Abstract
Investigation of the molecular basis of megakaryocyte (MK) and platelet biology has been limited by an inadequate source of genetically manipulable cells exhibiting physiologic MK and platelet functions. We hypothesized that ex vivo cultured MKs would exhibit agonist inducible glycoprotein (GP) IIb-IIIa activation characteristic of blood platelets and that these cultured MKs would be capable of transgene expression. Microscopic and flow cytometric analyses confirmed that human hematopoietic stem cells cultured in the presence of pegylated recombinant human MK growth and development factor (PEG-rHuMGDF) differentiated into morphologic and phenotypic MKs over 2 weeks. Cultured MKs expressed functional GPIIb-IIIa receptors as assessed by agonist inducible soluble fibrinogen and PAC1 binding. The specificity and kinetics of fibrinogen binding to MK GPIIb-IIIa receptors were similar to those described for blood platelets. The reversibility and internalization of ligands bound to MK GPIIb-IIIa also shared similarities with those observed in platelets. Cultured MKs were transduced with an adenoviral vector encoding green fluorescence protein (GFP) or β-galactosidase (β-gal). Efficiency of gene transfer increased with increasing multiplicities of infection and incubation time, with 45% of MKs expressing GFP 72 hours after viral infection. Transduced MKs remained capable of agonist induced GPIIb-IIIa activation. Thus, ex vivo cultured MKs (1) express agonist responsive GPIIb-IIIa receptors, (2) are capable of expressing transgenes, and (3) may prove useful for investigation of the molecular basis of MK differentiation and GPIIb-IIIa function.
INVESTIGATION OF megakaryocyte (MK) and platelet biology has been limited by an inadequate source of genetically manipulable cells capable of physiologic MK and platelet functions. Megakaryocytes comprise only 0.02% to 0.05% of human bone marrow,1 and the quantity of human marrow available for MK purification is insufficient for investigative purposes. As a result, the processes involved in MK and platelet development are incompletely understood. Although the abundance and accessibility of peripheral blood platelets has promoted an understanding of mature platelet physiology, information regarding many crucial aspects of platelet physiology remains unclear. One such area of incomplete knowledge is the process of activation-dependent high-affinity binding of soluble fibrinogen to platelet glycoprotein (GP) IIb-IIIa receptors (integrin αIIbβ3). Although numerous assays have been developed to characterize the function of this important adhesive receptor,2-4 information about the molecular signals involved in its activation is incomplete. The anucleate nature of platelets has contributed to this slow advance of knowledge, because molecular modification of mature platelets is not possible. Immortalized megakaryocytic5,6 and GPIIb-IIIa–transfected3 cell lines have limited use, because none demonstrates physiologic, agonist-inducible, inside-out exposure of soluble fibrinogen binding sites characteristic of GPIIb-IIIa activation on platelets.
To circumvent these problems, many investigators have developed in vitro MK culture systems using various growth factors to induce megakaryopoiesis from hematopoietic precursors.7-9 The cloning and expression of the c-Mpl ligand and its identification as megakaryocyte growth and development factor have been major advances in this endeavor.10-12 A number of investigators have demonstrated that stem/progenitor (CD34+) cells cultured in the presence of the c-Mpl ligand will differentiate into morphologic and antigenic MKs.9,13 14 We now show that ex vivo-generated MKs express functional GPIIb-IIIa receptors and are capable of expressing exogenously introduced transgenes.
MATERIALS AND METHODS
Human subjects.
Leukopheresis units were purchased from the Johns Hopkins Hemapheresis Center (Baltimore, MD). Processed bone marrow was obtained from the Johns Hopkins Oncology Center and the Division of Bone Marrow Transplantation of Washington University (St Louis, MO). This research was approved by the Johns Hopkins investigational review board. All donors provided informed consent.
Antibodies and reagents.
Biotinylated CD34 antibody was obtained from the Johns Hopkins Oncology Center. Phycoerythrin-labeled GPIb (PE-GPIb) antibody and fluorescein isothiocyanate-labeled GPIIb (FITC-GPIIb) antibody were obtained from Immunotech (Marseille, France). FITC-labeled PAC1 (FITC-PAC1) antibody and fluorescently labeled IgG isotype controls were obtained from Becton Dickinson (San Jose, CA). 10E5 antibody was the generous gift of Barry Coller (Mount Sinai Hospital, New York, NY). LM609 antibody was the generous gift of David Cheresh (Scripps Research Institute, La Jolla, CA). Fibrinogen (Sigma Chemical Co, St Louis, MO) was labeled with FITC (Pierce, Rockford, IL) as previously described.15Propridium iodide (PI) and ethydium bromide were from Boehringer Mannheim (Indianapolis, IN). Adenosine diphosphate (ADP), thrombin receptor activating peptide (TRAP), and arg-gly-asp-ser (RGDS) peptide were obtained from Sigma. Cyclic KGD heptapeptide (Integrilin) was the generous gift of Pascal Goldschmidt-Clermont (Columbus, OH).
Enrichment of CD34+ cells from leukopheresis units or bone marrow.
To obtain stem cells from leukopheresis units, low-density mononuclear cells were first separated by centrifugation over Histopaque 1077 (Sigma) as per the manufacturer's suggested protocol. Mononuclear cells were washed twice in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Cells were incubated with biotinylated CD34 antibody for 30 minutes and then washed free of excess antibody. CD34+ cells were isolated from the mononuclear fraction by passage over an avidin column (CellPro Inc, Bothell, WA) using the manufacturer's suggested protocol. In a second protocol, bone marrow for homologous transfusion was obtained and processed by our clinical oncology department (Johns Hopkins University, Baltimore, MD). Low-density mononuclear cells were separated from marrow by automated elutriation centrifugation. The mononuclear fraction was labeled with biotinylated CD34 antibody and passed over an automated avidin column. The CD34-depleted fraction, which contains 10% to 40% of the total CD34+ population, was obtained for further CD34 cell isolation. (The CD34+ fraction was retained by the oncology service for clinical use.) This CD34-depleted fraction was washed with 1% BSA/PBS and relabeled with biotinylated CD34 antibody. Excess antibody was washed free and cells were passed over an avidin column as described above for the leukopheresis product. In both preparations, CD34+ cells were recovered from the avidin column and resuspended in media for culture. Yields from the 2 separation techniques were as follows: leukopheresis: 0.8 to 1.2 × 106 CD34+ cells from 1 × 109mononuclear cells; CD34-depleted bone marrow fraction: 3 to 7 × 106 CD34+ cells from 1 × 109mononuclear cells.
Induction of megakaryopoiesis in liquid culture.
CD34+ cells isolated from leukopheresis or bone marrow were grown in StemPro Serum Free Media (Life Technologies, Gaithersberg, MD) reconstituted with nutrient supplement and 2 mmol/L L-glutamine as per the manufacturer's guidelines (formulation of this product is proprietary). Cells were plated in 1-mL aliquots on 24-well culture plates at an initial cell density of 2.5 to 5.0 × 105cells/mL. Pegylated recombinant human MK growth and development factor (PEG-rHuMGDF) was generously provided by Amgen, Inc (Thousand Oaks, CA) and was added to initial cell cultures at a final concentration of 50 ng/mL. Cells were maintained in a humidified incubator with 5% CO2 at 37°C. Cells were inspected on a daily basis and harvested for experiments on days 7 to 14 of culture.
Identification of cultured MKs by morphology, membrane phenotype, and ploidy.
Morphologic examination of cultured cells was performed on days 7 to 14. Cytospins of cultured cells were stained by modified Wright-Giemsa method and examined by light microscopy. MKs were characterized by membrane phenotype and ploidy using 2-color flow cytometry as previously described with modifications.8,16 Cultured cells were harvested on days 7 to 14 and diluted in culture media to 2.5 × 105 cells/mL. Cells were incubated with FITC-GPIIb antibody and/or PE-GPIb antibody for 30 minutes at 22°C. In ploidy experiments, cells were permeablized with Triton X-100 (0.02%) and incubated with PI (50 μg/mL) and RNAse (50 μg/mL) in hypotonic sodium citrate (0.1%). Cytometric analysis was performed on a FACScan flow cytometer (Becton Dickinson) equipped with a 15-mW argon laser set with excitation wavelength of 488 nm. Typically, 3 × 105 CD34+ cells yielded a heterogeneous population of 1 × 106 total cells by day 14 of culture. Preliminary experiments demonstrated maximal MK differentiation by days 12 to 14 of culture, with 75% of the total cell population demonstrating cell surface expression of GPIb and/or GPIIb. PI staining of cultured cells showed a ploidy pattern consistent with in vitro megakaryocytic differentiation (50%, 38%, 8%, and 2% of GPIIb-positive cells being 2N, 4N, 8N, and 16N, respectively). The rate and magnitude of MK differentiation observed in our culture system were similar to those reported by others.8-10 14
Functional studies of MK GPIIb-IIIa receptors.
Cultured cells were harvested for physiologic studies of GPIIb-IIIa function on days 7 to 14. Cells, diluted to 2.5 × 105/mL, were incubated with PE-GPIb antibody and FITC-PAC1 antibody or FITC-labeled fibrinogen (FITC-FGN) in the presence and absence of TRAP or ADP at 22°C. Preliminary studies demonstrated that the number of cultured cells capable of agonist-induced GPIIb-IIIa activation reached maximal levels between 10 and 14 days of culture. Therefore, all subsequent experiments assessing GPIIb-IIIa activation were performed during this interval. The specificity of fibrinogen binding was determined by incubating samples, in the presence and absence of TRAP, with a series of blocking antibodies and peptides. The kinetics of MK GPIIb-IIIa activation were determined by varying the concentration of agonist, activation interval, and fibrinogen concentration. Reversibility of FITC-FGN binding was determined by the addition of ethylenediaminetetraacetic acid (EDTA; final concentration, 10 mmol/L) to samples after 5 or 20 minutes of agonist stimulation. To determine if surface-bound FITC-FGN and FITC-PAC1 became internalized, ethidium bromide (final concentration, 50 μg/mL), a cell-impermeable quencher of FITC fluorescence,17 18 was added to samples after 5 or 20 minutes of TRAP stimulation. Samples were diluted with ice-cold PBS immediately before ethidium bromide and incubated on ice for 5 minutes before cytometric analysis. In preliminary studies on fixed TRAP-activated platelets, ethidium bromide quenched FITC-FGN fluorescence with an efficiency of 76%. Samples were examined using flow cytometry and 2-color analysis performed as described below (see analysis of cytometric data).
Analysis of cytometric data.
Two-color flow cytometry was used to determine MK phenotype and GPIIb-IIIa activation in cultured cells. Data from 25,000 to 50,000 events were collected from each sample with scatter data in linear mode and fluorescent data in logarithmic mode. In all experiments, data were analyzed using forward and side scatter gates to exclude dead cells and cell fragments. MKs were defined by GPIb positivity (PE fluorescence) in combination with their characteristic forward scatter as previously described.16 FITC fluorescence histograms were generated from the gated MK population and median fluorescence intensity (MFI) or percent positivity was used to determine differences in fluorescence between cell samples. Specific ligand binding was defined as the MFI of agonist-stimulated samples minus the MFI in unstimulated samples. In some experiments, MFI was converted to fluorochrome equivalents using standard calibration beads (Flow Cytometry Standards Corp, San Juan, Puerto Rico) as previously described.15 The fraction of fibrinogen irreversibly bound was determined by dividing the MFI of EDTA-treated samples by the MFI of agonist-treated samples in the absence of EDTA. The fraction of bound fibrinogen that became internalized was determined by dividing the MFI of ethidium bromide-treated samples by the MFI of activated samples without the fluorescein quencher and correcting for the quenching efficiency (×0.76).
Adenovirus vector preparation.
Preparation of an adenovirus vector encoding humanized green fluorescent protein (hGFP) driven by the cytomegalovirus (CMV) promoter has been described in detail previously.19,20 Briefly, cDNA for GFP was subcloned from pGreenLantern (Life Technologies, Gaithersberg, MD) into the adenovirus shuttle vector pE1CMV to create pE1CMVhGFP. The plasmids pE1CMVhGFP and pJM17, which contain the full human adenovirus serotype 5 genome, were cotransfected into HEK293 cells using LipofectAMINE (Life Technologies). As described previously, homologous recombination between the shuttle vector and pJM17 replaces the region of the adenovirus between map units 1.0 and 9.8 with the expression cassette cDNA. The recombinant virus designated AdCMVhGFP was expanded in HEK293 cells and the virus was purified by 3 rounds of cesium chloride banding as previously described.19
A recombinant replication defective adenovirus vector encoding nuclear targeted β-galactosidase (β-gal), AdCNLacZ, was generated by cotransfection of the shuttle vector pAdCNLacZ and full-length adenoviral DNA (serotype 5) into CRE8 cells (both a generous gift from Dr Stephen Hardy, Somatix Therapy Corp, Alameda, CA) as previously described.21 The pAdCNLacZ shuttle plasmid was constructed in a 2-step process. First, the Msc I fragment of pAAVRNLacZ (a generous gift from Dr Robert Kotin, NHLBI, Bethesda, MD) containing the cDNA encoding for nuclear targeted β-gal driven by an RSV promoter was cloned into the EcoRV site of pBluescript SK II+ (Stratagene, La Jolla, CA) to generate pBSRNLacZ. In the second step, pAdCNLacZ was generated by ligating the Sal I-Xba I fragment of pBSRNLacZ, containing the NLacZ cDNA, into the shuttle plasmid pAdlox (containing a CMV promoter; also a gift from Dr S. Hardy) digested with Xho I and Xba I. Recombinant virus was plaque purified and stocks of AdCNLacZ were prepared by propagation in 293 cells followed by double cesium chloride centrifugation.
Adenoviral transduction of cultured MKs.
Day-11 cultured cells were incubated with AdCMVhGFP, an adenoviral vector control lacking the sequence for GFP, or viral vehicle control at varying multiplicities of infection (MOI) for 24 to 72 hours. Cells were inspected daily for changes in morphology, cell number, and viability (Trypan blue staining). Expression of GFP was determined by flow cytometry at 24, 48, and 72 hours after viral incubation. Cell cultures incubated with virus or vehicle were harvested at the indicated times and incubated with PE-GPIb antibody for 30 minutes at 22°C before cytometric acquisition of data.
Cultured cells (day 10) were incubated with AdCNLacZ or vehicle control at varying MOI for 3 to 4 days. Expression of β-gal was determined as previously described.22 Briefly, cell suspensions were incubated in 2% formaldehyde/0.2% glutaraldehyde for 5 minutes. Cells were washed with PBS and then incubated in X-gal staining solution (5-bromo-4-chloro-3-indolyl-β-D-galacopyranoside) for 3 hours at 37°C. Stained cells were placed on a hemacytometer and transduction efficiency was estimated by determining the percentage of cells that appeared blue by light microscopy.
Immunofluorescence microscopy.
Cell cultures incubated with AdCNLacZ or vehicle for 3 days (day 13 of culture) were harvested and incubated with PE-GPIb antibody (or PE-IgG isotype control) and FITC-PAC1 in the presence and absence of 50 μmol/L TRAP for 15 minutes. Samples were fixed in 2% formaldehyde/0.2% glutaraldehyde for 5 minutes and then cytospun onto glass slides. Slides were rinsed in PBS and cells were stained in situ with X-gal solution for 3 hours at 37°C. Slides were viewed by conventional light and fluorescence microscopy using a Zeiss laser scanning microscope (LSM). Cells transduced with AdCNLacZ were identified by blue nuclear staining using conventional light, followed by immunofluorescent imaging using a krypton-argon excitation laser at 488 nn and emission filters of 505 to 530 nm (FITC) and 570 nm (PE). Fluorescent images for FITC and PE were obtained separately to ensure that signal overlap from the 2 fluorochromes could not occur.
RESULTS
Cultured MKs possess functional GPIIb-IIIa receptors.
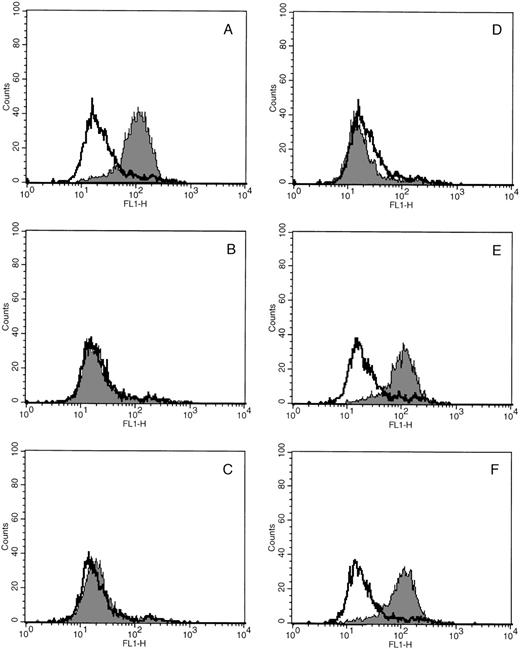
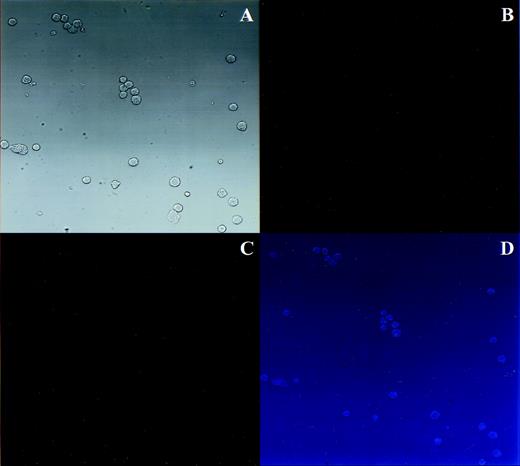
Preliminary studies demonstrated that cultured MKs stimulated with TRAP bound more FITC-FGN than unstimulated cells. The specificity of agonist induced soluble fibrinogen binding for GPIIb-IIIa on cultured MKs was verified by incubating cultured cells in the presence and absence of a series of GPIIb-IIIa blocking peptides and antibodies (Fig 1). In each case, KGD peptide, RGDS peptide, and 10E5 antibody (Fig 1B, C, and D, respectively), which are known to inhibit GPIIb-IIIa–mediated fibrinogen binding,23-25 blocked the TRAP-induced increase in MK FITC-FGN binding. In contrast, antibodies that lack specificity for GPIIb-IIIa, LM609 (block ligand binding to the vitronectin receptor26) and an irrelevant IgG, had no effect on the activation-dependent binding of FITC-FGN to cultured MKs (Fig 1E and F). None of the peptides or antibodies had any effect on the nonspecific FITC-FGN binding of unstimulated samples. In other control experiments, no agonist-induced increase in fluorescence was observed in samples incubated with a nonspecific fluorescent antibody instead of FITC-FGN (data not shown).
Soluble fibrinogen binding to cultured MKs is specific for GPIIb-IIIa. In each experiment, cultured cells were incubated with PE-GPIb antibody and FITC-FGN in the presence (shaded histograms) and absence (unshaded histograms) of TRAP and with or without the indicated blocking antibody. FITC fluorescence (FL1) histograms were generated from the MK population. (A) No blocking antibody. (B) KGD heptapeptide (Integrilin). (C) RGDS peptide. (D) 10E5 antibody. (E) LM609 antibody. (F) Nonspecific mouse monoclonal IgG. Data are representative of 3 experiments performed.
Soluble fibrinogen binding to cultured MKs is specific for GPIIb-IIIa. In each experiment, cultured cells were incubated with PE-GPIb antibody and FITC-FGN in the presence (shaded histograms) and absence (unshaded histograms) of TRAP and with or without the indicated blocking antibody. FITC fluorescence (FL1) histograms were generated from the MK population. (A) No blocking antibody. (B) KGD heptapeptide (Integrilin). (C) RGDS peptide. (D) 10E5 antibody. (E) LM609 antibody. (F) Nonspecific mouse monoclonal IgG. Data are representative of 3 experiments performed.
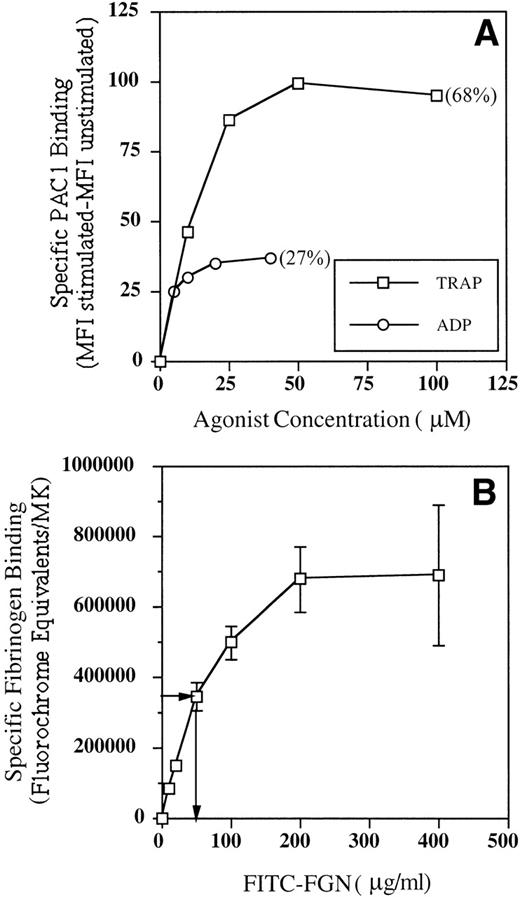
To determine if the kinetics of GPIIb-IIIa activation on MKs were similar to those described for platelets, we undertook a series of experiments to determine the following aspects of the receptor-ligand interaction: (1) the effect of agonist concentration on PAC1 binding, (2) the effect of duration of activation on PAC1 binding, and (3) the receptor affinity for fibrinogen. The effect of agonist concentration on MK GPIIb-IIIa activation was assessed by incubating cultured cells with PAC1 and increasing concentrations of TRAP or ADP. The results of these experiments are shown in Fig 2A and demonstrate a typical dose-response relationship for both agonists, with maximal PAC1 binding achieved in response to 50 μmol/L TRAP and 20 μmol/L ADP. TRAP activation routinely induced a greater number of PAC1-positive MKs than was observed with ADP stimulation (Fig 2A, percentage of PAC1 positivity). The effect of agonist stimulation time on MK GPIIb-IIIa activation was determined by incubating cultured cells with PAC1 and 50 μmol/L TRAP for increasing periods of time. These data demonstrated rapid receptor activation in the presence of TRAP, with maximal receptor binding occuring by approximately 20 minutes (data not shown). To determine the dissociation constant (Kd) of MK GPIIb-IIIa receptors for fibrinogen, cultured cells were incubated with increasing concentrations of FITC-FGN and 50 μmol/L TRAP for 20 minutes. Figure 2B shows that receptor binding was saturable and that half-maximal binding occurred at a fibrinogen concentration of approximately 50 μg/mL (ie, Kd = 1.5 × 10−7 mol/L).
Binding kinetics of activated GPIIb-IIIa receptors on cultured MKs. Cultured cells were incubated with PE-GPIb antibody and FITC-PAC1 or FITC-FGN in the presence and absence of TRAP or ADP. MFI was determined from MK fluorescence histograms. The percentage of PAC1-positive MKs is shown in parentheses. Fluorochrome equivalents were determined from the MFI as described in Materials and Methods. (A) Effect of agonist concentration on PAC1 binding. Data are representative of 2 experiments performed. (B) Fibrinogen binding kinetics. Arrows indicate fibrinogen concentration at which binding was half maximal (Kd). Data are the mean ± SEM of 3 experiments.
Binding kinetics of activated GPIIb-IIIa receptors on cultured MKs. Cultured cells were incubated with PE-GPIb antibody and FITC-PAC1 or FITC-FGN in the presence and absence of TRAP or ADP. MFI was determined from MK fluorescence histograms. The percentage of PAC1-positive MKs is shown in parentheses. Fluorochrome equivalents were determined from the MFI as described in Materials and Methods. (A) Effect of agonist concentration on PAC1 binding. Data are representative of 2 experiments performed. (B) Fibrinogen binding kinetics. Arrows indicate fibrinogen concentration at which binding was half maximal (Kd). Data are the mean ± SEM of 3 experiments.
Agonist-induced binding of fibrinogen to GPIIb-IIIa receptors on platelets has been reported to be reversible, particularly during the early stages of activation.27,28 To determine the reversibility of this receptor-ligand interaction on MKs, EDTA was added to cultured cells that had been incubated with FITC-FGN in the presence and absence of TRAP or ADP for 5 or 20 minutes. Approximately half of MK FITC-FGN binding was reversed after 5 minutes of TRAP activation, but binding was nearly irreversible by 20 minutes (Table 1). Similar results were observed when cells were activated with ADP. It has been reported that fibrinogen bound to platelet GPIIb-IIIa receptors becomes rapidly internalized after agonist stimulation.28 To determine if internalization of surface-bound fibrinogen follows MK activation, ethidium bromide was used to quench fluorescence from surface-bound FITC-FGN on TRAP-activated MKs. These experiments showed that 19% ± 6% and 33% ± 3% of surface-bound FITC-FGN was internalized by activated MKs at 5 and 20 minutes, respectively (Table 1). A similar reduction in FITC-PAC1 fluorescence was also detected in TRAP-stimulated MKs treated with ethidium bromide (data not shown). In parallel experiments on washed platelets, 37% ± 3% and 46% ± 12% of FITC-FGN was internalized by TRAP-activated platelets at 5 and 20 minutes, respectively (n = 3).
Successful transduction of cultured MKs with adenoviral vectors.
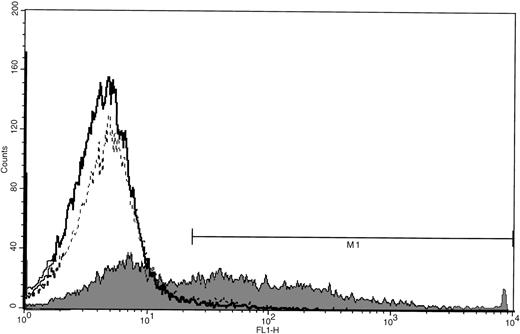
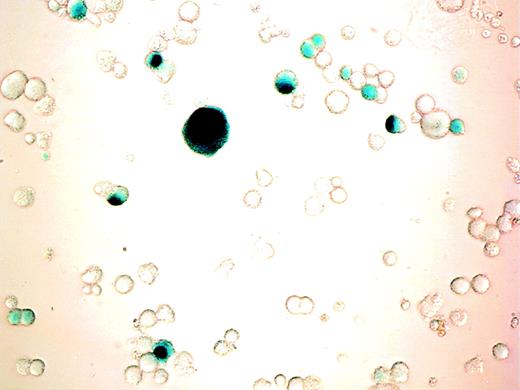
Cells were incubated with varying MOI of AdCMVhGFP, control virus, or vehicle on day 11 of culture and were harvested for cytometric analysis 24 to 72 hours later. MK expression of GFP was detected after 24 hours of viral incubation and increased over the next 48 hours (Fig 3). The efficiency of transduction increased with increasing MOI and incubation time (Table 2). There was a 10% reduction in cell viability for the highest MOI at 48 and 72 hours as determined by Trypan blue staining and scatter profile analyses. Incubation of cultured cells with AdCNLacZ also resulted in successful transduction with low cell toxicity (Fig4). Transduction efficiency at 4 days, as assessed by visualization of X-gal staining, was 9%, 18%, 22%, and 28% for MOI of 50, 100, 200, and 500, respectively.
GFP expression in cultured MKs after adenoviral transduction. CD34+ cells were cultured with PEG-rHuMGDF for 11 days before incubation with AdCMVhGFP (MOI of 200), viral control (MOI of 200), or vehicle control. Cells were harvested after 72 hours of viral infection and incubated with PE-GPIb antibody. FITC fluorescence (FL1) histograms were generated from the MK population for samples incubated with AdCMVhGFP (shaded), viral control (dotted line, unshaded), and vehicle control (solid line, unshaded). M1 is 1%, 2%, and 46% positivity, respectively, for vehicle control, viral control, and AdCMVhGFP. Data are representative of 2 experiments performed.
GFP expression in cultured MKs after adenoviral transduction. CD34+ cells were cultured with PEG-rHuMGDF for 11 days before incubation with AdCMVhGFP (MOI of 200), viral control (MOI of 200), or vehicle control. Cells were harvested after 72 hours of viral infection and incubated with PE-GPIb antibody. FITC fluorescence (FL1) histograms were generated from the MK population for samples incubated with AdCMVhGFP (shaded), viral control (dotted line, unshaded), and vehicle control (solid line, unshaded). M1 is 1%, 2%, and 46% positivity, respectively, for vehicle control, viral control, and AdCMVhGFP. Data are representative of 2 experiments performed.
β-gal expression in cultured cells after adenoviral transduction. CD34+ cells were cultured with PEG-rHuMGDF for 10 days before incubation with AdCNLacZ (MOI of 200). β-gal expression was visualized by light microscopy after cell fixation and X-gal staining as described in Materials and Methods. Photomicrograph of adenovirus infected cells (400×). Note the blue nuclear staining of successfully transduced cells. No blue coloration was detectable in any cell from samples that were similarly stained but were incubated with virus control.
β-gal expression in cultured cells after adenoviral transduction. CD34+ cells were cultured with PEG-rHuMGDF for 10 days before incubation with AdCNLacZ (MOI of 200). β-gal expression was visualized by light microscopy after cell fixation and X-gal staining as described in Materials and Methods. Photomicrograph of adenovirus infected cells (400×). Note the blue nuclear staining of successfully transduced cells. No blue coloration was detectable in any cell from samples that were similarly stained but were incubated with virus control.
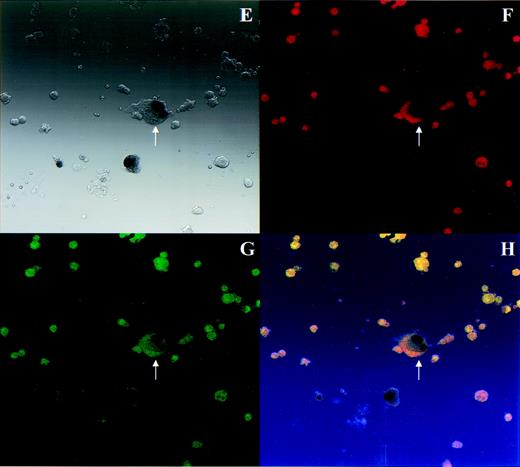
To determine if transduced MKs retained the capacity for agonist-induced GPIIb-IIIa activation, cells infected with AdCNLacZ were analyzed by microscopy for simultaneous expression of β-gal, GPIb, and TRAP-induced PAC1 binding. Results of these experiments are depicted in Fig 5. Although cells on each slide were heterogeneous, nuclear X-gal staining could be easily identified in transduced cells. (Blue X-gal stained nuclei appear black in black and white light transmission photograph.) As expected, the majority of cells were megakaryocytic by GPIb positivity at this point in culture (Fig 5F), and TRAP stimulation induced high-level PAC1 binding (Fig 5G). Successful transduction of a morphologic and phenotypic MK can be observed in Fig 5E through H. The arrow indicates an MK characterized by its bi-lobed X-gal–stained nucleus, strong PE-GPIb fluorescence, and bright FITC-PAC1 fluorescence after TRAP activation. Thus, exogenous gene transfer to cultured MKs was accomplished using adenoviral vectors and transduced MKs retained their characteristic morphologic and phenotypic features.
Transduced MKs retain typical MK morphology and phenotype. PEG-rHuMGDF–cultured cells that were incubated with AdCNLacZ or control were harvested for simultaneous analysis of β-gal expression and GPIIb-IIIa activation. Cells were incubated with PE-GPIb antibody (or PE-IgG control) and FITC-PAC1 in the presence or absence of TRAP before fixation and in situ X-gal staining. In each set of 4 photomicrographs, the first panel shows images using light transmission microscopy, the second and third show the same field using fluorescence microscopy, and the fourth shows a computer generated overlay of the first 3 images. (A through D) Virus control, PE-IgG control, FITC-PAC1, unstimulated for light transmission, PE fluorescence, FITC fluorescence, and overlay, respectively. (E through H) AdCNLacZ, PE-GPIb, FITC-PAC1, TRAP for light transmission, PE fluorescence, FITC fluorescence, and overlay, respectively. Note that in these black and white light transmission images X-gal–stained nuclei appear as black instead of blue. The arrow indicates a successfully transduced morphologic and phenotypic MK. All photomicrographs are 400×.
Transduced MKs retain typical MK morphology and phenotype. PEG-rHuMGDF–cultured cells that were incubated with AdCNLacZ or control were harvested for simultaneous analysis of β-gal expression and GPIIb-IIIa activation. Cells were incubated with PE-GPIb antibody (or PE-IgG control) and FITC-PAC1 in the presence or absence of TRAP before fixation and in situ X-gal staining. In each set of 4 photomicrographs, the first panel shows images using light transmission microscopy, the second and third show the same field using fluorescence microscopy, and the fourth shows a computer generated overlay of the first 3 images. (A through D) Virus control, PE-IgG control, FITC-PAC1, unstimulated for light transmission, PE fluorescence, FITC fluorescence, and overlay, respectively. (E through H) AdCNLacZ, PE-GPIb, FITC-PAC1, TRAP for light transmission, PE fluorescence, FITC fluorescence, and overlay, respectively. Note that in these black and white light transmission images X-gal–stained nuclei appear as black instead of blue. The arrow indicates a successfully transduced morphologic and phenotypic MK. All photomicrographs are 400×.
DISCUSSION
Most studies of MK and platelet biology have investigated separately the processes of MK differentiation and platelet physiology. Although the influence of MK development on platelet function is recognized, the ability to link these 2 processes has been limited by an inadequate source of genetically manipulable cells capable of physiologic platelet responses. This study demonstrates that ex vivo-cultured MKs are capable of (1) agonist-induced GPIIb-IIIa activation characteristic of blood platelets and (2) expression of an exogenous gene product after adenoviral transduction and that (3) transduced MKs retain physiologic GPIIb-IIIa activation. These data suggest that ex vivo-cultured MKs represent a genetically manipulable substrate suitable for investigation of GPIIb-IIIa function.
Cytometric studies demonstrated that cultured MKs were capable of inside-out signal transduction leading to binding of soluble fibrinogen and PAC1 to activated GPIIb-IIIa receptors. These studies confirm and extend the findings of previous investigators who have demonstrated that bone marrow-derived MKs are capable of GPIIb-IIIa–mediated fibrinogen binding.29,30 However, our data begin to address the functional similarities and dissimilarities between GPIIb-IIIa activation on MKs and platelets. Both the agonist concentration and time course required for maximal GPIIb-IIIa activation on MKs were similar to those described for receptor activation on platelets.2,31,32 Furthermore, the affinity of MK GPIIb-IIIa receptors for fibrinogen was similar to that reported for platelets.2 15 In the present assay, TRAP was substantially more effective at activating MK GPIIb-IIIa receptors than ADP, which is in accord with the relative strengths of thrombin and ADP as platelet agonists. Although MKs activated with TRAP aggregated in the presence of fibrinogen, ADP-activated MKs did not (data not shown). Thus, it is possible that this differential responsiveness to agonist stimulation may also be due to (1) inadequate numbers of MK ADP receptors, (2) immaturity of ADP-dependent second messenger signals, and/or (3) differences between MK and platelet cytoskeletal architecture.
Ligands induced to bind to MK GPIIb-IIIa receptors appear to share similarities with GPIIb-IIIa–bound ligands on activated platelets. Fibrinogen binding to MK GPIIb-IIIa receptors was reversible, and the extent of reversibility was dependent on the duration of cell activation, as has been described previously for platelets.27,28 The extent to which fibrinogen binding to activated MKs was irreversible was similar to that reported for platelets by some investigators,28 but more than others.27 Fluorescence quenching experiments determined that a substantial portion of the fibrinogen bound to MKs became internalized within a few minutes of activation, which is similar to what we observed in platelets. Rapid internalization of GPIIb-IIIa–bound fibrinogen has been described previously for activated platelets,28 as has internalization of GPIIb-IIIa on resting MKs29 and platelets.33 However, internalization of GPIIb-IIIa–bound fibrinogen can only account for some of the irreversibly bound ligands on MKs, because the fraction irreversibly bound was substantially greater than the fraction internalized. It is possible that cytoskeletal reorganization in activated MKs contributes to stablization of the GPIIb-IIIa–bound fibrinogen, as has been proposed to occur in activated platelets.27 Although such a determination is beyond the scope of this work, our studies suggest that ex vivo-cultured MKs may be a useful substrate for further characterization of platelet and MK functions.
Viral vectors have been successfully used for exogenous gene transfer to a number of cell types, including hematopoietic cells.34,35 Adenoviral vectors have been shown to be particularly useful for this purpose because of their ability to transform a variety of postmitotic cells, high multiplicity in culture, and ability to incorporate large inserts.36 Data from this study demonstrated that adenoviral vectors were useful for gene transfer to cultured MKs. Efficient transduction occurred at a variety of MOI over a 96-hour interval with only a modest reduction in cell viability. The efficiency, time course, and toxicity we observed with adenovirus-mediated gene transduction of cultured MKs were very similar to those recently reported by other investigators.37Transduction efficiency appeared to be greater with the GFP than with the β-gal vector. The observed difference in transduction efficiency more likely reflects greater sensitivity of the GFP assay than a true difference in protein expression; however, we cannot rule out the latter. The high transduction efficiency of MKs may be related to high-level αvβ3 expression, which has been shown to be important for adenoviral infection of a number of cell types, including MKs.37 Importantly, transduced cells were shown to retain morphologic and physiologic MK structure and function. GFP and β-gal were chosen as reporter genes because of the ease with which transformed cells can be identified. However, our data suggest that targeted gene transduction in cultured MKs is a methodology that may be useful for elucidating the molecular mechanisms underlying MK developmental biology and GPIIb-IIIa function.
Supported by National Institutes of Health Grants No. HL03454 and HL58564.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Nauder Faraday, MD, Department of Anesthesiology/CCM, Johns Hopkins University School of Medicine, 600 N Wolfe St, Baltimore, MD 21287.