Pharmacia), adherence to plastic for 1 hour at 37°C, and passage over a nylon wool column (Biotest A.G., Dreieich, Germany). Cells recovered after purification were on the mean greater than 90% CD3+ and greater than 60% CD45RA+, and were distributed at 5 × 104 per well into round-bottomed 96-well microplates (Nunc, Roskilde, Denmark). Cells were incubated for 5 days in the presence of graded numbers of irradiated DC stimulators (3,000 rad, 137Cs source) in 200 μL of medium containing 10% FCS. T-cell proliferation was assessed after 8 to 14 hours pulses of 3H-TdR (1 μCi/well; New England Nuclear, Boston, MA) by using standard procedures.
RESULTS
Cellular Suspensions Obtained After Primary Cultures of CD34+ Cells Contain Dendritic Cell Precursors
Cultures of CD34+ cells with FLT3-L plus TPO produced a mean increase in total cell number of 250-fold after 4 weeks of culture (mean of seven experiments, range, 53- to 612-fold) and of 16,600-fold after 8 weeks of culture (mean of three experiments; range, 1,500- to 47,000-fold). Cultures with FLT3-L plus TPO and SCF produced a mean increase in total cell number of 1,200-fold after 4 weeks of culture (mean of four experiments; range, 600- to 1,852-fold). Both types of culture were used as a source of DC precursors without distinction and are quoted as “primary cultures” in the remaining text. Cell suspensions obtained after 4 weeks of primary culture were 41% ± 9.4% CD33+, 71% ± 9.4% CD38+, 25% ± 7.5% CD40+, and 55% ± 2.6% HLA-DR+. CD34+ cells had decreased from 83% (see Materials and Methods) to 12% ± 3.5%. Thirty-five percent ± 2.6% of the cells were CD14+, and 20% ± 4.7% were CD86+. CD1a+ and CD83+ cells, respectively, represented 3.8% ± 2.7%, and 1.6% ± 0.5% of the total (these data are the mean of three experiments ± SD).
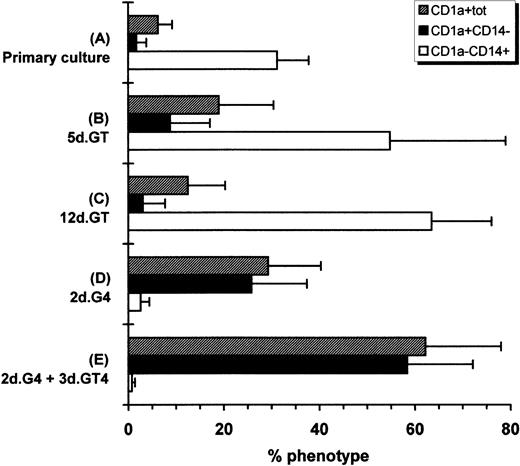
Hematopoietic progenitors amplified in the primary cultures were incubated with GM-CSF plus TNF, a combination which is known to induce the differentiation of CD34+ cells into DC.11Hematopoietic progenitors were also incubated with the combination of GM-CSF plus IL-4, which induces differentiation of adult peripheral blood CD14+ cells into immature DC and into mature DC if TNF is added after a few days of culture.10 As Fig1 shows, 5 or 12 days of culture with GM-CSF plus TNF induced mainly CD1a−CD14+cells. Total cell number increased by 1.2 ± 0.6-fold (mean ± SD of four experiments) after 5 days in such conditions. However, 2 days of culture with GM-CSF plus IL-4 induced a downmodulation of CD14+ cells relative to primary cultures and an increase of CD1a+CD14− cells reaching 26%. The total cell number was increased by 1.2 ± 0.6-fold. Thus, taking in account both the total cell proliferation occurring in the primary cultures and the efficiency of CD1a induction by GM-CSF and IL-4, the number of CD1a+CD14− cells generated represented on the mean 78-fold that of the input if cells were induced after 4 weeks of primary cultures, and 5,200-fold that of the input if cells were induced after 8 weeks of primary cultures. The induction with GM-CSF, IL-4, and TNF was performed after 2 to 10 weeks of primary cultures, and no change in the proportion of CD1a+CD14− cells induced was observed. If cultures with GM-CSF and IL-4 were supplemented with TNF after 2 days and conducted for 3 more days, CD1a+CD14−cells increased to 58%. Further analysis of surface molecules showed that markers related to DC function, ie, CD86, CD40, and HLA-DR, were already upregulated after 2 days of culture with GM-CSF plus IL-4 and their expression was further increased after the adjunction of TNF for the last 3 days of culture. Of interest, CD83, which is considered as a mature DC marker,35 was marginally induced with GM-CSF plus IL-4, but was markedly upregulated when TNF was added (Fig2). Phase contrast microscopy and cytospin smears showed that cells that were originally small and nonadherent in the primary cultures (not shown) increased in size and developed long dendritic processes when exposed to GM-CSF, IL-4, and TNF (Fig3A). Thus, the cell suspensions obtained after primary cultures contained a high proportion of hematopoietic progenitors capable of generating cells with the CD profile and the morphology of DC when exposed to appropriate cytokines.
Modulation of CD1a and CD14 expression. (A) CD34+ cells were cultured for 14 to 70 days with FLT3-L plus TPO with or without SCF. Cells were then cultured for (B) 5 or (C) 12 days with GM-CSF plus TNF or (D) for 2 days with GM-CSF plus IL-4. (E) Cells were also grown for 2 days with GM-CSF plus IL-4, then for 3 days with the latter cytokines plus TNF. Cytokines were renewed after 3 days of culture except for primary cultures. This is the mean of five experiments ± SD.
Modulation of CD1a and CD14 expression. (A) CD34+ cells were cultured for 14 to 70 days with FLT3-L plus TPO with or without SCF. Cells were then cultured for (B) 5 or (C) 12 days with GM-CSF plus TNF or (D) for 2 days with GM-CSF plus IL-4. (E) Cells were also grown for 2 days with GM-CSF plus IL-4, then for 3 days with the latter cytokines plus TNF. Cytokines were renewed after 3 days of culture except for primary cultures. This is the mean of five experiments ± SD.
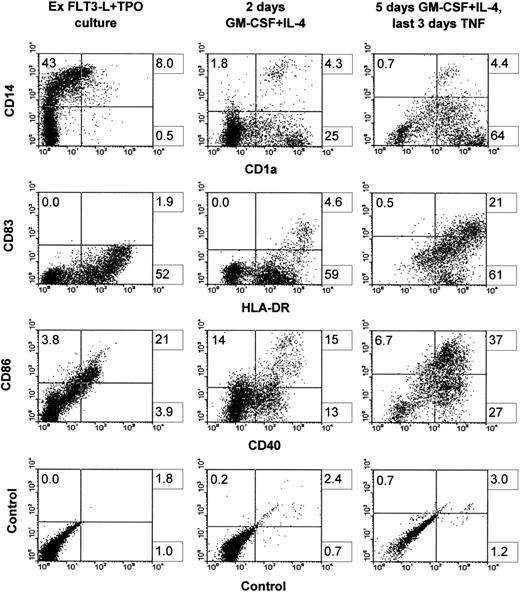
Surface phenotype before and after the induction into DCs. Cells were grown for 3 weeks in FLT3-L plus TPO, frozen, and thawed after 3 months of cryopreservation. Cells were then cultured back in the initial conditions for 10 days (left column). Cells were induced with GM-CSF plus IL-4 for 2 days (middle column) or with GM-CSF and IL-4 for 2 days, followed by 3 more days with the latter cytokines plus TNF (right column). One representative experiment is shown out of four.
Surface phenotype before and after the induction into DCs. Cells were grown for 3 weeks in FLT3-L plus TPO, frozen, and thawed after 3 months of cryopreservation. Cells were then cultured back in the initial conditions for 10 days (left column). Cells were induced with GM-CSF plus IL-4 for 2 days (middle column) or with GM-CSF and IL-4 for 2 days, followed by 3 more days with the latter cytokines plus TNF (right column). One representative experiment is shown out of four.
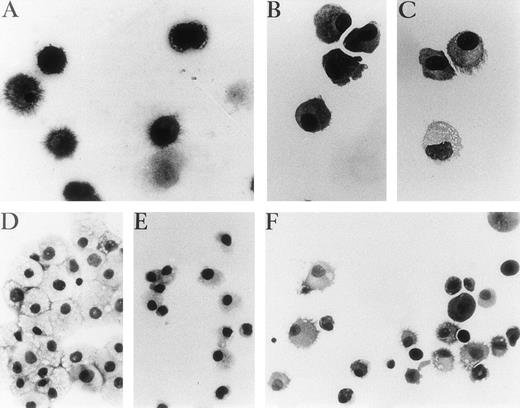
Representative photographs of May-Grünwald-Giemsa–stained cytospin preparations of cells cultured with different cocktails of cytokines after primary cultures. (A) Unsorted cells in GM-CSF, IL-4, and TNF for 7 days, (B) p14+ cells 3 days in GM-CSF plus IL-4. (C) p14+ cells, 7 days with the same cytokines as (B), with adjunction of TNF for the last 4 days. (D and E) p14−cells after 9 days of culture with GM-CSF plus TNF or GM-CSF plus FLT3-L, respectively. (F) p14− cells 3 days with GM-CSF plus FLT3-L, 4 days with GM-CSF and IL-4, then 4 days with GM-CSF plus IL-4 and TNF. (A, B, and C) Original magnification ×400. (D, E, and F) Original magnification ×250.
Representative photographs of May-Grünwald-Giemsa–stained cytospin preparations of cells cultured with different cocktails of cytokines after primary cultures. (A) Unsorted cells in GM-CSF, IL-4, and TNF for 7 days, (B) p14+ cells 3 days in GM-CSF plus IL-4. (C) p14+ cells, 7 days with the same cytokines as (B), with adjunction of TNF for the last 4 days. (D and E) p14−cells after 9 days of culture with GM-CSF plus TNF or GM-CSF plus FLT3-L, respectively. (F) p14− cells 3 days with GM-CSF plus FLT3-L, 4 days with GM-CSF and IL-4, then 4 days with GM-CSF plus IL-4 and TNF. (A, B, and C) Original magnification ×400. (D, E, and F) Original magnification ×250.
Dendritic Cells Generated After Long-Term Culture Are Functional
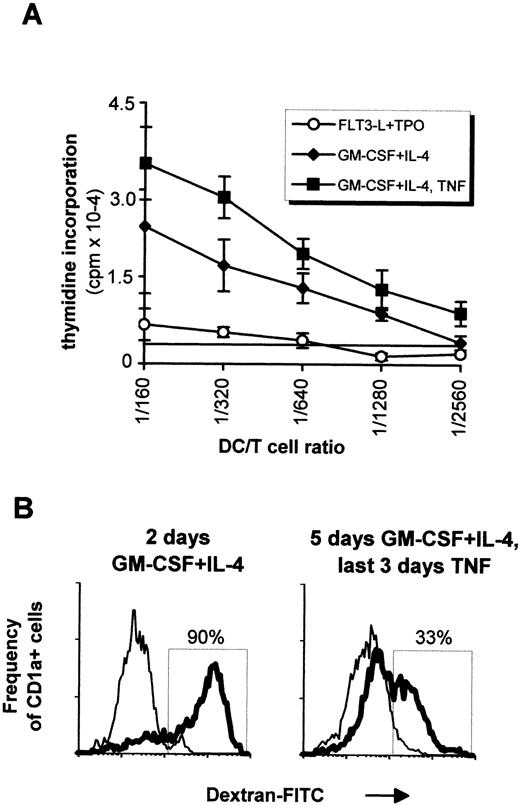
In vitro generated DC were tested in allogenic MLR. As shown in Fig4A, cells directly recovered after primary cultures induced a limited proliferation of T cells, whereas cells further incubated with GM-CSF plus IL-4 drastically enhanced the T-cell response, with a last significant DC:T-cell ratio of 1/1,280 without and 1/2,560 with TNF. Cells cultured with GM-CSF plus TNF also induced limited proliferation of T cells, comparable with that of the primary cultures (not shown). Internalization of carbohydrates via the DC mannose receptor, a process which is important in immature DC,34 was evaluated in vitro by monitoring the dextran-FITC uptake after different stimulations (Fig 4B). Ninety percent of CD1a+ cells obtained after a 2-day incubation with GM-CSF plus IL-4 became FITC-dextran-positive after 1-hour incubation at 37°C, while only 33% of th e CD1a+ cells obtained after further culturing for 3 days with GM-CSF plus IL-4 and TNF internalized FITC-dextran. This suggested that both immature and mature dendritic cells can be generated with our system.
In vitro function of DCs. (A) 5 × 104 naive T cells were incubated with graded amounts of irradiated cells obtained after 4 weeks of culture with FLT3-L plus TPO (○). Naive T cells were also incubated with cells that were additionally cultured with GM-CSF plus IL-4 for 5 days (⧫) or with GM-CSF plus IL-4 for 2 days followed by 3 days with the latter cytokines plus TNF (▪). Measurements were done in triplicates, and the last significant value over background (horizontal line) was determined as the 3H-TdR incorporation of T cells measured in absence of DCs plus 3 SD. Mean ± SD of one representative experiment out of five are shown. (B) Internalization of FITC-dextran by CD1a+ cells. Cells from a 4-week FLT3-L plus TPO culture were incubated for 2 days in GM-CSF plus IL-4 (left histogram) or for 5 days with the same cytokines, with adjunction of TNF for the last 3 days (right histogram). Cells were then incubated with FITC-dextran for 1 hour at 4°C (thin lines) or 37°C (bold lines) before labeling with PE-conjugated anti-CD1a antibody. This result is from one experiment representative of four. Experiments performed with sorted cells obtained as in Fig 6 gave similar results, that is, incubation in TNF always decreased dextran internalization irrespective of the origin of the CD1a+ cells.
In vitro function of DCs. (A) 5 × 104 naive T cells were incubated with graded amounts of irradiated cells obtained after 4 weeks of culture with FLT3-L plus TPO (○). Naive T cells were also incubated with cells that were additionally cultured with GM-CSF plus IL-4 for 5 days (⧫) or with GM-CSF plus IL-4 for 2 days followed by 3 days with the latter cytokines plus TNF (▪). Measurements were done in triplicates, and the last significant value over background (horizontal line) was determined as the 3H-TdR incorporation of T cells measured in absence of DCs plus 3 SD. Mean ± SD of one representative experiment out of five are shown. (B) Internalization of FITC-dextran by CD1a+ cells. Cells from a 4-week FLT3-L plus TPO culture were incubated for 2 days in GM-CSF plus IL-4 (left histogram) or for 5 days with the same cytokines, with adjunction of TNF for the last 3 days (right histogram). Cells were then incubated with FITC-dextran for 1 hour at 4°C (thin lines) or 37°C (bold lines) before labeling with PE-conjugated anti-CD1a antibody. This result is from one experiment representative of four. Experiments performed with sorted cells obtained as in Fig 6 gave similar results, that is, incubation in TNF always decreased dextran internalization irrespective of the origin of the CD1a+ cells.
Primary Cultures Contain a CD34−CD1a−CD14+ and a CD34−CD1a−CD14− DC Precursor
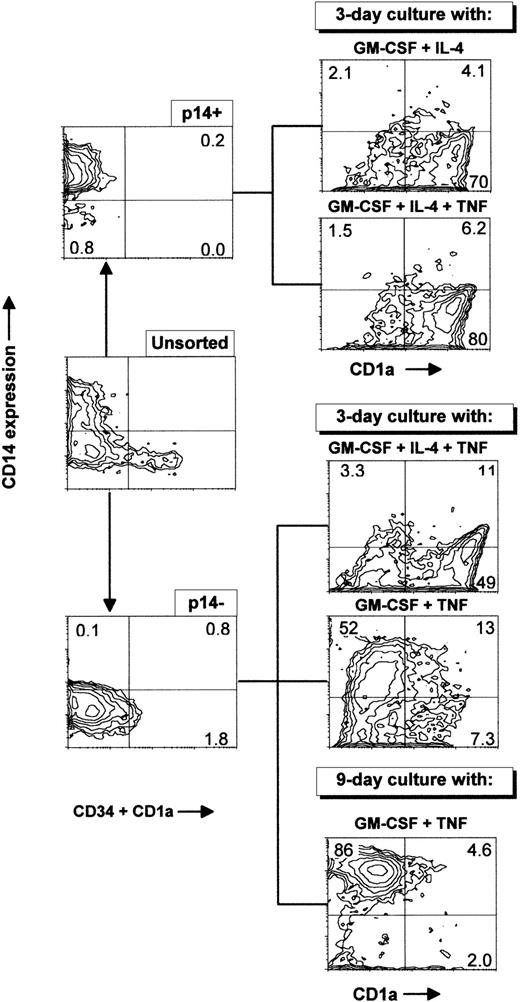
Primary cultures generated essentially CD1a−CD14− and CD1a−CD14+ cells (Fig 2). Both subsets were tested for DC induction. To this end, CD34−CD1a−CD14+ (p14+) cells, or CD34−CD1a−CD14−(p14−) cells were sorted by flow cytometry, and induced with different cytokine combinations as described in Fig5 and Table 1. As shown in Table 1, when incubated with GM-CSF plus IL-4 for 3 days, 74% of the p14+ cells became CD1a+CD14−, and 62% of the CD1a+ cells incorporated FITC-dextran, ie, immature DC were generated, which developed small dendritic processes (Fig 3B). When these cells were further incubated for 4 days with GM-CSF, IL-4, and TNF, CD1a+ cells increased to about 80% and only 30% of such cells internalized FITC-dextran; the percentage of CD83+ and CD80+ cells increased, and dendritic processes were more developed (Fig 3C). The allostimulatory capacity was high (with a last significant DC:T-cell ratio of 1/1,280). Thus, mature DCs were also generated from the p14+fraction. The total number of cells obtained after 7 days of induction represented 0.5-fold the input of p14+ cells (mean of three experiments; range, 0.4 to 1).
Sorting of p14+ and p14−cells present after long-term culture with FLT3-L plus TPO. Ex vivo–sorted CD34+ cord blood cells were grown for 21 days in FLT3-L plus TPO, with an increase in total cell number of 110-fold and were frozen. Cells were thawed 3 months later and grown for 26 extra days in the same conditions, with an increase in total cell number of 12-fold, giving an overall proliferation of 1,320-fold. Cells were then sorted in either CD1a−CD14+CD34−(p14+) or CD1a−CD14−CD34−(p14−) with a cocktail of anti–CD1a-PE, anti–CD34-PE, and anti–CD14-FITC MoAbs and cultured for 3 days with GM-CSF and IL-4, GM-CSF, IL-4, and TNF or for 3 and 9 days with GM-CSF and TNF. These data are from one experiment representative of seven. Sorting cells from primary cultures at different time points produced similar results.
Sorting of p14+ and p14−cells present after long-term culture with FLT3-L plus TPO. Ex vivo–sorted CD34+ cord blood cells were grown for 21 days in FLT3-L plus TPO, with an increase in total cell number of 110-fold and were frozen. Cells were thawed 3 months later and grown for 26 extra days in the same conditions, with an increase in total cell number of 12-fold, giving an overall proliferation of 1,320-fold. Cells were then sorted in either CD1a−CD14+CD34−(p14+) or CD1a−CD14−CD34−(p14−) with a cocktail of anti–CD1a-PE, anti–CD34-PE, and anti–CD14-FITC MoAbs and cultured for 3 days with GM-CSF and IL-4, GM-CSF, IL-4, and TNF or for 3 and 9 days with GM-CSF and TNF. These data are from one experiment representative of seven. Sorting cells from primary cultures at different time points produced similar results.
p14− cells incubated with GM-CSF plus IL-4 and TNF together for 3 days became 49% CD1a+CD14−and 42% of such cells incorporated FITC-dextran (Table 1). Cells cultured for 7 days in the same conditions became 60% CD1a+CD14− and only 25% of the CD1a+ cells incorporated dextran. However, CD83 and CD80 expression was increased compared with the 3-day cultures. Cells displayed an allostimulatory activity with a last significant DC:T-cell ratio of 1/640. The total cell number after 7 days was 0.5 ± 0.2-fold the input value (mean ± SD of three experiments). Analysis of early time points of induction showed that no CD14+ cells were generated during DC induction under these conditions (data not shown). Thus, p14− cells could be induced directly into DC with GM-CSF plus IL-4 and TNF, being mostly immature on day 3, and acquiring more extensively the features of mature DC on day 7.
p14− Cells Can Generate DC Via an Intermediate CD14+ DC Precursor
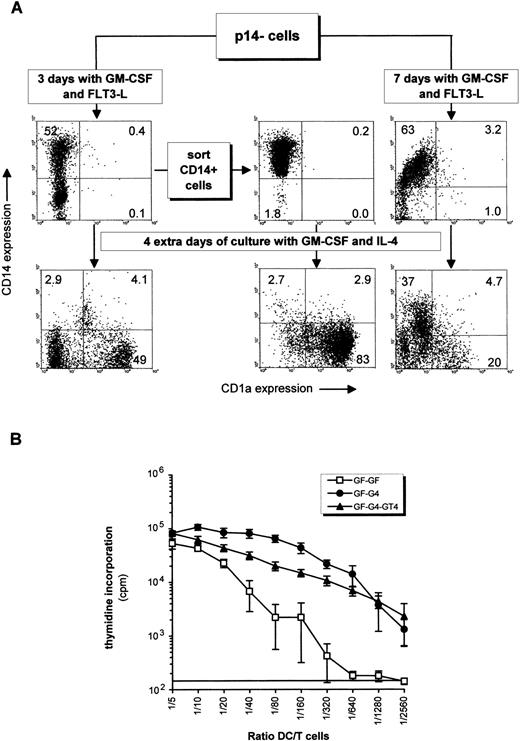
When p14− cells were first incubated with GM-CSF and TNF (or GM-CSF and FLT3-L) for 3 days, ie, without IL-4, 52% became CD1a−CD14+ and CD1a+CD14− cells were below 10% in both cases (Fig 5; Fig 6A, upper left panel). The total cell number increased, respectively, by 1.7 ± 0.8-fold and 3.4 ± 1.3-fold (mean ± SD of four experiments). When further exposed to GM-CSF and IL-4 for 4 days, the proportion of p14− becoming CD1a+CD14− raised to 31% by the mean (Table 1; 49% in Fig 6A, lower left panel), and the CD1a−CD14+ cells previously induced with GM-CSF and TNF (or FLT3-L) decreased (Table 1). The majority of the CD1a+ cells incorporated dextran. If p14−cells were cultured for 4 more days with GM-CSF, IL-4 and TNF, CD83 and CD80 were induced, and cells predominantly acquired a mature morphology as seen on cytospin smears (Fig 3F). The allostimulatory efficiency was high (Fig 6B). To evaluate if CD14+ cells obtained from the p14− cells could generate DC, such cells were sorted and were cultured with GM-CSF and IL-4 for 4 days. Consistent with the above hypothesis, these cells produced almost exclusively CD1a+CD14− cells (Fig 6A, middle panels). p14− cells cultured for 7 days with GM-CSF plus TNF (or FLT3-L) differentiated into CD14+ cells with a low allostimulatory activity (last significant DC:T-cell ratio 1/80 and 1/160, respectively), ie, these cells displayed a monocytic phenotype (Table 1). Most of these cells could no longer mature into DC (Fig 6A, lower right panel), as if the cells remained locked in the monocytic lineage. No significant CD1a+ cells were generated after 9 days of culture with GM-CSF plus TNF (Fig 5), whereas cells had increased in size, were partially adherent, and resembled macrophages (Figs 3D and E). These last observations suggested that p14− DC precursors could give rise to DC indirectly via a CD14+ precursor only if the exposure to GM-CSF and TNF or GM-CSF and FLT3-L was limited in time.
Indirect induction of DCs from p14− cells via a CD14+ intermediate precursor. (A) p14− cells sorted from a 28-day primary culture with FLT3-L plus TPO and SCF were incubated with GM-CSF plus FLT3-L for 3 and 7 days. CD14+ cells generated from the 3-day culture in GM-CSF plus FLT3-L were sorted out and all cell suspensions were additionally cultured for 4 days in GM-CSF plus IL-4 (bottom histograms). (B) Alloreactivity of some of the cellular fractions depicted in (A). GF-GF: p14− cells incubated for 7 days in GM-CSF plus FLT3-L, corresponding to the upper right histogram in (A); GF-G4: p14− fraction incubated 3 days in GM-CSF and FLT3-L, then 4 days with GM-CSF and IL-4, corresponding to the lower left histogram in (A); GF-G4-GT4: p14− fraction incubated 3 days in GM-CSF plus FLT3-L, 4 days with GM-CSF and IL-4, and 4 more days with these cytokines plus TNF. Counts per minute are displayed in a log scale to facilitate end point reading of low values. The last significant value over background is determined as in Fig 4A. Mean ± SD of quadruplicates are shown.
Indirect induction of DCs from p14− cells via a CD14+ intermediate precursor. (A) p14− cells sorted from a 28-day primary culture with FLT3-L plus TPO and SCF were incubated with GM-CSF plus FLT3-L for 3 and 7 days. CD14+ cells generated from the 3-day culture in GM-CSF plus FLT3-L were sorted out and all cell suspensions were additionally cultured for 4 days in GM-CSF plus IL-4 (bottom histograms). (B) Alloreactivity of some of the cellular fractions depicted in (A). GF-GF: p14− cells incubated for 7 days in GM-CSF plus FLT3-L, corresponding to the upper right histogram in (A); GF-G4: p14− fraction incubated 3 days in GM-CSF and FLT3-L, then 4 days with GM-CSF and IL-4, corresponding to the lower left histogram in (A); GF-G4-GT4: p14− fraction incubated 3 days in GM-CSF plus FLT3-L, 4 days with GM-CSF and IL-4, and 4 more days with these cytokines plus TNF. Counts per minute are displayed in a log scale to facilitate end point reading of low values. The last significant value over background is determined as in Fig 4A. Mean ± SD of quadruplicates are shown.
DC Can Be Generated in the Absence of Bovine Serum
All previous manipulations were repeated in the absence of FCS, with medium containing 5% pooled human serum AB+. CD34+ cells grown for 24 days in such conditions with FLT3-L plus TPO produced a 154-fold increase in total cell number (mean of two experiments; range, 100- 207-fold), not significantly different from cultures with FCS. CD14+, CD40+, and HLA-DR+ cells were slightly more numerous than with FCS (Table 2; see Table 1), but cells differentiated into DC in a similar manner. After a 3-day exposure to GM-CSF and IL-4, 47% of the cells were CD1a+CD14−, and 70% of the CD1a+cells actively internalized FITC-dextran. Further exposition to TNF upregulated the relative number of CD83+, CD86+, and CD40+ cells. These cells were highly efficient in MLR, with a last significant DC:T-cell ratio of 1/1,280. Therefore, DCs generated with or without FCS from CD34+cells were comparable. This last result may open up potential clinical applications by using in vitro amplified autologous DCs for immunotherapy.
DISCUSSION
Here, we describe the induction of DCs from CD34+ cells after a two-step process. The first step consists of the extensive amplification of hematopoietic progenitors with FLT3-L plus TPO with or without SCF, and the second consists of the differentiation of the amplified cells with GM-CSF and IL-4 into functional DCs. In the first step, a 16,600-fold amplification in total cell number was obtained after 8 weeks of culture with FLT3-L and TPO, and growth was further potentialized by SCF. Piacibello et al31 reported a 5 × 107-fold amplification in total cell number over a period of 25 weeks by using FLT3-L and TPO, suggesting that we are far from exhausting the amplification potential of this system. In the second step, cells acquired all the features of in vitro DCs in terms of surface phenotype, function, and morphology without further amplification in cell number, indicating that the final number of fully differentiated DCs obtained in this system relied essentially on the duration and the amplification rate of the primary cultures.
Primary cultures simultaneously produced CD34−CD1a− CD14+(p14+) and CD34−CD1a−CD14− (p14−) DC precursors, which were both capable of differentiating into immature and mature DCs, depending on the absence or presence of TNF, in addition to GM-CSF and IL-4. Thus, the combination of FLT3-L plus TPO was able to induce long-lasting DC precursors from CD34+cells, which maintained their growth potential and were not committed to a specific lineage as long as they remained in this cytokine mix. Furthermore, the addition of SCF to these cytokines increased the cellular yield of primary cultures without altering the ability of the cells to differentiate into DCs, as it has been reported in other systems where DCs are generated by direct exposure of CD34+cells to GM-CSF and TNF.13,15 p14+ cells readily differentiated into DCs in the same way as the CD14+ DC precursor found in the peripheral blood by Sallusto and Lanzavecchia,10 including the differentiation into immature DCs (CD83−, dextran uptake-positive cells) with GM-CSF and IL-4, and the subsequent maturation into CD83+, dextran uptake-negative cells after adjunction of TNF. It is likely that these precursors are the same.
p14− cells were able to generate DCs in two different ways, either directly with GM-CSF plus IL-4 and TNF, without the induction of a CD14+ cellular intermediate, or indirectly, via an intermediate CD14+ precursor, if cells were incubated first with GM-CSF plus TNF (or FLT3-L), and subsequently with GM-CSF plus IL-4. Contrary to the p14+ cells generated with FLT3-L plus TPO with and without SCF in the primary cultures, CD14+ cells generated with GM-CSF plus TNF, or GM-CSF plus FLT3-L in the absence of TPO, progressively lost their ability to mature into DC if the exposure to GM-CSF plus TNF (or FLT3-L) exceeded 3 days. Either the presence of GM-CSF or the absence of TPO seemed responsible for the irreversible commitment of CD14+ cells into the monocytic lineage. When FLT3-L was added during the core DC differentiation with IL-4, GM-CSF, and TNF, it did not qualitatively or quantitatively influence the process of DC induction. It is therefore likely that FLT3-L essentially acts on the amplification of early DC precursors, but does not influence the process of DC induction per se.
Activation markers such as CD83 and CD80 were, after DC maturation with TNF, upregulated on about 50% of the p14+ and 20% to 30% of the p14−, suggesting that both sets of progenitors did not display the same pattern of in vitro activation, particularly concerning CD83 upregulation. p14+ cells, which remain in the primary cultures for several weeks, may contain, as it has been recently reported for peripheral blood CD14+ DC precursors, preformed cytoplasmic CD83, which is readily translocated to the cellular surface upon activation.36 This may not hold true for the p14− cells, which represent a more immature DC progenitor, as demonstrated by their ability to give rise to a CD14+ DC progenitor. DCs maturating from p14−cells with GM-CSF, IL-4, and TNF may, therefore, require a longer inducing period or additional stimuli such as monocyte-conditioned medium,37 lipopolysaccharide,34 or supplementary cytokines38 to increase CD83 expression to a level corresponding to that of DCs generated from p14+cells.
Caux et al15 described the simultaneous production of a CD1a+CD14− and a CD1a−CD14+ DC precursor from highly purified CD34+ cells. With the same system, Rosenzwajg et al39 showed that both precursor populations were initiated presumably via a CD34−CD13+Lin−transient precursor. The reported phenotype would be compatible with that of the CD34−CD1a− CD14−(p14−) progenitor described in this work. However, because both investigators found that GM-CSF and TNF induced DCs (ie, without IL-4), it is unlikely that these precursors are the same. We were unable, even after long-term exposure to GM-CSF and TNF, to generate either CD1a+ cells or allostimulatory cells from p14− precursors. Thus, the cytokines used during the early stages of CD34+ cell culture affect the subsequent DC differentiation capacity. More recently, Herbst et al18have described the production of a CD33+, CD13+, CD4+, CD38+, CD44+, CD34−, CD14−, CD1a− DC/monocytic precursor after the culture of CD34+ cells for 7 days in IL-3, IL-6, and SCF and then for 9 days in GM-CSF with or without IL-4. These cells could then be induced into DCs with GM-CSF plus IL-4 or into monocytes with GM-CSF alone.40 This precursor may be identical to our p14− fraction. However, the cytokine mix used in the primary cultures did not support such extensive proliferation of DC precursors as observed with FLT3-L and TPO with and without SCF.
The system described in our study provides an additional tool for studying in detail the generation of DC precursors in vitro, as well as for certain biochemical characterization of DC, where a high number of cells is required. Alternatively, long-lasting primary cultures could be used as a reservoir of DC precursors, ready to be induced into DCs whenever needed for patients. This may be particularly relevant in cases of immunotherapy treatments requiring repetitive injections of autologous DCs loaded with specific antigens, where the long-term cultures of DC precursors would avoid repetitive stem cell mobilization, especially when tumor cell contamination can no longer be overcome.
ACKNOWLEDGMENT
We thank Dr O. Irion for access to cord blood samples, Dr F. Rousset for helpful comments, N. Brouwers for technical help, D. Wohlwend for cell sorting on FACStar, M. Pisteur and P. Carraux for the photographic work on cytospin, and T. Florestan for carefully reading the manuscript.
Supported in part by grant of the Swiss National Science Foundation (Grant No. 31-3343793) to R.H.Z., and by the Fondation pour la lutte contre le cancer et pour des recherches médico-biologiques, Genève.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Vincent Kindler, PhD, Division of Hematology, Geneva University Hospital, 25, Micheli-du-Crest, 1211 Geneva 14, Switzerland; e-mail: vincent.kindler@hcuge.ch.