Abstract
Agonists induce inside-out IIbβ3signaling resulting in fibrinogen binding and platelet aggregation. These in turn trigger outside-in signaling resulting in further platelet stimulation. Because the Syk tyrosine kinase is activated during both phases of integrin signaling, we evaluated its role in IIbβ3 function in murine platelets rendered null for Syk by gene targeting and in human platelets incubated with piceatannol, a tyrosine kinase inhibitor reportedly selective for Syk. Both Syk null murine platelets and piceatannol-treated human platelets exhibited a partial, but statistically significant defect in activation of IIbβ3 by adenine diphosphate (ADP) ± epinephrine as assessed by fibrinogen binding. Syk null platelets adhered normally to immobilized fibrinogen, and mice with these platelets exhibited normal tail bleeding times. In contrast, piceatannol treatment of human platelets completely inhibited platelet adhesion to immobilized fibrinogen. The discrepancy in extent of integrin dysfunction between murine and human platelet models may be due to lack of specificity of piceatannol, because this compound inhibited the activity of Src and FAK as well as Syk and also reduced tyrosine phosphorylation of multiple platelet proteins. These results provide genetic evidence that Syk plays a role in IIbβ3 signaling in platelets and pharmacological evidence that, although piceatannol also inhibits IIbβ3 signaling, it does so by inhibtion of multiple protein tyrosine kinases.
THE MOST ABUNDANT integrin on platelets, αIIbβ3 (glycoprotein [GP] IIb-IIIa), participates in signaling events that are critical for successful platelet aggregation, consolidation of the platelet aggregate, and hemostasis. Upon receiving an inside-out signal during the stimulation of platelets by agonists such as adenosine diphosphate (ADP), epinephrine, and thrombin, the receptor function of αIIbβ3 is activated, resulting in the binding of soluble fibrinogen or von Willebrand factor. Ligand binding, together with subsequent platelet-platelet interactions during aggregation, trigger the αIIbβ3-mediated outside-in signaling processes that generate stable platelet aggregates.1 Although a variety of signaling events occur in activated platelets, including phosphoinositide turnover, calcium mobilization, arachadonic acid metabolism, activation of MAP kinases, and phosphorylation of numerous proteins on serine/threonine and tyrosine residues,2 3 a major unsolved problem is the identification of the pathways used for the signal transduction to and from αIIbβ3.
A preponderance of circumstantial evidence supports a role for Syk, a 72-kD protein tyrosine kinase, in both inside-out and outside-in αIIbβ3 signaling. For example, Syk is phosphorylated early in response to stimulation of platelets by thrombin, ADP, or collagen, regardless of the activation and/or ligand binding status of the αIIbβ3integrin.4,5 Because each of these agonists is capable of inducing αIIbβ3 activation, Syk has become a candidate for involvement in inside-out αIIbβ3 signal transduction. Additionally, Syk is thought to have a proximal position in the outside-in αIIbβ3 signal transduction cascade, because tyrosine phosphorylation and activation of Syk occurs rapidly after platelet aggregation mediated by fibrinogen binding and signaling through αIIbβ3. Syk is the only tyrosine kinase in platelets that has been shown to be activated directly in response to αIIbβ3 ligation by soluble ligand.2,4 In addition to αIIbβ3 signaling, Syk has recently been implicated in collagen-induced platelet signaling. Syk becomes tyrosine phosphorylated upon collagen-induced platelet activation and associates with GP VI through the intermediary immune receptor tyrosine-based activation motif (ITAM)-containing Fcγ signaling subunit and indeed Syk-deficient murine platelets fail to respond to collagen.6 Further evidence to support a role of Syk in platelet function comes from studies using piceatannol, a tyrosine kinase inhibitor reported to exhibit selectivity toward Syk. Piceatannol has been shown to inhibit platelet aggregation induced by collagen, thrombin, or the thromboxane analogue U46619.5
The present study was designed to determine the role of Syk in αIIbβ3 signal transduction using two complementary approaches. First, Syk-deficient murine platelets, generated using gene targeting methodology, were subjected to functional analysis. Second, a pharmacologic model of Syk inhibition was used involving pretreatment of human platelets with the tyrosine kinase inhibitor, piceatannol. Interestingly, although piceatannol was found to inhibit both αIIbβ3 activation by platelet agonists and αIIbβ3-dependent platelet adhesion and tyrosine phosphorylation, Syk deficiency was found to have an effect only in one of the events studied herein, namely the activation-induced binding of soluble fibrinogen to αIIbβ3 in platelets stimulated with ADP ± epinephrine. These observations establish a contributory role for Syk in αIIbβ3 signaling. Furthermore, they demonstrate that the discrepancy between the effects of piceatannol and Syk deficiency can be explained by a heretofore unappreciated lack of selectivity of this inhibitor.
MATERIALS AND METHODS
Reagents.
Antibodies to ZAP-70 (M-20), Syk (C-20 or 4D10), and Fyn (FYN3) were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Antibody AB-1 to v-Src and purified Src enzyme were from Oncogene Sciences (Cambridge, MA). The antiphosphotyrosine antibody 4G10 and the purified enzymes Fyn and Lyn were from UBI (Lake Placid, NY). PY-20 was purchased from Transduction Laboratories (Lexington, KY). The rabbit antimouse Syk polyclonal, #2131; the αIIbβ3 monoclonal antibody, A2A9; and the rabbit anti-FAK polyclonal have been previously described.7-9 Rabbit antihuman αIIbβ3 (#41), which cross-reacts with mouse αIIbβ3, was obtained by immunizing a rabbit with purified αIIbβ3 protein. PAC-1 antibody specific for the activated conformation of αIIbβ3 was fluorescein isothiocyanate (FITC) conjugated as described.10 Horseradish peroxidase (HRP)-conjugated antirabbit IgG and antigoat IgG were from Jackson Immunoresearch Laboratories (West Grove, PA). HRP-conjugated antimouse IgG, ECL detection kits, and radiolabeled 33P γ-adenosine triphosphate (ATP) were from Amersham Life Science Inc (Arlington Heights, IL). Baculo-virus produced murine Syk was from S. Harmer and A. L. DeFranco (University of California San Francisco, San Francisco, CA). Piceatannol was the kind gift of Mark Cushman (Purdue University, West Lafayette, IN) and had a purity greater than 98% by high-performance liquid chromatography (HPLC). Additional piceatannol was purchased from Boehringer Mannheim Corp (Indianapolis, IN). ADP, epinephrine, phorbol myristate acetate (PMA), and enolase were from Sigma Chemical Co (St Louis, MO). Fibrinogen was purified from human plasma.11 The cyclic RGD peptide Mpr-RGDWP-Pen-NH2 was previously described.12
Generation of Syk-deficient chimeric mice.
Radiation chimeras were generated as previously described.6Briefly, 8- to 10-week-old BALB/c mice received two doses of irradiation from a 60Co source (each of 500 rads at 3 hours apart). The mice were then reconstituted with an intravenous injection of 1.5 × 106 fetal liver cells obtained from 16.5-daySyk−/−,Syk+/−, or Syk+/+ mouse fetuses. These were generated by intercrossing mice heterozygous for the Syktm1Tyb mutation (Syk+/−) backcrossed for at least five generations onto a B10.D2 background.8 Reconstituted mice received neomycin sulfate (0.16%) in their drinking water for 4 weeks after irradiation and were used for experiments between weeks 5 and 6 after irradiation. The genotype of the reconstituting liver cells was confirmed in each case by Southern blotting.8
Platelet preparation and pretreatment with piceatannol.
To obtain human platelets, blood was collected from healthy donors and resting platelets were prepared as in Law et al.13 For pretreatment with piceatannol, the rested platelets (at 4 to 5 × 108/mL) were incubated with the desired concentration of piceatannol, or control dimethyl sulfoxide (DMSO) vehicle, for 10 to 15 minutes.
In the case of murine platelets, blood was collected by cardiac puncture from anesthetized mice. For fluorescence-activated cell sorting (FACS) experiments, 700 μL of blood was drawn into a syringe containing 1/10th volume of 3.8% trisodium citrate (TSC). The blood was transfered to a 1.5-mL eppendorf tube, 700 μL of saline was added, and the sample was centrifuged at approximately 90g for 10 minutes. The platelet-rich plasma (PRP) layer was carefully removed and used in the FACS experiments detailed below. To obtain washed platelets, 700 μL of blood was drawn into a syringe containing 200 μL ACD (2.5% trisodium citrate, 2% dextrose, 1.5% citric acid [monohydrate]), 500 μL saline, and prostaglandin E1 (PGE1; to give a final concentration of 50 ng/mL). The mixture was transfered into a 1.5-mL microcentrifuge tube and centrifuged at 90g for 10 minutes, and the PRP layer was removed to a new tube. For maximal recovery of platelets, the red blood cell pellet from the first spin was diluted to 1.4 mL with CGS (0.038% trisodium citrate, 0.6% dextrose, 0.72% NaCl, pH to 7.0) containing PGE1 at 25 ng/mL and recentrifuged, and the supernatant was collected. The pooled supernatant samples were made up to a volume of 1.4 mL with CGS and after gentle mixing were spun at 16,000g for 7 seconds. The pelleted platelets were resuspended in Ca2+- and Mg2+-free Tyrodes buffer, counted, and diluted to 1.2 × 108/mL. MgCl2 was then added to a final concentration of 1 mmol/L.
Adhesion to immobilized fibrinogen.
For assay with human platelets, the platelets were resuspended (at 109/mL) in phosphate-buffered saline (PBS), pH 7.4, and labeled with 6 μmol/L BCECF-AM [2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester; Molecular Probes Inc, Eugene, OR] for 30 minutes at 37°C. They were then pelleted and resuspended at 4 × 108/mL in Tyrodes buffer supplemented with 1 mmol/L Ca2+. Human fibrinogen in PBS, pH 8.0, was plated onto Immulon-2 microtiter plates at concentrations ranging from 1 ng to 2 μg/well and incubated overnight at 4°C. Plates were washed two times with PBS, pH 7.4, and blocked for 2 hours at room temperature with 20 mg/mL radioimmunoassay (RIA) grade bovine serum albumin (BSA; Sigma Chemical Co) in PBS. Fifty microliters of labeled platelets (2 × 108 total) was added per well. After 1 hour of incubation, nonadherent platelets were removed by aspiration and the wells were washed twice with 150 μL Tyrodes buffer supplemented with 1 mmol/L Ca2+. Adherent platelets were then quantitated on a Fluorescence Concentration Analyser (Pandex, Mundelein, IL) at excitation/emmission wavelengths of 485/535 nm.
Adherence of murine platelets to immobilized fibrinogen was determined using BSA-blocked fibrinogen microtiter wells plates prepared as described above, followed by the addition of 50 μL of platelets at 1.2 × 108/mL. After 1 hour of incubation, nonadherent platelets were removed and the wells were washed twice with 150 μL Tyrodes buffer supplemented with 1 mmol/L Mg2+. One hundred fifty microliters of pNpp buffer (0.1 mol/L citrate, pH 5.4, 0.1% Triton X-100, 5 mmol/L para-nitropheylphosphate) was added for 1 hour at room temperature. Then, 100 μL of 2 mol/L NaOH was added and adherent platelets were quantitated in a microplate reader (Molecular Devices, Menlo Park, CA) at 405 nm. In assays using piceatannol, the murine platelets were pretreated with the indictated concentration of inhibitor for 10 minutes before addition to the fibrinogen-coated microtiter wells.
The percentage of platelets adhering was determined by calculating the ratio of bound/maximal signal at 405 nm, where maximal reading was obtained from a microtiter well containing 2 × 108platelets that was not subjected to washing procedures.
FACS analysis of FITC–PAC-1 and FITC-fibrinogen binding to platelets.
The analysis of PAC-1 binding to agonist-activated human platelets was performed as described.10 To assess the binding of fibrinogen to murine platelets, human fibrinogen was labeled with FITC using a similar method to that described for PAC-1 labeling.10 Basically, human fibrinogen (0.4 mL at 5mg/mL) was mixed with 60 μL 1 mol/L HaHCO3, pH 9.3, and 120 μL of FITC-cellite at 20 mg/mL in PBS for 1 hour at room temperature. The mix was then loaded onto a PD10 column, fractions were collected from PBS washing, and the fluorophore-labeled fibrinogen fractions were pooled. On the day of an experiment mixes were made up consisting of the desired stimuli and a 1/6 dilution of FITC-fibrinogen in Walsh buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2.6H2O, 3.3 mmol/L NaH2PO4.H2O, 3.8 mmol/L HEPES, pH 7.4) containing 0.1% BSA and 0.1% dextrose. Twenty microliters of PRP was added to 30 μL of these mixes, and samples were incubated for 30 minutes at room temperature in the dark. Samples were then diluted with 0.5 mL Tyrodes buffer and analyzed on a FACScan (Becton Dickinson, Mountain View, CA).
Syk expression levels in murine platelets.
A 100-μL sample of PRP from each mouse was washed with CGS (16,000g for 2 minutes) and lysed in RIPA buffer (1% Triton X-100, 1% deoxycholate acid [sodium form; DOC], 0.1% sodium dodecyl sulfate [SDS], 20 mmol/L Tris, pH 7.5, 5 mmol/L EDTA) containing 1 mmol/L phenylmethylsulfonyl fluoride, 20 μmol/L leupeptin, and 0.15 U/mL aprotinin. Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 7.5% gel), transfered to nitrocellulose, and immunoblotted with either rabbit anti-Syk antiserum (1/1,000) to determine whether the Syk protein was expressed in the platelets or an anti-αIIbβ3 polyclonal antibody (#41 at 2 μg/mL) to determine expression levels of αIIbβ3. After detection with enhanced chemiluminescence (ECL) reagent, immunoblots were stripped (according to the manufacturer’s recommendation; Amersham) and reprobed with antiserum to ZAP-70 (2 μg/mL).
Determination of protein tyrosine phosphorylation in piceatannol-treated human platelets.
Platelets pretreated with piceatannol, as described above, were incubated at 5 × 108/mL with PBS, 0.1 U/mL thrombin, or 0.1 U/mL thrombin with stirring for 2 minutes before lysis in RIPA lysis buffer. For antiphosphotyrosine analysis of total protein, the proteins were separated on gels, transferred to nitrocellulose, and immunoblotted with 4G10 and PY-20. For assessment of FAK phosphorylation, FAK was immunoprecipitated from platelet lysates by incubating the lysates with anti-FAK antiserum and protein A/G sepharose. The sepharose-bound material was washed twice with RIPA buffer and the samples were boiled before separation by SDS-PAGE. After transfering to nitrocellulose the blots were subjected to antiphosphotyrosine immunoblotting.
Determination of kinase activity of piceatannol-treated enzymes.
Either purified enzymes or enzymes obtained by immunoprecipitation with relevant antibodies from platelet lysates were incubated with the desired concentration of piceatannol or DMSO (0.5% vol/vol final) for 10 minutes. In some experiments, platelets were pretreated with piceatannol before immunoprecipitating the kinases from lysates. The samples were then subjected to an in vitro kinase assay by the addition of 0.5 μCi 33P γ-ATP, 10 μmol/L ATP with or without exogenous substrate (enolase at 1.5 μmol/L). After 15 minutes, reactions were stopped by the addition of laemmli sample buffer and boiled. Proteins were separated by SDS-PAGE and bands were visualized by autoradiography. Densitometry was performed using a Bio-Rad Imager (Bio-Rad, Hercules, CA) with Molecular Analyst software (Bio-Rad).
Bleeding times.
Mice were anesthetized with SQ ketamine cocktail (Ketamine, Xylazine, AcePromazine) and 6 minutes later the tail was completely transected 0.5 cm from the tip with a scalpel. Blood was blotted onto SurgiCut blotting paper (International Technidyne Corp, Edison, NJ) every 30 seconds and the bleeding time was defined by the time required for cessation of blood flow. Gentle blotting every 30 seconds was continued for 1 minute to detemine whether stable hemostasis had been achieved. If bleeding continued for 30 minutes it was stopped manually to prevent loss of life.
RESULTS
Genetic analysis of the role of Syk in murine platelet function.
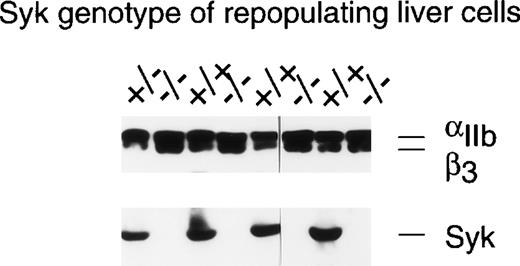
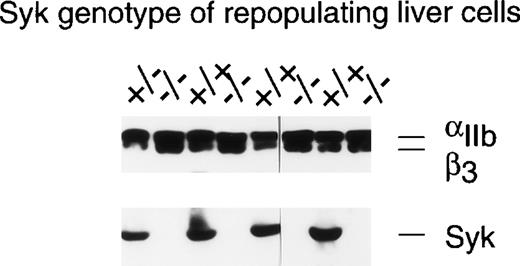
Although recent data have indicated a role for Syk in the signaling pathways activated by the binding of collagen to platelet GP VI,6 support for the role of Syk in αIIbβ3-mediated signaling events remains circumstantial. To address this issue in a more definitive fashion, we sought a genetic approach where studies could be performed on murine platelets in which the Syk protein was not expressed due to gene targetting. Because genetically engineered mice that lack Syk either die perinatally or within 1 to 5 days of birth,8 14a radiation chimera system was used in which all hematopoietic cells, including platelets, were Syk deficient (see Materials and Methods). Syk expression in platelets from individual chimeras was evaluated by Western blotting and, as can be seen from the examples in Fig 1, platelets from animals repopulated with Syk−/− liver cells did not express detectable levels of Syk, whereas appreciable levels of Syk were present in mice repopulated with liver cells genotyped asSyk+/− or Syk+/+. It was determined that 3% contamination of Syk-deficient platelets by Syk-expressing platelets could be detected in these Western blots (data not shown). Similar levels of αIIbβ3 were present in the platelets from all reconstitutions (top panel, Fig 1), indicating that the lack of Syk had no deleterious effect on αIIbβ3 expression. Two of the 38 BALB/c mice repopulated with Syk−/− liver cells were rejected for further study because they had detectable levels of Syk protein in the platelet lysates, whereas samples from 4 other mice were eliminated due to inadequate sample size.
Determination of IIbβ3protein expression in murine platelets using immunoblotting. This figure shows the analysis of platelets from 8 different radiation chimeras that were repopulated with liver cells of the indicated Syk genotype. One hundred microliters of PRP was washed with CGS and then solubilized in RIPA buffer. The proteins were separated by SDS-PAGE on a 7.5% gel and transferred to nitrocellulose. The resulting blot was cut in two at approximately the 80-kD point. The upper portion of the blot was immunoblotted with the anti-IIbβ3polyclonal #41. The lower portion of the blot was probed with the anti-Syk antiserum #2131. As little as 3% contamination of Syk null platelets with Syk-expressing platelets could be detected by this method.
Determination of IIbβ3protein expression in murine platelets using immunoblotting. This figure shows the analysis of platelets from 8 different radiation chimeras that were repopulated with liver cells of the indicated Syk genotype. One hundred microliters of PRP was washed with CGS and then solubilized in RIPA buffer. The proteins were separated by SDS-PAGE on a 7.5% gel and transferred to nitrocellulose. The resulting blot was cut in two at approximately the 80-kD point. The upper portion of the blot was immunoblotted with the anti-IIbβ3polyclonal #41. The lower portion of the blot was probed with the anti-Syk antiserum #2131. As little as 3% contamination of Syk null platelets with Syk-expressing platelets could be detected by this method.
Effect of lack of Syk on murine tail bleed times.
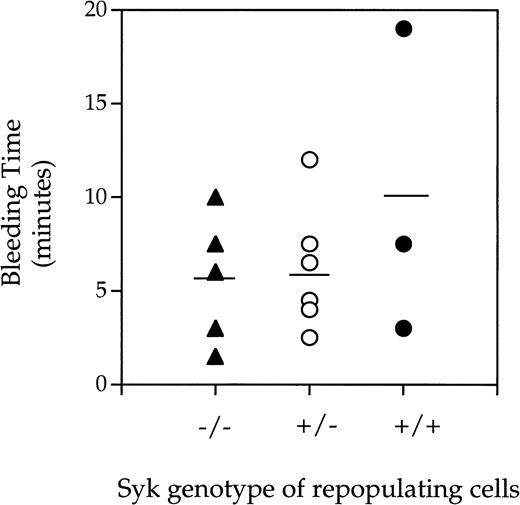
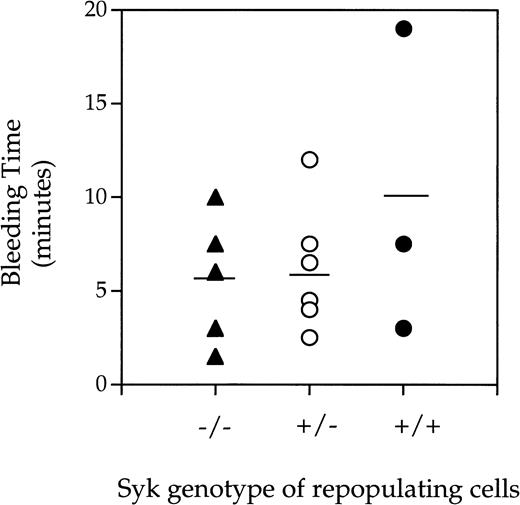
A bleeding time model was used to assess the effect of lack of Syk expression on platelet-dependent hemostasis. Prolonged bleeding time can be a result of low platelet count or defective platelet function. Indeed, mice deficient in β3, as a result of gene targetting, have prolonged bleeding times similar to that observed in humans lacking αIIbβ3expression.15 Treating mice with aspirin or a low molecular weight αIIbβ3-inhibitor also results in prolonged bleeding times (Ministri and Hollenbach, manuscript in preparation). As can be seen in Fig 2, there was no difference in bleeding times when chimeric mice lacking the Syk tyrosine kinase were compared with those expressing Syk (either heterozygotes or homozygotes). However, 2 of the 5 Syk−/− mice rebled within the 1 minute after the primary endpoint. The result given above indicates that Syk is not required for cessation of bleeding after tail transection in the mouse but suggests that Syk deficiency may decrease the stability of the hemostatic plug.
Bleeding times generated fromSyk−/−, Syk+/−, andSyk+/+ mice. Radiation chimera mice repopulated with either (▴) Syk−/−, (○)Syk+/−, or (•) Syk+/+fetal liver cells were anesthetized and 6 minutes later had 0.5 cm from the tip of the tail removed with a scalpel blade. Blood was gently blotted onto surgical blotting paper every 30 seconds until cessation of bleeding. The mean and standard deviations obtained for the bleeding times were 5.6 ± 3.4 (forSyk−/−, where n = 5), 5.9 ± 3.1 (forSyk+/−, where n = 7), and 9.8 ± 8.2 (forSyk+/+, where n = 3).
Bleeding times generated fromSyk−/−, Syk+/−, andSyk+/+ mice. Radiation chimera mice repopulated with either (▴) Syk−/−, (○)Syk+/−, or (•) Syk+/+fetal liver cells were anesthetized and 6 minutes later had 0.5 cm from the tip of the tail removed with a scalpel blade. Blood was gently blotted onto surgical blotting paper every 30 seconds until cessation of bleeding. The mean and standard deviations obtained for the bleeding times were 5.6 ± 3.4 (forSyk−/−, where n = 5), 5.9 ± 3.1 (forSyk+/−, where n = 7), and 9.8 ± 8.2 (forSyk+/+, where n = 3).
Inside-out signaling in Syk null murine platelets.
The Syk null platelets were then used to address the role that Syk might play in specific αIIbβ3-mediated signaling events. In the last decade there has been a surge of work using gene targetted mice; however, relatively little has been published on examining the function of murine platelets. Thus, we developed and adapted two assays to examine platelet function that took into account the relatively small number of platelets obtained per mouse. The first assay examined whether the absence of Syk expression had any effect on inside-out αIIbβ3signaling and used a FACS-based assay. The αIIbβ3 present on murine platelets, like its human counterpart, needs to be activated to bind soluble fibrinogen. The binding of FITC-labeled fibrinogen to murine platelets stimulated by either 1 μmol/L ADP alone or a combination of 10 μmol/L ADP plus 25 μmol/L epinephrine was used as a measure of inside-out signaling. Preliminary studies showed that the binding of FITC-fibrinogen was saturable and inhibitable by EDTA and inhibited by immunoblocking of αIIbβ3, characteristics typical of unmodified fibrinogen.
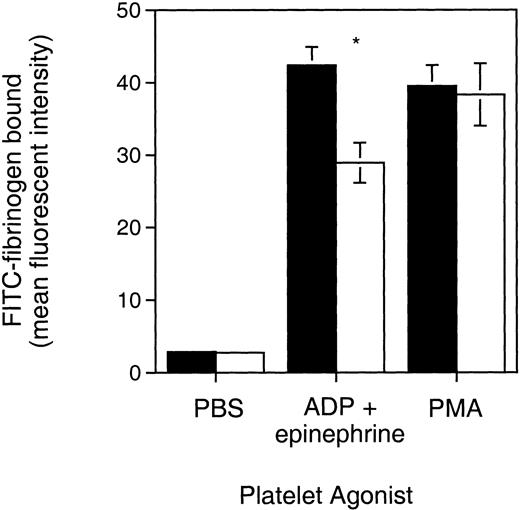
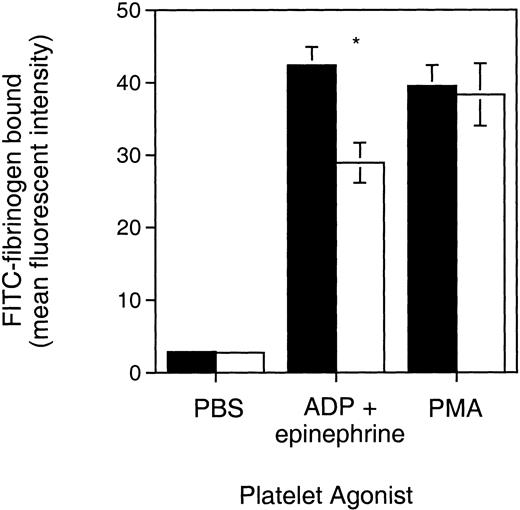
As demonstrated in Fig 3, Syk null platelets activated by ADP plus epinephrine bound 32% less fibrinogen than control. This functional defect, although partial, was statistically significant (P = .0029, Student’st-test) and was observed in the platelets from two independent batches of Syk null radiation chimeras. Similar results were observed when Syk null platelets were stimulated with ADP alone, with 28.6% less fibrinogen being bound compared with control platelets (P= .08), although this experiment was limited to a single batch of mice (data not shown). In contrast, Syk null platelets demonstrated the same fibrinogen binding as control platelets in response to direct activation of protein kinase C by PMA. This indicates that the defective activation of αIIbβ3 observed with ADP ± epinephrine was not due to some defect in αIIbβ3 per se. We conclude from these results that Syk does play a role in inside-out αIIbβ3 signaling when ADP ± epinephrine are used as agonists.
FITC-fibrinogen binding to Syk null and Syk positive platelets. PRP from (▪) Syk positive or (□) Syk null mice was treated with the indicated platelet agonists, PBS (control), ADP (10 μmol/L) plus epinephrine (25 μmol/L), or PMA (20 μmol/L). FITC-labeled fibrinogen was added with the agonists. After 30 minutes of incubation in the dark at room temperature, samples were diluted in 0.5 mL Tyrodes buffer and analyzed on a FACScan. Bars represent the geometric mean ± standard error fluorescent channel for PAC-1 binding and were 2.75 ± 0.26 (control), 28.94 ± 2.75 (ADP + epi), and 38.31 ± 4.33 (PMA) for the Syk null mice (where n = 7), and 2.85 ± 0.22 (control), 42.4 ± 2.49 (ADP + epi), and 39.5 ± 2.89 (PMA) for Syk positive mice (where n = 12, including 10 mice repopulated with Syk+/− liver cells and 2 repopulated with Syk+/+ liver cells). *P = .0029 (Student’s t-test). Similar results were obtained with a different batch of radiation chimeras.
FITC-fibrinogen binding to Syk null and Syk positive platelets. PRP from (▪) Syk positive or (□) Syk null mice was treated with the indicated platelet agonists, PBS (control), ADP (10 μmol/L) plus epinephrine (25 μmol/L), or PMA (20 μmol/L). FITC-labeled fibrinogen was added with the agonists. After 30 minutes of incubation in the dark at room temperature, samples were diluted in 0.5 mL Tyrodes buffer and analyzed on a FACScan. Bars represent the geometric mean ± standard error fluorescent channel for PAC-1 binding and were 2.75 ± 0.26 (control), 28.94 ± 2.75 (ADP + epi), and 38.31 ± 4.33 (PMA) for the Syk null mice (where n = 7), and 2.85 ± 0.22 (control), 42.4 ± 2.49 (ADP + epi), and 39.5 ± 2.89 (PMA) for Syk positive mice (where n = 12, including 10 mice repopulated with Syk+/− liver cells and 2 repopulated with Syk+/+ liver cells). *P = .0029 (Student’s t-test). Similar results were obtained with a different batch of radiation chimeras.
Binding of murine Syk null platelets to immobilized fibrinogen.
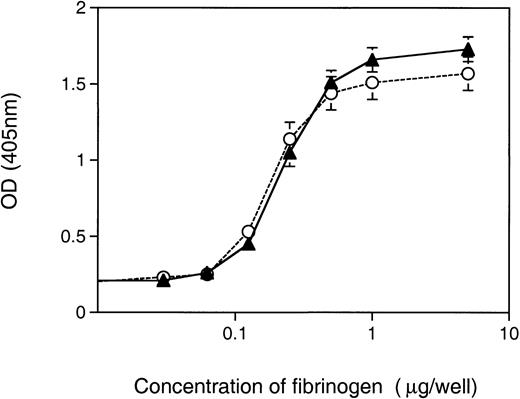
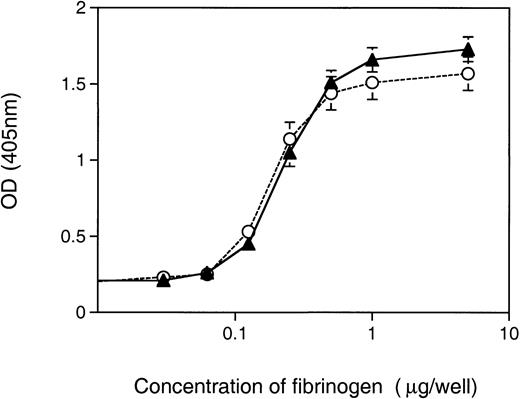
The second assay to measure αIIbβ3 function in Syk-deficient platelets assessed the role of Syk in the binding of platelets to immobilized fibrinogen. Unstimulated platelets can bind to immobilized fibrinogen and the initial recognition of immobilized fibrinogen by unstimulated platelets is possible because of the activation of fibrinogen by immobilization.16 We developed an assay that, using an acid phosphoatase detection system,17 was sufficiently sensitive to generate fibrinogen-dependent adhesion curves with platelets from a single mouse. When Syk−/− platelets were compared with control, either Syk+/− orSyk+/+, for their ability to adhere to immobilized fibrinogen, no differences were observed (Fig 4). The concentration of fibrinogen required to induce half-maximal binding was similar for Syk null (0.26 ± 0.05 μg/well) and control (0.22 ± 0.07 μg/well) platelets. Approximately 28% to 32% of Syk null or control platelets adhered to the immobilized fibrinogen at optimal fibrinogen concentrations. These results suggest that αIIbβ3-adherence to immobilized fibrinogen under static conditions does not require Syk.
Adherence of Syk null and Syk positive platelets to immobilized fibrinogen. Murine platelets (6 × 106), either (▴) Syk null or (○) Syk positive, were added to microtiter wells coated with fibrinogen (at a range of concentrations from 1 ng to 2 μg per well). After 1 hour of incubation at room temperature, wells were washed twice and 150 μL of pNpp buffer was added for a further 1 hour at room temperature. One hundred microliters of 2 N NaOH was added and adherent platelets were quantitated in a microplate reader at 405 nm. Platelets from single mice where used to generate duplicate points for platelet adherence to each fibrinogen concentration. The graph depicts the mean ± standard error at each fibrinogen concentration, where for Syk null mice n = 10 and for Syk positive mice n = 18.
Adherence of Syk null and Syk positive platelets to immobilized fibrinogen. Murine platelets (6 × 106), either (▴) Syk null or (○) Syk positive, were added to microtiter wells coated with fibrinogen (at a range of concentrations from 1 ng to 2 μg per well). After 1 hour of incubation at room temperature, wells were washed twice and 150 μL of pNpp buffer was added for a further 1 hour at room temperature. One hundred microliters of 2 N NaOH was added and adherent platelets were quantitated in a microplate reader at 405 nm. Platelets from single mice where used to generate duplicate points for platelet adherence to each fibrinogen concentration. The graph depicts the mean ± standard error at each fibrinogen concentration, where for Syk null mice n = 10 and for Syk positive mice n = 18.
Previous studies have shown that members of the same family of tyrosine kinases can compensate for a deleted kinase.18 The kinase related to Syk is ZAP-70, a 70-kD protein tyrosine kinase with 73% homology to Syk.19 To explore the possibility that ZAP-70 was compensating for the lack of Syk in the Syk null platelets, the PRP lysates were immunoblotted with an anti–ZAP-70 antibody. A very low level of ZAP-70 protein was detected in the PRP lysates, possibly due to contaminating T cells. The expression levels were comparable in both the Syk null and control samples.
Effect of piceatannol on platelet intergin signaling and protein tyrosine kinases.
Piceatannol is a tyrosine kinase inhibitor with a reported selectivity for Syk.20 Previous studies showing that this inhibitor blocks the platelet aggregation induced by several different agonists have been used to infer a role for Syk in platelet function.5 21 However, on the basis of the results obtained with Syk null murine platelets, we decided to re-examine the effect of piceatannol on a number of platelet functions.
Consistent with results obtained with Syk null murine platelets, piceatannol treatment of human platelets resulted in a partial inhibition of αIIbβ3 activation. Inhibition was dose-dependent such that PAC-1 binding to ADP-stimulated platelets was reduced by approximately 50% at a piceatannol concentration of 30 μg/mL, and consistent inhibition was observed even at 10 μg/mL (Fig 5). Similar results were obtained when TRAP, an agonist peptide to the PAR-1 thrombin receptor, was used as the agonist (data not shown). Piceatannol had no effect on the level of PAC-1 binding to platelets stimulated with 0.2 μmol/L PMA. Thus, the data obtained with both the Syk null platelets and with piceatannol suggest a role for Syk in the activation of αIIbβ3 through ADP receptors but indicate that Syk is independent of, or proximal to, agonist-induced activation of protein kinase C.
Effect of piceatannol on PAC-1 binding to platelets. Human platelets were pretreated for 10 minutes at room temperature with control DMSO vehicle or piceatannol at 1, 10, or 30 μg/mL. They were then stimulated with the desired agonist ([○] PBS control, [▴] 0.1 μmol/L ADP, [□] 1 μmol/L ADP, [•] 10 μmol/L ADP, [⧫] 0.2 μmol/L PMA) and incubated with FITC-labeled PAC-1 antibody. The level of PAC-1 bound is expressed as a percentage of maximal PAC-1 binding obtained when the DMSO-pretreated platelets were stimulated with the highest concentration of agonist (either 10 μmol/L ADP for the ADP-treated samples or 0.2 μmol/L PMA for the PMA-treated samples). Results shown are the mean ± SE values from three separate experiments.
Effect of piceatannol on PAC-1 binding to platelets. Human platelets were pretreated for 10 minutes at room temperature with control DMSO vehicle or piceatannol at 1, 10, or 30 μg/mL. They were then stimulated with the desired agonist ([○] PBS control, [▴] 0.1 μmol/L ADP, [□] 1 μmol/L ADP, [•] 10 μmol/L ADP, [⧫] 0.2 μmol/L PMA) and incubated with FITC-labeled PAC-1 antibody. The level of PAC-1 bound is expressed as a percentage of maximal PAC-1 binding obtained when the DMSO-pretreated platelets were stimulated with the highest concentration of agonist (either 10 μmol/L ADP for the ADP-treated samples or 0.2 μmol/L PMA for the PMA-treated samples). Results shown are the mean ± SE values from three separate experiments.
In stark contrast to the data obtained with Syk null murine platelets, in which lack of Syk had no effect on platelet adhesion to fibrinogen, piceatannol inhibited the binding of human platelets to immobilized fibrinogen in a dose-dependent manner, with a 30 μg/mL piceatannol pretreatment leading to complete abrogation of platelet adherence to fibrinogen (data not shown). The αIIbβ3-dependency of the platelet adherence was confirmed using either the αIIbβ3blocking antibody, A2A9, or an RGD inhibitory peptide, both of which abolished platelet adherence to the fibrinogen. Cytochalasin E (4 μmol/L), which disrupts the actin cytoskeleton, also blocked platelet adherence to fibrinogen when added to the isolated platelets (not shown).
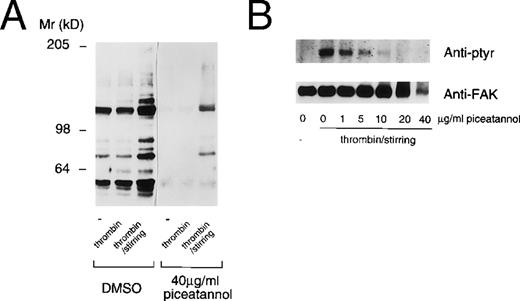
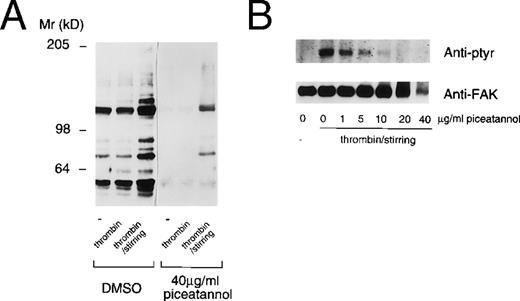
This result suggested that the effects of piceatannol might not be limited to an inhibition of Syk function. To further address this issue, we assessed the effect of piceatannol on protein tyrosine phosphorylation in platelets. The effect of piceatannol on both total protein tyrosine phosphorylation, as well as on the phosphorylation of the specific substrate, FAK, was examined. Piceatannol pretreated platelets were stimulated by the addition of thrombin (with or without stirring). Surprisingly, pretreatment of platelets with 40 μg/mL piceatannol had a profound effect on the extent of protein tyrosine phosphorylation, with the level of phosphorylation of most proteins rendered less than basal (Fig 6A). Pretreating platelets with various concentrations of piceatannol established that doses greater than 5 μg/mL decreased the tyrosine phosphorylation of a number of proteins that are induced by thrombin-induced aggregation, including FAK (Fig 6B). It is unlikely that Syk is responsible for maintaining the tyrosine phosphorylation state of proteins in the unstimulated platelets or for all the tyrosine phosphorylations induced by thrombin stimulation. Thus, the ablation of tyrosine phosphorylation of almost all proteins in control, activated, or aggregated platelets again suggests that the effects of the inhibitor are not specific to Syk.
Piceatannol inhibition of protein tyrosine phosphorylation in platelets. Human platelets were pretreated with DMSO or piceatannol (40 μg/mL) for 10 minutes at room temperature before the addition of PBS (−), 0.1 U/mL thrombin, or 0.1 U/mL thrombin plus stirring. After 2 minutes, the platelets were solubilized in RIPA buffer and the proteins were separated by SDS-PAGE on an 8% gel and transferred to nitrocellulose. (A) Total protein lysates. (B) FAK immunoprecipitated from platelet lysates. In each case the blots were probed with the antiphosphotyrosine antibodies 4G10 and PY-20. (B) The blot stripped and reprobed with the anti-FAK antibody to confirm equal loading of protein. Proteins were visualized using ECL detection methods. The molecular weight standards are indicated to the left.
Piceatannol inhibition of protein tyrosine phosphorylation in platelets. Human platelets were pretreated with DMSO or piceatannol (40 μg/mL) for 10 minutes at room temperature before the addition of PBS (−), 0.1 U/mL thrombin, or 0.1 U/mL thrombin plus stirring. After 2 minutes, the platelets were solubilized in RIPA buffer and the proteins were separated by SDS-PAGE on an 8% gel and transferred to nitrocellulose. (A) Total protein lysates. (B) FAK immunoprecipitated from platelet lysates. In each case the blots were probed with the antiphosphotyrosine antibodies 4G10 and PY-20. (B) The blot stripped and reprobed with the anti-FAK antibody to confirm equal loading of protein. Proteins were visualized using ECL detection methods. The molecular weight standards are indicated to the left.
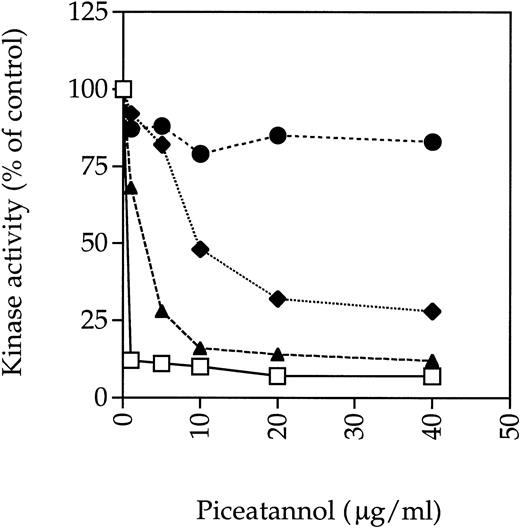
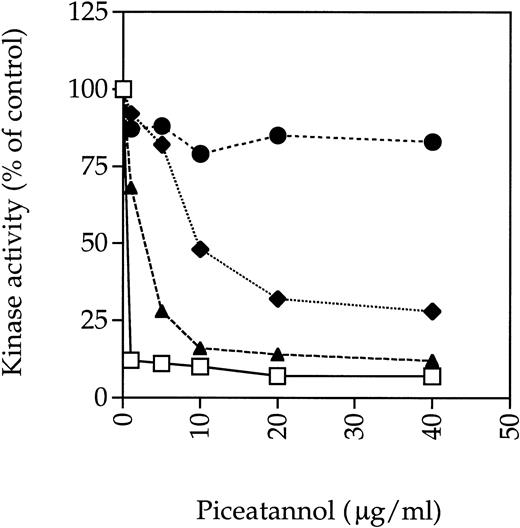
To assess whether piceatannol might be exerting an inhibitory effect through some kinases in addition to Syk, the effect of piceatannol on the activity of other tyrosine kinases known to be present in platelets was assessed. Previously, investigators have suggested that piceatannol selectively inhibits Syk, because doses in the 30 to 50 μg/mL range, which totally abrogated Syk kinase activity, did not inhibit the activity of the Src-family tyrosine kinase Lyn in in vitro kinase assays.20 We repeated this experiment and found that piceatannol at 40 μg/mL profoundly inhibited Syk kinase activity by greater than 90%, as assessed by autophosphorylation, whereas Lyn activity was unaffected. Furthermore, the kinase activity of Fyn, another member of the Src-family of tyrosine kinases, was inhibited by only approximately 20% at 40 μg/mL of piceatannol. However, the kinase activity of Src was inhibited by greater than 75% at 40 μg/mL of piceatannol and by 50% at 10 μg/mL (Fig 7).
Effect of piceatannol on tyrosine kinase activity. (⧫) Src, (•) Fyn, (□) Syk, or (▴) FAK were incubated with the indicated concentration of piceatannol for 10 minutes at room temperature. The enzymes were then incubated with 0.5 μCi33P γ-ATP for 15 minutes at room temperature. Reactions were stopped by the addition of Laemmli sample buffer and the proteins were separated by SDS-PAGE. Gels were dried down and the radioactive bands were visualized by autoradiography. Densitometry was preformed using a Bio-Rad Imager equipped with Molecular Analyst software. The graph shows the levels of autophosphorylation of relevant enzymes expressed as a percentage of control, where control was the level of autophosphorylation obtained in the absence of piceatannol but with DMSO vehicle present. Similar results were obtained in at least two separate experiments with each enzyme and also when enolase was used as an exogenous substrate.
Effect of piceatannol on tyrosine kinase activity. (⧫) Src, (•) Fyn, (□) Syk, or (▴) FAK were incubated with the indicated concentration of piceatannol for 10 minutes at room temperature. The enzymes were then incubated with 0.5 μCi33P γ-ATP for 15 minutes at room temperature. Reactions were stopped by the addition of Laemmli sample buffer and the proteins were separated by SDS-PAGE. Gels were dried down and the radioactive bands were visualized by autoradiography. Densitometry was preformed using a Bio-Rad Imager equipped with Molecular Analyst software. The graph shows the levels of autophosphorylation of relevant enzymes expressed as a percentage of control, where control was the level of autophosphorylation obtained in the absence of piceatannol but with DMSO vehicle present. Similar results were obtained in at least two separate experiments with each enzyme and also when enolase was used as an exogenous substrate.
These data were in contrast to previous reports concluding that Src was not inhibited by piceatannol based on observations showing that the kinase activity of Src immunoprecipitated from platelets was not affected by piceatannol pretreatment of the cells.5 To determine whether different assay protocols accounted for the different results obtained in these studies because our experiments used purified enzymes, Src was immunoprecipitated from lysates made from platelets pretreated with various concentrations of piceatannol. Significant piceatannol inhibition of Src was still observed in these experiments, although the dose-response curve was shifted to right, with approximately 50% inhibition of Src activity seen at 20 μg/mL piceatannol. Under the same conditions, the dose-response curve for Syk was also shifted with at least 5 μg/mL piceatannol being required to inhibit 90% of Syk activity compared with approximately 1 μg/mL when purified enzyme was incubated directly with the inhibitor (data not shown).
The effect of piceatannol on FAK, another prominent platelet tyrosine kinase, was also examined. FAK was immunoprecipitated from platelet lysates and then incubated with piceatannol before performing an immune-complex in vitro kinase assay. Both FAK autophosphorylation and the phosphorylation of exogenous substrate were dramatically decreased by 5 μg/mL piceatannol (Fig 7). Similar results were obtained with two different sources of piceatannol. These results show that piceatannnol can significantly inhibit FAK and Src at the 30 μg/mL dose often used to determine the role of Syk in various cellular function; indeed, even at doses of piceatannol lower than 10 μg/mL, FAK kinase activity is still significantly impaired.
DISCUSSION
The purpose of this study was to determine the functional importance of Syk in the αIIbβ3-mediated signaling processes that occur upon platelet activation. Using a genetic approach, we found that the specific deficiency of this tyrosine kinase led to a partial defect in the activation of αIIbβ3 in murine platelets. However, lack of Syk did not affect the ability of platelets to adhere to immobilized fibrinogen and neither did it have an effect on αIIbβ3-mediated primary hemostasis, as assessed by tail bleeding times. These data were surprising in light of previous reports, based on the kinetics of Syk phosphorylation and activation and on the effects of piceatannol, that concluded that Syk was important for all αIIbβ3-mediated signaling events.2 5 However, the present results indicate that discrepancies in αIIbβ3 dysfunction observed between the genetic and pharmacological approaches are due, at least in part, to a lack of specificity of piceatannol. Regardless, both experimental approaches yielded results consistent with Syk playing an essential role in achieving maximal αIIbβ3 activation.
Our data draw into question the validity of using piceatannol to infer specific roles for Syk in cell function. Piceatannol has been widely used to study Syk and results obtained with this inhibtor have been used to predict the role of Syk in a number of cell signaling events.5,20,22 23 Experiments examining the activity of tyrosine kinases immunoprecipitated from piceatannol-pretreated platelet lysates and the activity of kinases directly treated with piceatannol both demonstrated that this inhibitor is not selective for Syk and can inhibit other kinases, in particular FAK, at the concentrations often used to infer the role of Syk in a particular system. Such results highlight the problem of using piceatannol, even at relatively low concentrations (5 to 10 μg/mL), as a reagent to infer crucial roles for Syk in cell function and suggest that alternative experimental approaches need to be considered. One such approach is the study of platelets from genetically engineered mice.
In the present study we show that murine platelets lacking Syk are defective in αIIbβ3 signaling. This defective activation of αIIbβ3 was observed when ADP ± epinephrine were used as agonists. This combination of agonists can induce several signaling pathways, including calcium mobilization and phosphoinositide hydrolysis, and results in platelet shape change and αIIbβ3activation.24 It remains unclear whether Syk plays a role in initiating one of the aforementioned signaling pathways, is involved in a secondary signaling pathway, or takes part in an event directly involving the αIIbβ3 integrin. In other systems in which Syk plays a critical role in signaling, such as via the B-cell and Fc receptors, an interaction between Syk and proteins containing a tyrosine-based motif sequence (ITAM) has always been documented.25-28 Syk binds to and is activated by the phosphorylated ITAMs via its tandem SH2 domains, allowing for its localization to signaling complexes at the cell membrane.29Indeed, in the one pathway in platelets in which Syk clearly plays a role, namely collagen-induced signaling, an ITAM-containing protein, Fcγ, is thought to be involved.6 Thus, whereas Syk is required for maximal αIIbβ3 activation, the partial phenotype displayed by the Syk null platelets suggests that other pathways, and presumably other protein tyrosine kinases, must also be involved in integrin activation. Further studies will be necessary to characterize these pathways in more detail.
Our experiments with Syk-deficient platelets failed to demonstrate a role for Syk in other αIIbβ3-mediated events, such as adherence to immobilized fibrinogen. In addition, work by Poole et al6 demonstrated the ability of Syk-deficient platelets to aggregate and release arachidonic acid in response to 10 U/mL thrombin stimulation. It is conceivable that some other kinase can compensate for the lack of Syk in these platelets. Indeed, it has been observed that the thymocytes from people who genetically lack the tyrosine kinase ZAP-70 express Syk at higher than normal levels.18 However, no increased expression of ZAP-70, the most likely candidate for a compensating kinase because it belongs to the same family as Syk, was observed in the Syk-deficient platelets. This does not rule out the possibility of some unrelated kinase being able to take over Syk’s function in an ADP plus epinephrine-induced signaling pathway. However, there are data that suggest that in some cases loss of Syk is not compensated for by any other tyrosine kinase. For example, Syk-deficient B cells give the expected phenotype based on loss of Syk, ie, they fail to progress past the pre-B–cell stage, in keeping with the importance of Syk for signaling via the pre-B–cell receptor complex.8,14 In addition, the complete loss of response towards collagen in Syk null platelets also indicates that Syk cannot be compensated for in this particular signaling reaction.6
Further evidence indicating a lack of importance of Syk in certain αIIbβ3-mediated processes comes from bleeding time data. It is clear that in the mouse, as in the human, prolonged bleeding times can correlate with defective αIIbβ3 function or defective platelet signaling. Mice genetically engineered to lack β3 have increased bleeding times.15 In addition, mice that lack Gq have a defect in platelet activation and also have prolonged bleeding times.30 No increase in tail bleeding time was observed when Syk−/− animals were compared with Syk+/− orSyk+/+ controls, as had previously been observed.6 Whereas Syk-deficiency had no effect on bleeding times here, it is possible that this is due to a limitation of the model, because it is known that these Syk-deficient platelets fail to mount responses to collagen and, thus, by analogy to humans with defective collagen-induced platelet responses,31,32 might be expected to have prolonged bleeding times on that basis alone. However, Syk deficiency was assocaited with rebleeding after primary hemostasis in 2 of 5 animals. It is not known whether this indicates unstable hemostatic plug formation in these animals, and neither is it known whether this is a contributing factor in death due to hemorrhage that happens to most Syk knockout mice shortly after birth.8 14
The discrepancies between the pharmacological and genetic analysis of the role of Syk in αIIbβ3-mediated platelet processes reiterate the need for caution when interpreting results obtained with inhibitors. They also highlight the strength of a genetic approach that, as gene targeting technology becomes more commonplace, is an increasingly viable strategy for determining protein function. This method, incorporating the micro-assays of platelet function used herein, may be particularly useful for studying the role of proteins in platelet function, because platelets are not amenable to more traditional genetic manipulation such as transfection.
ACKNOWLEDGMENT
The authors thank Mark Smyth (Cor medicinal chemistry group) for checking the purity of piceatannol and all the members of the Tybulewicz laboratory for their help during this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David R. Phillips, PhD, COR Therapeutics, Inc, 256 E Grand Ave, South San Francisco, CA 94080; e-mail: david_phillips@corr.com.

![Fig. 5. Effect of piceatannol on PAC-1 binding to platelets. Human platelets were pretreated for 10 minutes at room temperature with control DMSO vehicle or piceatannol at 1, 10, or 30 μg/mL. They were then stimulated with the desired agonist ([○] PBS control, [▴] 0.1 μmol/L ADP, [□] 1 μmol/L ADP, [•] 10 μmol/L ADP, [⧫] 0.2 μmol/L PMA) and incubated with FITC-labeled PAC-1 antibody. The level of PAC-1 bound is expressed as a percentage of maximal PAC-1 binding obtained when the DMSO-pretreated platelets were stimulated with the highest concentration of agonist (either 10 μmol/L ADP for the ADP-treated samples or 0.2 μmol/L PMA for the PMA-treated samples). Results shown are the mean ± SE values from three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/8/10.1182_blood.v93.8.2645/5/m_blod40813005x.jpeg?Expires=1770228019&Signature=0IkLChgvhmX-BX6WVOAGR2jeeCxLo1LdB~4JjHx1LWxR3NLVc4z2Q1qzRylUzKKHxEP5IRmG~jeuQGwqYe8oAg-y2Tjkue8OrwL~~HVC35AAXk3a2nAIg8kxWSyBRguQppeo5RgYCaSJWm1Z2HylJsrq3ISK7Ygu7-9q5ov2mvWZkoFU8gIAho0Ti6Vb8-ub-jjf~9lrcgVZGANI9LWGL0aogi~hnp7RreUezl3tXcfgeF-Y1kMBX3wwLofQMaS4RYdxefaKlhMyhw43tXNZ5pBLKLhSTVALN3bUbIhJcTciLGvGBEMi7NGcmnw1mIdyKf2b1yEKDAJA6hVDWGlHxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Effect of piceatannol on PAC-1 binding to platelets. Human platelets were pretreated for 10 minutes at room temperature with control DMSO vehicle or piceatannol at 1, 10, or 30 μg/mL. They were then stimulated with the desired agonist ([○] PBS control, [▴] 0.1 μmol/L ADP, [□] 1 μmol/L ADP, [•] 10 μmol/L ADP, [⧫] 0.2 μmol/L PMA) and incubated with FITC-labeled PAC-1 antibody. The level of PAC-1 bound is expressed as a percentage of maximal PAC-1 binding obtained when the DMSO-pretreated platelets were stimulated with the highest concentration of agonist (either 10 μmol/L ADP for the ADP-treated samples or 0.2 μmol/L PMA for the PMA-treated samples). Results shown are the mean ± SE values from three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/8/10.1182_blood.v93.8.2645/5/m_blod40813005x.jpeg?Expires=1770682122&Signature=cbWRmxaUGv~iZ9Gc4UcfXHtAHTM0Hgw2oXds8~qkjB9wY6SwMQAl1-SJAzC1i5bbWJAffm6zJO9ullhABbTTQvcyTN0o3Idn6vmeXpQVlnFHb8ZMqA9Ub-sHxGx0tRmuxc516kiIukLD1dPZSAoEws52u5Oymk5rW7gZeNytWtkf1hd4pIHJ2wvh20DCwbsvdkgTOW5rNwW~oFRjXquC6W8aIWktBhjoRvCgoyq6bM0NAGaGprtkEEFMPzzezcxpFsLoHWgt3qEMhE1I8fDzMEv0AaEkjNK-oHgRTGhlAYBP3Yj7SyFqeIFMfkLBu2la-azqAOLeT5jAhagmA1vwNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)