Abstract
Kell blood group protein shares a consensus sequence (H.E.X.X.H) with a large family of zinc-dependent endopeptidases. Kell has closest homology with neutral endopeptidase 24.11, endothelin converting enzyme-1 (ECE-1), and the PEX gene product that, as a group, comprise the M13 subfamily of mammalian neutral endopeptidases. The proteolytic activity of the M13 members, but not of Kell, has been previously demonstrated. A secreted form of wild-type Kell protein (s-Kell), devoid of the intracellular and transmembrane domains, was expressed in sf9 cells. As a negative control, an inactive mutant Kell protein (E582G) was expressed. As determined by N-terminal amino acid sequencing and mass spectrometry of the cleaved products, wild-type s-Kell, but not the control mutant protein, specifically cleaved big endothelin-3 (ET-3) at Trp21-Ile22, yielding ET-3, and, to a much lesser extent, also cleaved big ET-1 and big ET-2 at Trp21-Val22, yielding ET-1 and ET-2. Enzymatic activity was partially inhibited by phosphoramidon. s-Kell has an acidic pH optimum (pH 6.0 to 6.5). Like the recombinant protein, red blood cells of common Kell phenotype also preferentially process big ET-3, in contrast to Ko (null) cells that do not. These data demonstrate that the Kell blood group protein is a proteolytic enzyme that processes big ET-3, generating ET-3, a potent bioactive peptide with multiple biological roles.
THE KELL BLOOD GROUP protein is a 93-kD, type II, membrane glycoprotein that shares a pentameric zinc-binding consensus sequence (H.E.X.X.H) with a large family of metalloendopeptidases.1-3 Within this large group, Kell is homologous to 4 other type II membrane glycoproteins; neutral endopeptidase 24.11 (NEP), two different endothelin converting enzymes (ECE-1 and ECE-2), and the product of the PEXgene.4-7 This subfamily of proteins has been classified as the M13, or neprilysin family, of zinc endopeptidases. Kell has 32% to 36% amino acid identity with NEP and ECE-1 in a C-terminal extracellular domain (residues 550 to 732 of Kell) that contains the zinc-binding enzymatic active site. In addition to amino acid sequence homology, there are striking structural similarities, because 10 extracellular cysteine residues are conserved in the M13 family.
NEP is a promiscuous enzyme with wide tissue distribution and specificity, cleaving small peptides at the amino-terminal side of hydrophobic amino acids and also hydrolyzing a variety of physiologically active peptides.8 The specificity of NEP appears to be dependent on its cellular location and the availability of suitable substrates. NEP has been implicated in the hydrolysis of the enkephalins, substance P, bombesin-like peptides, atrial natriuretic factor, oxytocin, bradykinin, angiotensin I and II, and the bacterial chemotactic peptide, fMet-Leu-Phe.4,9 10
ECE-1, by contrast, has a narrow specificity and is primarily involved in the processing of the intermediate precursors of endothelin, termed big endothelins, to produce bioactive endothelins. The endothelins are potent vasoconstrictors, affecting vascular tone, and have several additional biological roles, including proliferative effects on many cells and regulation of embryogenesis by affecting the development of neural crest-derived cells.11-14 There are 3 distinct genes that encode the different endothelins (endothelin-1 [ET-1], ET-2, and ET-3). Initially, the endothelins are synthesized as large (∼200 amino acids) prepro-endothelins that are intracellularly processed by cleavage of paired basic sites by furin-type proteases into intermediate (37 to 41 amino acids) inactive peptides termed big endothelins (big ETs). ECE-1 then processes the big ETs generating bioactive 21 amino acid peptides. ET-1 and ET-2 are formed by cleaving a Trp21-Val22 bond and ET-3 is generated by cleaving a Trp21-Ile22 bond. ECE-1 cleaves big ET-1 more efficiently than it processes big ET-2 or big ET-3.7,15,16 Three different isoforms of ECE-1 have been described, all with similar properties.17-19 ECE-2, which has 59% amino acid identity to ECE-1, has similar specificity as ECE-1, but differs in having an acidic pH optimum and may function intracellularly instead of on the cell surface.6 Recently, a third endothelin converting enzyme, with preference for big ET-3 rather than for big ET-1, was purified from bovine iris, but its primary structure has not yet been determined.20 Although the primary function of ECE-1 and ECE-2 is to process the endothelins, recombinant ECE-1, which is expressed in CHO cells, has been shown to also cleave bradykinin at the Pro7-Phe8 bond, but not to affect a number of other bioactive peptides.21
Mutations in PEX are associated with X-linked hypophosphatemic rickets.22,23 The product of the PEX gene also has endopeptidase activity and on expression in transfected COS cells does not have NEP-like activity but can hydrolyze human parathyroid hormone-derived peptides.24 PEX is preferentially expressed in bone tissue but is also present in kidney, ovary, lung, fetal skeletal muscle, and tumor tissues associated with hypophosphatemic osteomalacia.
The enzymatic activities of NEP and ECE-1 are contained within the extracellular domain. Expression of recombinant truncated forms of NEP and ECE-1 has been reported, and the soluble forms, lacking the intracellular and transmembrane domains, retain proteolytic activity and substrate specificity.25-27
Several amino acid residues in both NEP and ECE-1 have been identified as necessary for zinc-binding and proteolytic activity. Essential amino acid residues are the 2 zinc-coordinating histidines and glutamic acid in the H.E.X.X.H motif. A third zinc ligand, E647 in NEP, and E650 in ECE-1 are also essential for proteolytic activity, as are H712 of NEP, H715 of ECE-1, R718 of NEP, and R721 of ECE-1. These studies have been previously reviewed.5 28 The amino acids necessary for endopeptidase activities are all conserved in Kell.
Kell proteins differ from the other members of the M13 family in that they are covalently linked by a disulfide bond to a 50.9-kD protein, XK, that spans the membrane 10 times.29 Kell Cys72, which is on the extracellular domain, close to the transmembrane region, is linked to XK Cys347, present in the fifth extracellular loop of XK.30 ECE-1 is associated with itself as a dimer through Cys412 but is not linked to a different protein.31 The absence of XK, which occurs in McLeod patients, is correlated with acanthocytic red blood cells (RBCs) and a late-onset form of nerve and muscle disorders, but its specific cellular functions are not known.2 32 We report here that Kell is involved in the processing of bioactive peptides and can process and activate the endothelins. In contrast to ECE-1, Kell preferentially generates ET-3.
MATERIALS AND METHODS
Construction of Expression Vectors
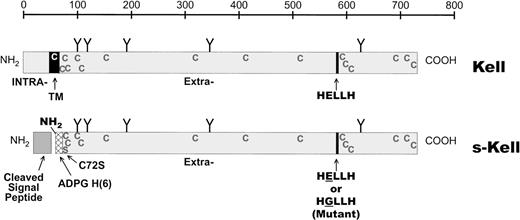
cDNA encoding truncated versions of Kell protein, lacking the intracellular and transmembrane domains but containing a secretion signal sequence and tagged at the amino-terminal end of the protein with 6 histidines, were placed in pAcGP67A transfer vector. Three different constructs were prepared encoding the extracellular domains of wild-type Kell, the Jsa Kell phenotype, and, as a negative control, a E582G mutant that replaces the glutamic acid residue in the H.E.L.L.H. consensus sequence with glycine. All of these constructs also contained an additional point mutation, Cys72Ser, to avoid intermolecular associations. (See diagram in Fig 1.)
Diagrams of Kell protein and of the recombinant expressed extracellular domain. The top diagram is of Kell protein showing the transmembrane region (TM), separating the short intracellular domain from a large extracellular segment. Also shown are the positions of cysteine residues (C), carbohydrate moieties (Y), and the zinc-binding enzymatic active site (HELLH). The bottom diagram depicts the expressed extracellular domain, devoid of intracellular and transmembrane domains and containing 6 histidines as a tag, plus an additional 4 amino acids (ADPG). The location of the introduced C72S mutation and a gp67 secretion signal that is cleaved after translation are also shown.
Diagrams of Kell protein and of the recombinant expressed extracellular domain. The top diagram is of Kell protein showing the transmembrane region (TM), separating the short intracellular domain from a large extracellular segment. Also shown are the positions of cysteine residues (C), carbohydrate moieties (Y), and the zinc-binding enzymatic active site (HELLH). The bottom diagram depicts the expressed extracellular domain, devoid of intracellular and transmembrane domains and containing 6 histidines as a tag, plus an additional 4 amino acids (ADPG). The location of the introduced C72S mutation and a gp67 secretion signal that is cleaved after translation are also shown.
Wild-Type s-Kell
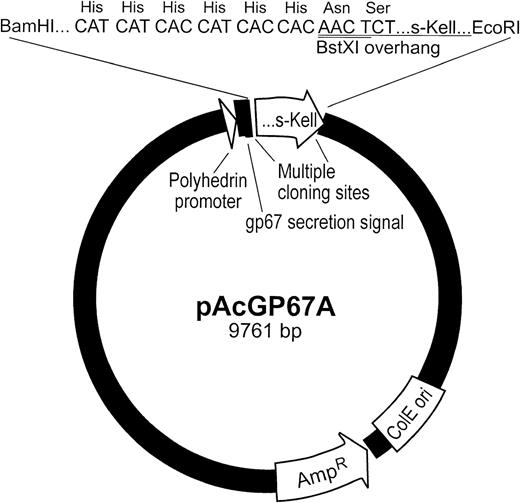
A pRc/CMV vector containing wild-type Kell with the Cys72Ser mutation was the starting material.30 The areas encoding the intracellular and transmembrane regions were excised by cutting withHindIII and BstXI (Kell nt327). An adapter containing aBamHI site, encoding 6 histidine residues, and HindIII and BstXI overhangs was ligated to the 5′ end of the remaining Kell cDNA. On treatment with BamHI and Stu I (Kell nt 1406), a 1.1-kb fragment was released from the pRc/CMV vector. The released fragment, which encodes the extracellular domain of Kell (s-Kell) and the adaptor, was placed in BamHI and Stu I sites of Kell cDNA in pAcGP67A vector. A map of the pAcGP67A transfer vector containing the s-Kell cDNA is shown in Fig 2. The construct was confirmed by DNA sequencing.
Vector containing Kell cDNAs used for expression of sKell by sf9 cells. Diagram of pAcGP67A vector containing s-Kell cDNA. The locations of s-Kell cDNA, gp67 secretion signal, polyhedrin promoter, and cloning sites are shown. Also depicted, in expanded form, is the 5′ end of s-Kell cDNA encoding the histidine tag and the first 2 codons of s-Kell.
Vector containing Kell cDNAs used for expression of sKell by sf9 cells. Diagram of pAcGP67A vector containing s-Kell cDNA. The locations of s-Kell cDNA, gp67 secretion signal, polyhedrin promoter, and cloning sites are shown. Also depicted, in expanded form, is the 5′ end of s-Kell cDNA encoding the histidine tag and the first 2 codons of s-Kell.
E582G Kell Mutant
A Kell cDNA that contains a Hpa I site at Kell nt 1735 was used as the starting material for construction of an A1865G mutation. Forward and reverse primers harboring the A1865G mutation were used to amplify, by a 3-step procedure, a 376-bp product. The 376-bp product was digested with Hpa I (nt 1735) and Nhe I (nt 2043) to create a 309-bp fragment that was inserted at Hpa I andNhe I sites of Kell cDNA. The resulting plasmid was digested with Stu I and Nhe I and a 638-bp fragment obtained was placed in the Stu I (nt 1406) and Nhe I (nt 2043) sites of wild-type s-Kell pACGP67A (described above).
Jsa-Phenotype s-Kell
The s-Kell containing the Jsa Kell phenotype33was constructed similarly to wild-type s-Kell, except that a pRC/CMV vector containing the cDNA encoding Jsa Kell phenotype was used as the starting material. The Jsa cDNA was prepared from total RNA obtained from reticulocytes by a reverse transcriptase reaction.
Preparation of High-Titer Viral Stocks and Expression of Recombinant Proteins
sf9 cells (9 × 105 cells) were coinfected with pAcGP67A containing s-Kell cDNA (1.3 μg in 100 μL of Grace Basic medium; Invitrogen, Carlsbad, CA) and BaculoGold (0.17 μg; Pharmingen, San Diego, CA). Infection occurred in 35-mm plates with 5 μL of Cellfection (GIBCO BRL, Gaithersburg, MD) as recommended by the manufacturer. High titer viral stocks were prepared basically following the protocol provided by Pharmingen. Briefly, media containing the virus was harvested 5 days after transfection by centrifugation at 2,000g for 5 minutes. The virus stock was amplified 2 times using end point dilution methods in which 1, 10, and 100 μL of virus stock was used to infect 0.5 × 105 sf9 cells in 1 mL of Grace media grown in 12-well plates. The first amplified batch of virus stock, ranging from 2 to 8 × 107 multiplication of infection (MOI) per milliliter, was used to prepare the final high titer viral stock by infection of sf9 cells, which were grown in monolayers in Excell 400 media (JRH Biosciences, Lenexa, KS) with approximately 0.9 MOI of the viral stock.
Recombinant protein was expressed by infecting the sf9 cells, grown in Excell 400 and 420 mixed media (1/1 vol/vol), with approximately 10 MOI of the high titer viral stock. The media containing recombinant protein was collected, centrifuged at 2,000g for 5 minutes to remove cell debris, centrifuged at 40,000g for 25 minutes to remove virus particles, and stored at −70°C.
Isolation of s-Kell From the Cell Media
Affinity chromatogrphy on nickel columns.
s-Kell in the cell media (600 μL) was applied to a Ni-NTA resin column (QIA express kit; Qiagen, Valencia, CA) that had been equilibrated with cell culture media. The column was washed with 50 mmol/L sodium phosphate buffer, pH 8.0, containing 0.3 mol/L NaCl and 20 mmol/L imidazole. Bound protein was eluted with 50 mmol/L sodium phosphate buffer, pH 8.0, 0.3 mol/L NaCl, and 250 mmol/L imidazole.
Immunoabsorbtion.
Mouse monoclonal antibody to KEL1434 was tagged with biotin using biotin hydrazide coupling reagent (Pierce Chemical Co, Rockford, IL). s-Kell, which was present in the cell culture medium, was immunoabsorbed by anti-KEL14 by incubation for 3 hours at 4°C. The antibody-antigen complex was linked to ImmunoPure immobilized streptavidin beads (Pierce Chemical Co) by further incubation at 4°C for 3 hours. The beads were washed with phosphate-buffered saline.
Endoprotease Assay Using 7-Amino-4-Methyl Coumarin (AMC)-Conjugated Synthetic Peptides
A 2-step enzyme reaction was employed using 96-well plates to determine endopeptidase activity. In the first step, synthetic peptides with blocked amino termini and coupled with AMC (125 μmol/L; Enzyme Systems Products Inc, Livermore, CA) were incubated at 30°C for 2 hours with ZnCl2 (12.5 μmol/L), 10 μL of cell medium containing recombinant s-Kell media, and 74 mmol/L HEPES buffer, pH 7.4, in a total volume of 80 μL. In a second step, 0.125 U aminopeptidase I from Streptomyces griseus (Sigma, St Louis, MO) was added and the plates were further incubated at 30°C and read for 2 hours in a FL-500 Microplate Flourescence Reader (Bio-Tek Instruments, Winooski, VT) at an excitation wavelength of 360/40 and an emission wavelength of 460/40.
Processing of Big ET-1, Big ET-2, and Big ET-3 With s-Kell
High performance liquid chromatography (HPLC) analysis.
An enzyme reaction mixture was made by mixing 8 to 16 μL of 0.5 mg/mL big ET-1 (1-38), big ET-2 (1-37), or big ET-3 (1-41) (American Peptide Co [Sunnyvale, CA] and Sigma), 5 μL of 1 mmol/L ZnCl2, and 70 μL cell media containing recombinant s-Kell in a total volume of 116 μL. The mixture was incubated at 37°C for different time periods, and 20- to 30-μL aliquots of the incubation mixtures were assayed by reversed-phase HPLC. When Km values were determined, the concentrations of big ET-2 ranged from 1 to 16 μmol/L and the incubation was 30 minutes; for big ET-1, the concentrations ranged from 8 to 32 μmol/L and the incubation time was 1 hour. When s-Kell antibody complex, bound to ImmunoPure beads, was assayed for processing activity, 100 μL of packed beads was incubated with 16 μg of big ET-1 for 3 hours at 37°C, as described above.
The condition of HPLC analysis was as follows: solvent A: 0.1% trifluoroacetic acid/2.5% 1-propanol/ H2O; and solvent B: 0.09% trifluoroacetic acid/2.5% 1-propanol/90% acetonitrile/ H2O.
The gradient condition was 1% to 61% A to B over a 0- to 60-minute period at a flow rate of 0.15 mL/min. A 2.1 × 150 mm C18 column (Vydac, Hesperia, CA) was used.
Endothelin enzyme immunoassay (EIA).
Big ET-1, big ET-2, or big ET-3 (0.1 μmol/L) were incubated at 37°C for 15 minutes with assay buffer (50 mmol/L HEPES, pH 6.0, 50 μmol/L ZnCl2, and 150 mmol/L NaCl containing 0.25% [vol/vol] of supernatant of boiled bovine serum albumin [BSA; 1 mg /mL]) and various amounts of media containing s-Kell (corresponding to 0.0625 to 0.5 μL of the original undiluted media). The final volume was 200 μL. At the end of the incubation time, an equal volume of 5 mmol/L EDTA was added to terminate the reaction, and the samples were kept in ice until EIA was performed. Various amounts of the sample up to 100 μL were used to determine the amount of ET-1, ET-2, or ET-3 generated using the ET-1 EIA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. The antibody to ET-1, which is used in the kit, cross-reacts equally well with ET-2 and ET-3.
When Km values were determined for big ET-3, the substrate concentrations ranged from 0.1 to 0.6 μmol/L and the incubation time was 10 minutes.
When s-Kell antibody complex bound to ImmunoPure beads was assayed, 20 μL of a 1:1 (vol/vol) suspension was used and the beads were removed by centrifugation at the end of the initial 15 minutes of incubation.
Processing of Big Endothelins by Wild-Type and Ko(null) RBCs
Droplet frozen wild-type and Ko(null) RBCs were recovered from liquid nitrogen storage, thawed in warm phosphate-buffered saline, and washed, and a 0.5% (vol/vol) RBC suspension was filtered 3 times through a Leukosorb filter (Pall Biomedical Products, East Hills, NY) to remove white blood cells. A suspension of 8 × 107 cells in 200 μL was incubated at 37°C for 15 minutes with 0.1 μmol/L big ET-1, big Et-2, or big ET-3, as described above. The RBCs were removed by centrifugation and an equal volume of 5 mmol/L EDTA was added to the supernatant fraction to stop the reaction. Aliquots were used for EIA as described above.
Other Methods
Amino acid sequences were obtained using an ABI 477A/120A protein sequencer with a 100-μL sample loop and ABI reagents (Applied Biosystems Division of Perkin-Elmer Inc, Foster City, CA).
Mass spectrometric analyses were performed using a Perseptive Biosystems Voyager DE MALDI mass spectrometer (Perkin Elmer Biosystems, Framingham, MA). Spectra were calibrated against an external standard.
Chromatographic analyses or purifications were performed using a Hewlett Packard 1100 or 1090 with a HP ChemStation (Hewlett Packard, Palo Alto, CA). C18 reverse-phase columns (2.1 × 150 mm) were from Vydac (Hesperia, CA). All reagents were of HPLC-grade quality.
RESULTS
Expression and Secretion of s-Kell
The presence of s-Kell in the sf9 cell culture media was determined by separation of the proteins on reduced 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblots using a polyclonal antibody to 93-kD Kell protein. Wild-type s-Kell, the Jsa-Kell phenotype, and the E582G mutant all showed near equal amounts a protein of the expected size for s-Kell (∼80 kD) that reacted with the antibody (Fig 3, left panel, lanes 2 through 4). This protein was not present in the cell medium of noninfected sf9 cells (Fig 3, left panel, lane 1).
Western immunoblots of expressed s-Kell. The left panel shows Western immunoblots with a rabbit polyclonal antibody to human Kell protein. Proteins present in the media from sf9 cells expressing wild-type, Jsa Kell phenotype and E582G mutant s-Kell were separated by SDS-PAGE. Molecular size markers are shown. Lane 1 is a control from medium of sf9 cells that were not infected. Lane 2 is medium from cells expressing from wild-type s-Kell. Lane 3 has medium from cells expressing Jsa Kell phenotype and lane 4 has medium from those expressing E582G mutant. The right panel shows proteins isolated on nickel columns eluted with imidazole and analyzed as described above. Lane 1 is from cells expressing Jsa Kell phenotype, lane 2 is from cells expressing wild-type, and lane 3 is from cells expressing the E582G mutant. Lane 4 is a control from uninfected sf9 cells.
Western immunoblots of expressed s-Kell. The left panel shows Western immunoblots with a rabbit polyclonal antibody to human Kell protein. Proteins present in the media from sf9 cells expressing wild-type, Jsa Kell phenotype and E582G mutant s-Kell were separated by SDS-PAGE. Molecular size markers are shown. Lane 1 is a control from medium of sf9 cells that were not infected. Lane 2 is medium from cells expressing from wild-type s-Kell. Lane 3 has medium from cells expressing Jsa Kell phenotype and lane 4 has medium from those expressing E582G mutant. The right panel shows proteins isolated on nickel columns eluted with imidazole and analyzed as described above. Lane 1 is from cells expressing Jsa Kell phenotype, lane 2 is from cells expressing wild-type, and lane 3 is from cells expressing the E582G mutant. Lane 4 is a control from uninfected sf9 cells.
The presence of s-Kell in the cell media was also determined by immunoprecipitation with a biotinylated mouse monoclonal antibody to KEL14 and subsequent adsorption on agarose beads containing immobilized streptavidin (ImmunoPure beads; Pierce). The bound proteins were extracted from the agarose beads with 8 mol/L urea, 1% SDS, and 5% mercaptoethanol in Tris-HCl, pH 6.7; separated by SDS-PAGE; and analyzed by Western immunoblotting using a rabbit polyclonal antibody to the 93-kD Kell protein. A protein band with the approximate expected size of s-Kell was detected for the wild-type, E582G mutant and the Jsa Kell phenotype, but not from samples obtained from control noninfected sf9 cells (data not shown).
Similar results were obtained when the proteins were first captured on nickel columns, eluted with imidazole, and analyzed by SDS-PAGE and Western immunoblot using a rabbit polyclonal antibody to human Kell protein (Fig 3, right panel).
Small Synthetic Peptides and Bradykinin Are Not Cleaved by s-Kell
More than 20 small, synthetic peptides, ranging from 3 to 8 amino acids, coupled to the fluorescent dye, AMC, were tested as possible substrates for s-Kell. The cell media containing either wild-type, Jsa Kell phenotype s-Kell, or the E562G mutant s-Kell, as a negative control, were incubated with the synthetic peptides and assayed for endopeptidase activity. Included were peptides that are cleaved by trypsin, chymotyrypsin, cathepsin G, and neutral endopeptidases 24.11, a close homolog of Kell protein. As compared with the E562G mutant, there was no significant endopeptidase activity displayed by wild-type or the Jsa Kell phenotype s-Kell (data not shown).
After 2 hours of incubation at 37°C, Bradykinin (Sigma), which is hydrolyzed by both NEP and ECE-1,21 was not cleaved by s-Kell or Jsa phenotype s-Kell, as determined by HPLC analysis (data not shown).
Wild-Type and Jsa-Kell Phenotype, But Not the E562G Mutant, Process Big Endothelins
Big ET-1, ET-2, and ET-3 were incubated with cell medium from sf9 cells expressing wild-type, Jsa phenotype or E582G mutant s-Kell and the reaction products were separated by HPLC using a reverse-phase C18 column. The separated peptides were analyzed by mass spectrometry (Table 1) and amino acid sequencing (Table 2).
Big ET-1 (38 amino acids) was not cleaved by the E582G s-Kell mutant, and a single peptide (retention time, 43.2 minutes) was obtained by HPLC (Fig 4, lower panel). On incubation with wild-type or Jsa phenotype s-Kell, 2 other peptides with retention times of 28.5 and 45.1 minutes were obtained (Fig 4, upper panel). Mass spectrometry of the products derived from incubation with wild-type s-Kell determined that the peptide with a retention time of 28.5 minutes (peak C, Fig 4) had a mass of 1,810.3 Daltons (Table1), which corresponds to the theoretical mass of a peptide consisting of amino acids 22-38 of big ET-1. The peptide with a retention time of 45.1 minutes (peak B, Fig 4) had a mass of 2,494.6 Daltons, corresponding to a peptide with amino acids 1 to 21 of big ET-1. The major peptide (retention time, 43.2 minutes; peak A, Fig 4) had a mass of 4284.5 Daltons and corresponds to uncleaved big ET-1 containing 38 amino acids. The mass spectrometry determination indicates that wild-type s-Kell cleaved big ET-1 at the Trp21-Val22 site. The mass spectrometry results are summarized in Table 1.
HPLC separation of the cleavage products of big ET-1. Big ET-1 was incubated for 2 hours with either wild-type s-Kell or the E582G mutant, and the peptides were separated by HPLC as described in Materials and Methods. The top panel is with wild-type s-Kell and the bottom panel is with the E582G mutant. Peak A is big ET-1 and peaks B and C, noted in the top panel, are the cleaved products.
HPLC separation of the cleavage products of big ET-1. Big ET-1 was incubated for 2 hours with either wild-type s-Kell or the E582G mutant, and the peptides were separated by HPLC as described in Materials and Methods. The top panel is with wild-type s-Kell and the bottom panel is with the E582G mutant. Peak A is big ET-1 and peaks B and C, noted in the top panel, are the cleaved products.
Cleavage at this site was confirmed by amino acid sequencing of the peptide products. Peak B (Fig 4, top panel) had an amino acid sequence that corresponds to amino acids 1 to 21 of big ET-1 (Table 2). The cysteine residues were not detected, because they were not derivatized and were degraded during the sequencing procedure. Also, not detected was the carboxy-terminal tryptophan, which should appear in the cycle 21. The recovery of tryptophan is customarily less than that of the other amino acids and its low recovery from a late and C-terminal cycle is not unusual. The mass spectrum of the product confirmed the presence of tryptophan. Cleavage at Try21-Val22 was confirmed by detecting valine in the amino-terminus of the peptide in peak C (Fig 4, top panel). The amino acids sequence of peak C corresponded with that expected for a peptide representing amino acids 22 to 38 of big ET-1.
Mass spectrometry and amino acid sequence of the major peptides produced by incubation of big ET-2 (1-37) with wild-type s-Kell also demonstrated cleavage at Trp21-Val22. Peak A (Fig 5 and Tables 1 and 2) had valine at its N-terminus and the amino acid sequence expected of peptide 22-37 of big ET-2. The amino acid sequence of peak C corresponds with that expected for amino acids 1 to 21 of big ET-2. As noted for big ET-1, the recovery of the C-terminal tryptophan in cycle 21 was low and not detected. Peak B in Fig 5 was characterized by its retention time and mass spectrometry as uncleaved big ET-2.
HPLC separation of the peptides produced by cleavage of big ET-2 by wild-type s-Kell. Big ET-2 was incubated with wild-type s-Kell, and the peptides were separated by HPLC as described in Fig 4. Peak B is big ET-2, and peaks A and C are the cleaved products.
HPLC separation of the peptides produced by cleavage of big ET-2 by wild-type s-Kell. Big ET-2 was incubated with wild-type s-Kell, and the peptides were separated by HPLC as described in Fig 4. Peak B is big ET-2, and peaks A and C are the cleaved products.
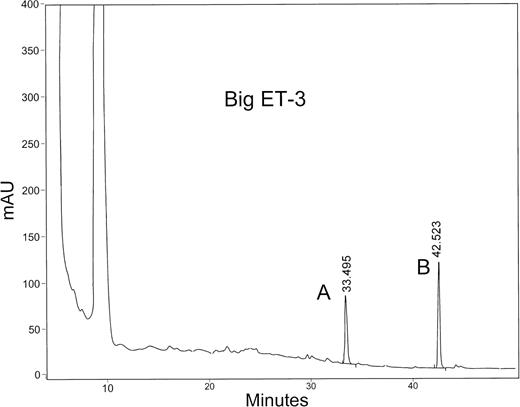
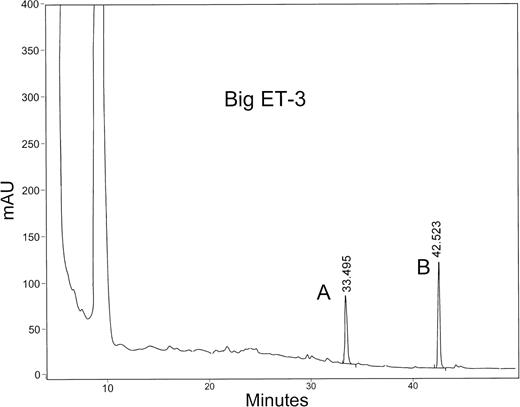
The two peptides produced by cleavage of big ET-3 by wild-type s-Kell corresponded to peptides containing amino acids 1 to 21 and 22 to 41 of big ET-3, indicating cleavage of the Trp21-Ile22 bond. Amino acid sequencing showed that peak A (Fig 6) had N-terminal isoleucine and the remainder of the sequence corresponded to amino acids 22 to 41 of big ET-3 (Table 2). The amino acid sequence of peak B corresponded to amino acids 1 to 21 of big ET-3. Again, the cysteine residues, as predicted, were not detected and were absent from the expected cycles and the C-terminal tryptophan was not detected due to low recovery. The results of mass spectrometry analysis agreed with the amino acid sequence of the 2 peptides (Table 1).
HPLC separation of the cleaved products of big ET-3 by wild-type s-Kell. Big ET-3 was incubated with wild-type s-Kell as described in Figs 4 and 5. Big ET-3 was completely processed yielding the cleaved products, peaks A and B.
The above-noted results were obtained using cell media as the enzyme source. Isolation of s-Kell by affinity chromatography using a nickel column and elution with imidazole inactivated the enzyme. Enzyme activity, which was measured by the ability to cleave the big ETs as determined by HPLC, as described above, or EIA, was lost both in the pass-through and the eluted fractions. Enzymatic activity of purified s-Kell was demonstrated by immunoprecipitation. When wild-type s-Kell was immunoabsorbed with biotin-tagged monoclonal antibody to KEL14 and Streptavidin-ImmunoPure beads, the bound protein retained proteolytic activity. Bound wild-type s-Kell processed big ET-1, as measured by HPLC, and preferentially processed big ET-3, as determined by EIA (data not shown).
Preferential Processing of Big ET-3
Processing of the different big endothelins by wild-type s-Kell was measured by incubation (15 minutes at 37°C) of big ET-1, ET-2, or ET-3 with increasing amounts of either wild-type s-Kell or the E582G mutant and determination of the amount of ET-1, ET-2, or ET-3 generated by EIA. Big ET-3 was nearly 10 times as effective a substrate as big ET-2 or big ET-1. Although not shown, similar results were obtained with Jsa Kell phenotype s-Kell. When the mutant E562G was used, there was no significant processing of big-ET-3 (Fig 7).
Preferential processing of big ET-3 by wild-type s-Kell. Big ET-1, big ET-2, or big ET-3 was incubated for 15 minutes with wild-type s-Kell. As a control, the E582G mutant was also incubated with big ET-3. Endothelins produced were measured by EIA. A legend is included in the top left side of the figure.
Preferential processing of big ET-3 by wild-type s-Kell. Big ET-1, big ET-2, or big ET-3 was incubated for 15 minutes with wild-type s-Kell. As a control, the E582G mutant was also incubated with big ET-3. Endothelins produced were measured by EIA. A legend is included in the top left side of the figure.
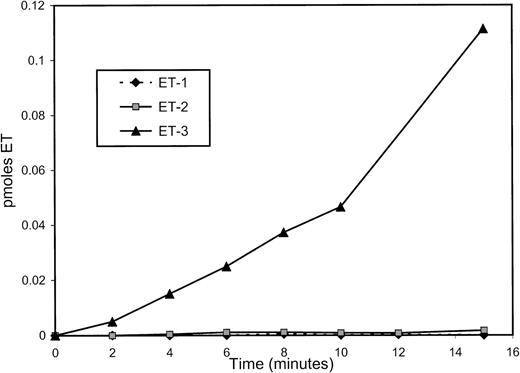
A time course of incubation using big ET-1, big ET-2, or big ET-3 as substrates also showed marked preferential processing of big ET-3 (Fig 8).
Time course of incubation. Big ET-1, big ET-2, or big ET-3 was incubated with wild-type s-Kell and endothelins measured by EIA. The box in the figure includes a legend.
Time course of incubation. Big ET-1, big ET-2, or big ET-3 was incubated with wild-type s-Kell and endothelins measured by EIA. The box in the figure includes a legend.
The Km value of s-Kell with big ET-3 as substrate was 0.33 ± 0.16 μmol/L, as determined by EIA. It was difficult to determine Km using big ET-1 and big ET-2 as substrates with EIA, due to low processing. Therefore, the Km values were obtained by measuring the cleavage products of big ET-1 and big ET-2 by HPLC. The approximate values were 43 μmol/L for big ET-1 and 20 μmol/L for big ET-2.
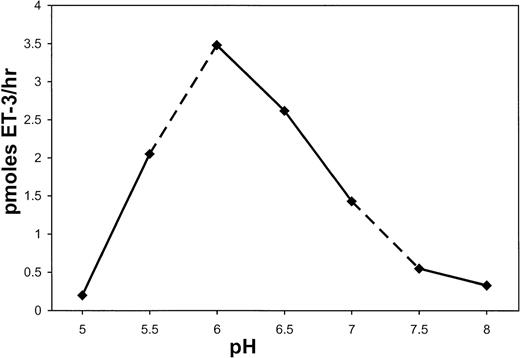
s-Kell Has an Acidic pH Optimum
pH optimum using big ET-3, ET-2, or ET-1 as substrates was determined using the following buffers: 50 mmol/L MES for pH 5.0 to 5.5; 50 mmol/L HEPES for pH 6.0 to 7.0; and 50 mmol/L Tris-HCl for pH 7.5 to 8.0. Enzyme activity was measured using the EIA assay. The pH optimum for s-Kell, with big ET-3 as substrate, was between 6.0 and 6.5 (Fig 9). Similar results (data not shown) were obtained with big ET-2 and big ET-1 as substrates when processing was measured by HPLC .
pH optimum of wild-type s-Kell. Processing of big ET-3 by wild-type s-Kell was measured at various pH by EIA determination of ET-3. Incubation conditions are described in Materials and Methods.
pH optimum of wild-type s-Kell. Processing of big ET-3 by wild-type s-Kell was measured at various pH by EIA determination of ET-3. Incubation conditions are described in Materials and Methods.
Proteolytic Activity Is Inhibited by Phosphoramidon
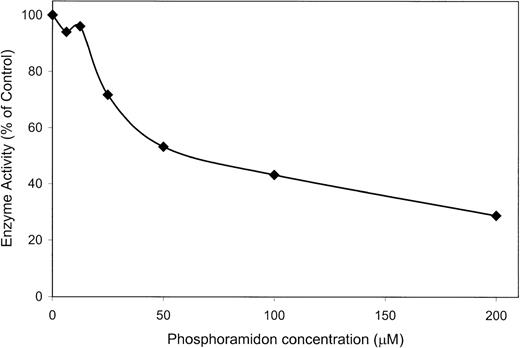
Processing of big ET-3 by wild-type s-Kell was partially inhibited by phosphoramidon. Big ET-3 was incubated for 15 minutes at 37°C with wild-type s-Kell, and at a phosphoramidon concentration of 50 μmol/L the endopeptidase activity was reduced to approximately 50%. At 200 μmol/L phosphoramidon, approximately 30% of the enzymatic activity remained (Fig 10).
Partial inhibition by phosphoramidon. Big ET-3 was incubated with wild-type s-Kell and varying concentrations of phosphoramidon for 15 minutes at 37°C, and the amount of processing was determined by EIA. A 100% activity is that which occurs in the absence of phosphoramidon.
Partial inhibition by phosphoramidon. Big ET-3 was incubated with wild-type s-Kell and varying concentrations of phosphoramidon for 15 minutes at 37°C, and the amount of processing was determined by EIA. A 100% activity is that which occurs in the absence of phosphoramidon.
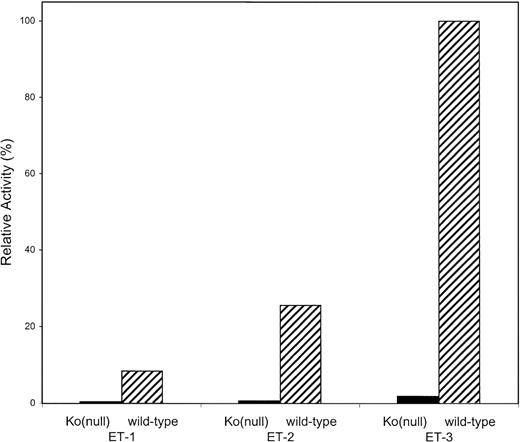
RBCs of Common Kell Phenotype, But Not Ko(null) RBCs, Preferentially Process Big ET-3
RBCs of common Kell phenotype (wild-type) were compared with Ko(null) RBCs for the ability to process the big ETs and generate endothelins. Frozen RBCs were thawed and washed; residual white blood cells were removed and incubated with big ET-1, bigET-2, or big ET-3; and the endothelins generated were measured by EIA. Wild-type RBCs preferentially processed big ET-3. Big ET-3 was processed 4.5× more than big ET-2 and nearly 10× more than big ET-1. Strikingly, Ko(null) RBCs had little or no processing ability (Fig 11).
Preferential processing of big ET-3 by RBCs of common Kell phenotype: comparison with Ko(null) phenotype. Common Kell phenotype and Ko(null) RBCs were incubated for 15 minutes at 37°C with big ET-1, big ET-2, or big ET-3, and the endothelin generated was measured by EIA. A 100% activity is that of wild-type RBCs with big ET-3 as substrate. (▨) RBCs of common Kell phenotype (wild-type); (▪) Ko(null) RBCs.
Preferential processing of big ET-3 by RBCs of common Kell phenotype: comparison with Ko(null) phenotype. Common Kell phenotype and Ko(null) RBCs were incubated for 15 minutes at 37°C with big ET-1, big ET-2, or big ET-3, and the endothelin generated was measured by EIA. A 100% activity is that of wild-type RBCs with big ET-3 as substrate. (▨) RBCs of common Kell phenotype (wild-type); (▪) Ko(null) RBCs.
Further evidence for the specific processing of big ET-3 by native Kell protein on RBCs was obtained by comparing membrane ghosts prepared from RBCs of common Kell phenotype and from Ko(null) RBCs. Similar to intact RBCs, big ET-3 was processed by membranes from normal, but not from Ko(null), RBCs (data not shown).
DISCUSSION
Cell surface proteases occur in many cell types and play important roles in cell-cell and cell-matrix interactions and in the activation and degradation of a wide range of bioactive peptides. Many of the cell-surface proteases are zinc-dependent and have been implicated in a variety of cellular functions, including cell growth and differentiation, inflammation, and regulation of vascular tone.5 9 Based on structural and amino acid sequence similarities, the Kell blood protein was classified, together with NEP, ECE-1, ECE-2, and the product of the PEX gene, as a member of the M13 or neprilysin subfamily of zinc metalloproteases. Our studies now show that Kell is a cell-surface endopeptidase that shares substrates with ECE-1 and ECE-2, processing the inactive intermediate precursors of the endothelins (big ETs) and generating bioactive peptides. However, Kell protein differs from ECE-1 and ECE-2 in that it preferentially activates ET-3 rather than ET-1.
Kell, unlike NEP and similar to ECE, has a narrow endopeptidase specificity in that it specifically cleaves big ET-1 and big ET-2 at Trp21-Val22 and big ET-3 at Trp21-Ile22. This specific cleavage is necessary to produce the 21 amino acid bioactive endothelins. Evidence that Kell protein cleaves at these sites was obtained by isolation of the cleaved products by HPLC, mass spectrometry, and amino acid sequencing.
Zinc metalloproteases all contain a H.E.X.X.H. consensus sequence that is present in all zinc endopeptidases and is mandatory for catalytic activity.35,36 The glutamic acid residue in this sequence is essential for proteolytic activity, and its substitution with other amino acids has been shown to inactivate NEP and ECE-1.31 37 We used the E582G mutation as a negative control, because it would inactivate the proteolytic ability of Kell. The E582G Kell mutant did not process the big ETs. This was a necessary control, because the cell medium of sf9 cells that was used as an enzyme source may contain other proteases. A histidine tag was attached to the N-terminus of s-Kell to facilitate isolation of the protein, which would separate it from contaminating proteases, but unfortunately the procedure, which entails elution from a nickel-column with a chelating agent, imidazole, inactivated the Kell endopeptidase activity. Kell, a zinc-binding protein, appears to be very sensitive to chelating agents. However, proteolytic activity of the purified Kell proteins could be demonstrated by immunoabsorption of s-Kell on agarose beads containing a specific antibody to Kell. The absorbed s-Kell retained endopeptidase activity and specifically processed big ET-1 and big ET-3, with preference for bigET-3. This, together with the negative result of the E582G mutant, demonstrates that the processing of the endothelins is due to s-Kell in the cell media and not due to other proteases.
Further compelling evidence for the processing of big ET-3 by Kell was obtained by comparing wild-type RBCs with the rare Ko(null) RBCs that have little or no Kell protein. These studies showed that big ET-3 is not only processed by recombinant s-Kell, but also by native Kell on intact RBCs. Nature has provided an appropriate control and the rare Ko(null) RBCs, which have undetectable levels of Kell protein, did not proteolytically process big ET-3.
Interestingly, s-Kell has an acidic pH optimum (pH ∼6.0 to 6.5) that differs from native ECE-1, which has a pH optimum of 6.7 to 6.9, and from ECE-2, which has the very acidic pH optimum of 5.5.6It has been noted that a soluble recombinant form of ECE-1 lacking the intracellular and transmembrane domains has a lower pH optimum (pH 6.1 to 6.4) than native ECE-1 (pH 6.7 to 6.9).27 Another study with soluble ECE-1 showed different pH optima for different substrates, with an optimum pH of 6.6 to 6.8 for big ET-1 and an optimum pH of 6.0 for big ET-2 and big ET-3.26 This differs from s-Kell that has approximately the same pH optima for the 3 forms of big ET. It has been suggested that ECE-2 acts as an intracellular enzyme, because its low pH optimum of 5.5 is consistent with the pH of intracellular organelles involved in the late stages of secretion and in endocytosis. The pH optimum of s-Kell is intermediate between that required for optimal intracellular processing and that expected for a surface-exposed membrane proteins The pH optimum of an enzyme from bovine iris, specific for big ET-3, is approximately 6.6.20
Another difference between Kell and ECE-1 is that s-Kell is less sensitive to phosphoramidon, an inhibitor of zinc metalloproteases. For example, 50% inhibition of soluble ECE-1 occurs at phosphoramidon concentrations ranging from 0.03 μmol/L at pH 5.8 to 40 μmol/L at pH 7.2,27 with complete inhibition at 100 μmol/L,26 27 whereas approximately 50 μmol/L phosphoramidon is required to inhibit s-Kell processing of big ET-3 and 40% activity remains at 100 μmol/L phosphoramidon.
The endopeptidase activity of Kell protein is retained in the extracellular domain, similar to NEP and ECE-1,25-27 again demonstrating that the intracellular and transmembrane domains are not necessary for catalytic activity. On RBCs, Kell is part of a complex composed of a 93-kD glycoprotein (Kell) covalently linked, by a single disulfide bond, to a 53-kD protein, XK,29,30 that spans the membrane 10 times. Because the complex is covalently linked, it may be considered as a single entity and not as 2 distinct proteins. Our studies show that the Kell domain proteolytically processes big ET-3 and that XK is not necessary for the processing of big ET. We do not know if the remaining domains of the Kell/XK complex influence the enzymatic functions of Kell, but it is known that absence of XK is associated with abnormal RBC shape and with late onset forms of muscular and neurological abnormalities. XK has structural similarities to membrane transporters,32 and it is possible that the Kell/XK complex may have dual, or multiple, complementary functions, one of which is the activation of ET-3.
The 3 different endothelin isopeptides are encoded by separate genes. ECE-1 and ECE-2 are both capable of specifically cleaving the 3 different big ETs, but they have a strong preference for big ET-1 as substrate.7,15,16 Although all 3 endothelins are normally present in the same tissues, there are some regions of the brain, and other tissues, such as the iris, in which prepro-ET-3 is expressed in higher amounts than prepro-ET-1 and ET-2. This observation led to the biochemical isolation of a 140-kD enzyme from bovine iris (ECE-3) that preferentially activate ET-3.20 This 140-kD protein has not yet been characterized, but its large size differentiates it from Kell protein. Although Kell is thought to be primarily expressed in erythroid tissues,38 recent studies from our laboratory (unpublished data) demonstrate that Kell, linked to XK, is present, in lesser amounts, in many other tissues. We do not yet know if there is a correlation between the distribution of ET-3 and Kell/XK complex in different tissues.
ET-3, like ET-1 and ET-2, is a potent vasoconstrictor, but the endothelins are also mitogenic and appear to be involved in a number of other biological activities. The endothelins act on 2 distinct G protein-coupled receptors, ETA and ETB, that are present in many target cells. At physiological concentrations, ETA binds ET-1 and ET-2 but not ET-3, whereas ETB binds ET-1, ET-2, and ET-3 equally well. Mutations in the ETB receptors are associated with Hirschsprung disease, a congenital intestinal disease, and ET-3 is involved in the development of the enteric nervous system and the migration of neural crest-derived cells.11-14 39 Ko(null) persons, whose RBCs lack Kell protein, are healthy, suggesting that alternate pathways for activation of ET-3 are present. We do not yet know if Ko(null) persons express Kell protein in nonerythroid tissues or if they exhibit abnormal levels of plasma endothelins. Our studies demonstrate that the extracellular domain of the Kell moiety and the native protein on intact RBCs avidly process big ET-3 generating ET-3; however, the Kell/XK complex may have additional functions in both erythroid and nonerythroid tissues.
Supported by a National Institutes of Health Specialized Center of Research (SCOR) Grant in Transfusion Biology and Medicine (HL54459) and by the Robert Leet and Clara Guthrie Patterson Trust.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Soohee Lee, PhD, The New York Blood Center, 310 E 67 St, New York, NY 10021; e-mail: slee@nybc.org.