Abstract
Chronic myeloid leukemia (CML) is characterized by an increased proliferative activity of the leukemic progenitors that produce an elevated number of mature granulocytes. Nevertheless, cell cycle-active agents, even in very high doses, are alone unable to eradicate the leukemic clone, suggesting the presence of a rare subset of quiescent leukemic stem cells. To isolate such cells, we first used Hoechst 33342 and Pyronin Y staining to obtain viable G0 and G1/S/G2/M fractions of CD34+cells by fluorescence-activated cell sorting (FACS) from 6 chronic-phase CML patients’ samples and confirmed the quiescent and cycling status of the 2 fractions by demonstration of expected patterns of Ki-67 and D cyclin expression. Leukemic (Ph+/BCR-ABL+) cells with in vitro progenitor activity and capable of engrafting immunodeficient mice were identified in the directly isolated G0 cells. Single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) analysis showed that many leukemic CD34+ G0cells also expressed BCR-ABL mRNA. CD34+ from 8 CML patients were also labeled with carboxyfluorescein diacetate succinimidyl diester (CFSE) before being cultured (with and without added growth factors) to allow viable cells that had remained quiescent (ie, CFSE+) after 4 days to be retrieved by FACS. Leukemic progenitors were again detected in all quiescent populations isolated by this second strategy, including those exposed to a combination of flt3-ligand, Steel factor, interleukin-3, interleukin-6, and granulocyte colony-stimulating factor. These findings provide the first direct and definitive evidence of a deeply but reversibly quiescent subpopulation of leukemic cells in patients with CML with both in vitro and in vivo stem cell properties.
CHRONIC MYELOID leukemia (CML) is a myeloproliferative disorder that appears after the deregulated clonal expansion and differentiation of a totipotent hematopoietic stem cell.1,2 The cytogenetic hallmark of the disease is the Philadelphia (Ph) chromosome, which forms as a result of a reciprocal translocation between the long arms of chromosomes 9 and 22.3 At the DNA level, the corresponding change is the creation of a novel BCR-ABL fusion gene.4,5 Despite overgrowth of the marrow in CML patients by the Ph+/BCR-ABL+ clone, a residual population of primitive normal (Ph−/BCR-ABL−) hematopoietic stem cells typically persists (reviewed in Eaves et al2).
In recent years, considerable interest has focused on the observation that Ph+/BCR-ABL+ cells can show a reduced or absent growth factor dependence for their survival.6,7 We have demonstrated that highly purified populations of phenotypically very primitive Ph+/BCR-ABL+ cells obtained from CML patients will, in fact, proliferate in vitro for several weeks in the absence of added growth factors8 and recently showed that this involves an autocrine interleukin-3 (IL-3) and granulocyte colony-stimulating factor (G-CSF) mechanism.9 These observations could explain both the increased proliferative activity exhibited by many types of Ph+/BCR-ABL+progenitors in vivo2,10,11 and the decreased self-renewal exhibited by very primitive Ph+/BCR-ABL+ cells identified as long-term culture-initiating cells (LTC-IC),12,13 because exposure of primitive normal hematopoietic cells to excess concentrations of IL-3 promotes their differentiation as well as stimulating their proliferation.14,15 Constitutive activation of the leukemic progenitors in CML is also consistent with the ability of intensive chemotherapy regimens to induce cytogenetic remissions in chronic-phase patients.16,17 However, such remissions have generally been short-lived and CML is considered incurable by conventional chemotherapy alone. Therefore, it seemed likely that CML patients might possess a rare subpopulation of quiescent Ph+/BCR-ABL+ stem cells that had not been identified by previous strategies used to purify primitive populations from CML samples. To try to isolate such cells, we pursued 2 approaches for obtaining quiescent CD34+ cells. The first of these exploited the dual staining procedure with Hoechst 33342 (Hst) and Pyronin Y (Py) that allows G0 and G1/S/G2/M cells to be separated.18-20 The second exploited the resolving power of 5- (and 6-) carboxyfluorescein diacetate succinimidyl diester (CFSE) staining21 to distinguish undivided and divided cells in short-term cultures of CD34+ cells.
MATERIALS AND METHODS
Cells.
Heparinized blood and bone marrow cells were obtained from 8 patients with untreated BCR-ABL+ chronic-phase CML (see Table 1 for details). Samples of normal adult marrow were either from harvests taken for allogeneic marrow transplants or were from cadaveric marrow samples (Northwest Tissue Center, Seattle, WA). Before use, all samples were enriched for CD34+ cells by negative immunomagnetic depletion of lineage marker+ (lin+) cells using StemSep columns (StemCell Technologies, Vancouver, British Columbia, Canada). For the CML samples, antibodies to CD15 and IgE were added to the recommended cocktail to enhance the removal of mature cells.22 All human cell samples were obtained with informed consent according to institutional guidelines.
Flow cytometry.
CD34+ cells were fractionated into G0 and G1/S/G2/M fractions according to their staining with Hst and Py.18-20 Briefly, cryopreserved lin+-depleted (lin−) cells were thawed and incubated overnight in a serum-free medium (SFM) consisting of Iscove’s medium (StemCell), a serum substitute (BIT; StemCell), 40 μg/mL low-density lipoproteins (Sigma Chemicals, St Louis, MO), 10−4 mol/L 2-mercaptoethanol, 300 ng/mL each of recombinant human Steel factor (SF; Amgen, Thousand Oaks, CA) and Flt3-ligand (FL; Immunex, Seattle, WA), and 60 ng/mL each of recombinant human IL-3 (Novartis, Basel, Switzerland), IL-6 (Cangene, Mississauga, Ontario, Canada), and G-CSF (StemCell) to allow reactivation of RNA synthesis. The following day, the cells were washed once in Hank’s balanced salt solution supplemented with 2% fetal calf serum (HF/2) and then resuspended in HF/2 containing 10 μmol/L Hst (Molecular Probes, Eugene, OR). After 45 minutes at 37°C, Py (Sigma) was added to give a final concentration of 2.5 μg/mL and cells were incubated for an additional 45 minutes. Anti-CD34-fluorescein isothiocyanate (FITC) (8G12-FITC; Dr P. Lansdorp, Terry Fox Laboratory, Vancouver, British Columbia, Canada) was then added at 10 μg/mL for a final 20 minutes. Cells were washed once in HF/2 containing Hst (10 μmol/L) and Py (2.5 μg/mL) and a second time in the same medium containing 1 μg/mL propidium iodide (PI; Sigma). Cells were finally resuspended in HF/2 without PI and kept on ice in the dark until being sorted on a 3 laser FACStar Plus (Becton Dickinson [BD], San Jose, CA). Gates were set to collect Hstlo Pylo (G0) and all remaining (G1/S/G2/M) cells as separate fractions within the PI− CD34+ population.23
Relatively homogeneously CFSE (Molecular Probes)-labeled fractions of CD34+ cells were also obtained and then cultured for 4 days in SFM, with or without the following growth factors: 50 ng/mL thrombopoietin (TPO; Genentech, San Francisco, CA), or FL, SF, IL-3, IL-6, and G-CSF at the concentrations described above and with or without 100 ng/mL colcemid (GIBCO BRL, Burlington, Ontario, Canada), as indicated. At the end of the 4 days, cells were harvested, washed once in HF/2, and washed a second time in HF/2/PI. Cultures containing colcemid were used to establish the range of fluorescence expected for all cells that had not divided during the 4-day interval.21For both sorting strategies, single cells (and defined numbers of cells where indicated) were collected using the automated cell deposition unit of the fluorescence-activated cell sorter (FACS).
Detection of Ki-67 and D cyclins.
To detect intracellular Ki-67, the staining procedure described by Jordan et al24 was followed. Cells were analyzed for expression of cyclins D1, D2, and D3 after fixation and staining with an FITC-conjugated antibody (Ab) (Pharmingen, Mississauga, Ontario, Canada) according to the manufacturer’s directions.
Analysis of BCR-ABL mRNA expression.
Cells were centrifuged and lyzed in guanidinium isothiocyanate solution (5 mol/L GIT, 20 mmol/L 1,4-diothioerythritol [DTT], 25 mmol/L sodium citrate, pH 7.0, 0.5% Sarcosyl) before the application of a 2-step (nested) reverse transcriptase-polymerase chain reaction (RT-PCR) procedure using an initial oligo (dT)-based primer and poly (A) tailing strategy.25,26 After electrophoresis of amplified products, BCR-ABL–specific and actin-specific fragments were detected by Southern blotting and hybridization with BCR-ABL and actin probes.25 26 Blots were exposed for 6 to 16 hours.
In vitro progenitor assays.
Assays for different types of colony-forming cells (CFC) capable of generating pure or mixed colonies of erythroid cells, granulocytes, and macrophages in fetal calf serum (FCS)-containing methylcellulose medium (H4330; StemCell) supplemented with 50 ng/mL SF and 20 ng/mL each of IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis), and G-CSF, and 3 U/mL of erythropoietin (StemCell) were performed as previously described.27 Cells were assayed for LTC-IC by measurement of the CFC content of 6-week cocultures containing pre-established, irradiated murine fibroblasts genetically engineered to produce human IL-3, G-CSF, and SF.27 28
Transplantation and detection of human cells in immunodeficient mice.
Breeding pairs of NOD/LtSz-scid/scid mice with homozygous disruption of their β2 microglobulin genes (NOD/SCID-β2M−/−mice29; kindly provided by Dr L. Schultz, Jackson Laboratory, Bar Harbor, ME) were expanded and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, British Columbia, Canada) in microisolators under defined sterile conditions. Six- to 8-week-old mice were irradiated with 300 cGy of137Cs γ-rays not more than 24 hours before being injected intravenously with human cells. In each case, 106irradiated (1,500 cGy) normal human bone marrow cells (as carriers) were coinjected into each mouse. After 6 weeks, the cells from both femurs and tibias from each mouse were labeled with various antimouse and antihuman antibodies and depleted immunomagnetically of all murine cells using a StemSep column,22 and human CD34−CD45RA+ and/or CD71+(total human) and CD34+ (primitive human) cells were specifically identified and sorted using the FACS.26Aliquots of the sorted human CD34− and CD34+ cells were analyzed by RT-PCR for BCR-ABL mRNA as described above.
Statistics.
Error estimates shown on mean values from greater than 2 samples in a group are the SEM.
RESULTS
Direct isolation of G0 leukemic cells from chronic-phase CML patients.
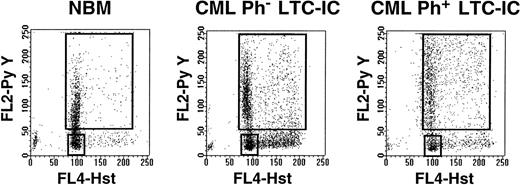
In a first series of experiments, CD34+ cells from normal marrow (n = 3) and CML samples (no. 1 through 6, Table 1) were separated into 2 fractions: 1 containing HstloPylo (G0) cells and 1 containing all other (HstloPy+ and all Hst+) (G1/S/G2/M) cells (Fig 1). In all 9 samples, a relatively discrete population of HstloPylo cells was identified. On average, this population was largest in the 3 normal samples (39% ± 18%), intermediate in the 2 CML samples with predominantly normal (Ph−) LTC-IC (21% and 31%), and smallest in the 4 CML samples with predominantly leukemic LTC-IC (4% ± 2%).
Representative examples of Hst and Py staining of viable CD34+ cells from normal marrow (left) and from CML patients with predominantly Ph− (middle, no. 2) or Ph+ (right, no. 3) LTC-IC.
Representative examples of Hst and Py staining of viable CD34+ cells from normal marrow (left) and from CML patients with predominantly Ph− (middle, no. 2) or Ph+ (right, no. 3) LTC-IC.
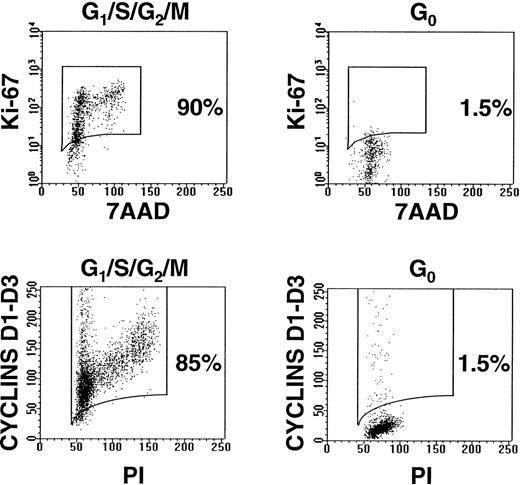
To further characterize the cycling status of these 2 subpopulations of CD34+ cells, particularly in the CML samples with no detectable normal elements, their expression of intracellular Ki-67 and D cyclins was investigated. Ki-67 is a nuclear antigen found exclusively in proliferating cells,24,30 and the D cyclins are induced upon mitogenic stimulation of quiescent cells.31 Less than 2% of the HstloPylo cells from all 3 CML samples examined (no. 4 through 6, Table 1) were found to contain detectable levels of Ki-67 or any of the D cyclins, whereas a high level of expression of both Ki-67 and at least 1 of the D cyclins was consistently evident in the majority of the remaining CD34+ cells (Fig 2).
Representative examples of G0 and G1/S/G2/M fractions of CD34+cells after staining with anti-Ki-67-FITC/7AAD and anti-cyclins D1, D2, and D3-FITC/PI (CML no. 4).
Representative examples of G0 and G1/S/G2/M fractions of CD34+cells after staining with anti-Ki-67-FITC/7AAD and anti-cyclins D1, D2, and D3-FITC/PI (CML no. 4).
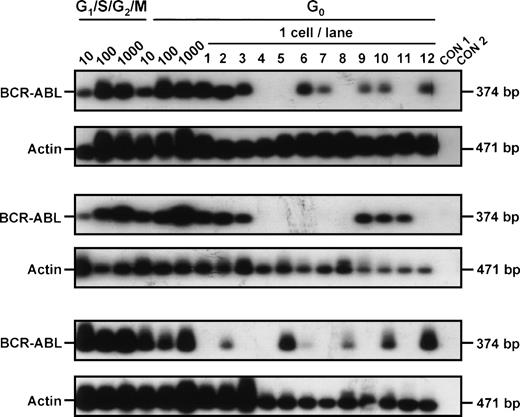
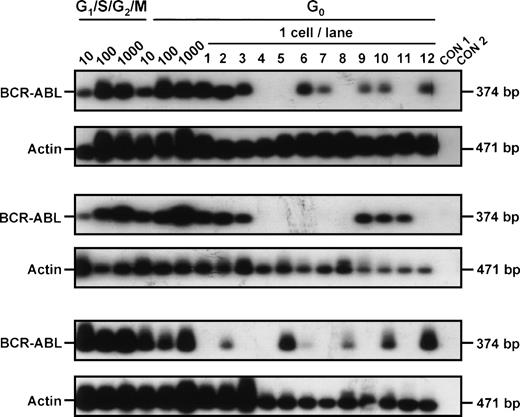
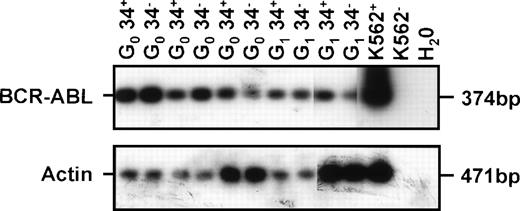
In vitro assays for progenitor activity showed that most of the CML CFC and many of the LTC-IC were cycling (Hst+ and/or Hstlo Py+). However, as summarized in Table 2, both CML progenitor types could also be detected in the quiescent (HstloPylo) fraction of the CD34+ cells, although proportionately less so than was the case for normal marrow. Cytogenetic analyses32 of the colonies generated in the assays of the cells isolated from the CML samples showed that leukemic (Ph+) progenitors were present in 4 of 5 cases in which analyzable colony metaphases were obtained (Table 2). To determine whether these quiescent cells also produce BCR-ABL transcripts, RT-PCR analyses were performed directly on the freshly isolated cells from 3 patients (all of whom had predominantly Ph+ LTC-IC). BCR-ABL mRNA was detected in aliquots of 10, 100, and 1,000 CD34+ cells from all 3 patients. Analyses of single HstloPylo cells showed 8 of 12 (67%), 6 of 12 (50%), and 6 of 12 (50%) of these (from each of the 3 patients, respectively) to be BCR-ABL+(Fig 3).
BCR-ABL expression in G0 and G1/S/G2/M CD34+ cells isolated from CML patient samples no. 3 through 5. Controls included 1,000 G1/S/G2/M cells without RT (CON 1) and H2O blank (CON 2).
BCR-ABL expression in G0 and G1/S/G2/M CD34+ cells isolated from CML patient samples no. 3 through 5. Controls included 1,000 G1/S/G2/M cells without RT (CON 1) and H2O blank (CON 2).
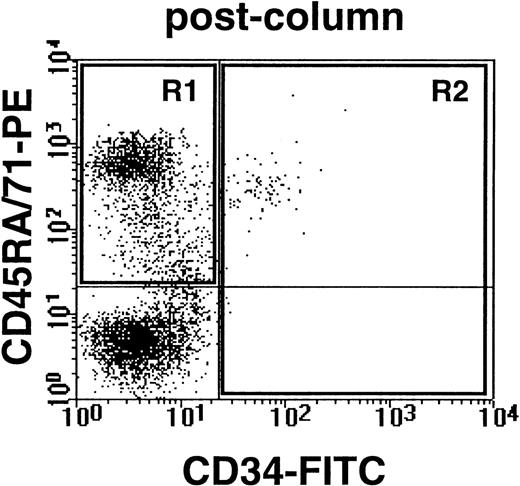
To determine whether the quiescent cells isolated from CML patients could also generate leukemic populations in vivo, 30 sublethally irradiated immunodeficient NOD/SCID-β2M−/− mice were injected either with HstloPyloCD34+ cells (1 to 2 × 105/mouse) or with the remaining CD34+ cells (1 to 20 × 105/mouse) isolated from 3 CML samples (no. 4 through 6, Table 1). When assessed 6 weeks later, all of the mice showed evidence of engraftment with human cells, including CD34+ as well as CD34− phenotypes (see example shown in Fig 4). Both of these subpopulations were then isolated by FACS and examined for the presence of leukemic (BCR-ABL+) cells by RT-PCR. Most mice transplanted with either G0 cells (6/6) or G1/S/G2/M cells (14/15) from 2 of the CML samples were found to contain both CD34− and CD34+ leukemic cells (see example in Fig 5). In the third case (CML no. 5, Table 1), no leukemic cells were detected in the CD34− population of human cells present in 1 of the 2 mice transplanted with G0 cells or in any of the 7 mice transplanted with G1/S/G2/M cells (despite the consistent presence of detectable human actin transcripts). Thus, some normal transplantable human hematopoietic cells must have been present in both the quiescent and cycling populations isolated from this CML sample (no. 5). The known ability of transplantable stem cells from normal human marrow and cord blood to generate predominantly CD34− B-lineage progeny in NOD/SCID mice33-35 may explain the prevalence of normal human CD34− cells in mice containing BCR-ABL+CD34+ cells.
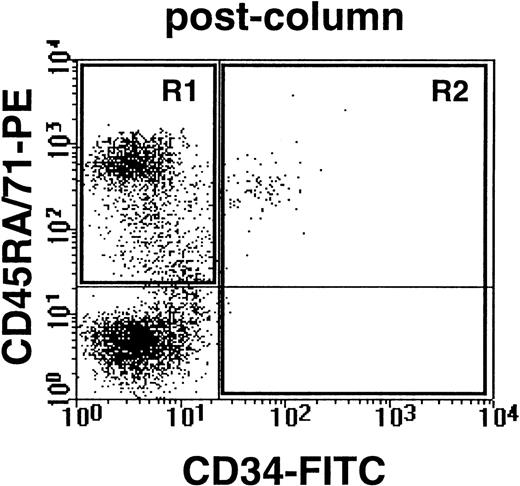
Example of human CD34− cells (expressing either CD45RA or CD71) and human CD34+ cells obtained from the marrow of a NOD/SCID-β2M−/−mouse transplanted 6 weeks previously with 105G0 cells from CML no. 5. Cells were first depleted of coexisting mouse cells by immunodepletion as described in Materials and Methods.
Example of human CD34− cells (expressing either CD45RA or CD71) and human CD34+ cells obtained from the marrow of a NOD/SCID-β2M−/−mouse transplanted 6 weeks previously with 105G0 cells from CML no. 5. Cells were first depleted of coexisting mouse cells by immunodepletion as described in Materials and Methods.
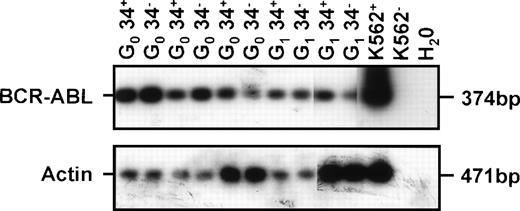
Examples of BCR-ABL expression in CD34− and CD34+ cells isolated from the marrow of immunodeficient mice transplanted 6 weeks earlier with G0 or G1/S/G2/M fractions of CD34+ CML cells (CML no. 6 in Table 1). Controls included 1,000 K562 cells (K562+), 1,000 K562 cells without RT (K562−), and H2O blank.
Examples of BCR-ABL expression in CD34− and CD34+ cells isolated from the marrow of immunodeficient mice transplanted 6 weeks earlier with G0 or G1/S/G2/M fractions of CD34+ CML cells (CML no. 6 in Table 1). Controls included 1,000 K562 cells (K562+), 1,000 K562 cells without RT (K562−), and H2O blank.
Ability of some leukemic progenitors from CML patients to remain quiescent in vitro for at least 4 days independent of exposure to exogenous growth factors.
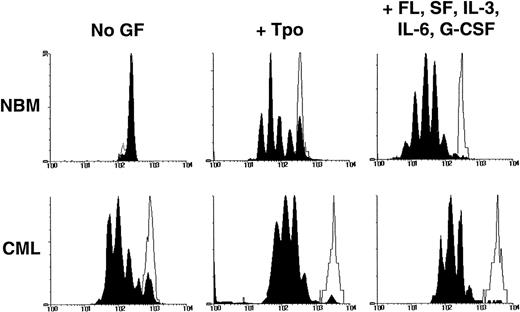
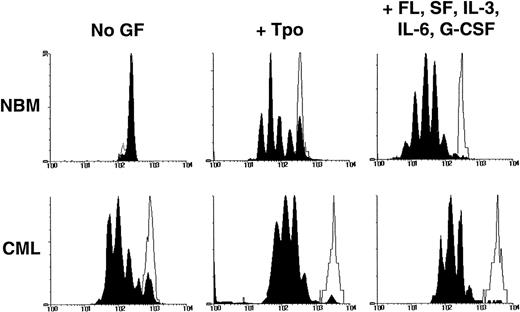
In a second series of experiments, the ability of quiescent leukemic CD34+ cells to survive and resist mitogenic activation for at least 4 days under different conditions of growth factor stimulation was compared with that characteristic of normal CD34+cells. Cells were labeled with CFSE, incubated overnight, sorted to obtain a homogeneously CFSE-labeled CD34+ population, and then cultured for another 4 days in SFM containing either no other factors, TPO only, or a combination of FL, SF, IL-3, IL-6, and G-CSF. The cultured cells were then harvested and resorted into 2 fractions according to their proliferative histories, as indicated by their individual levels of persisting CFSE fluorescence (Fig 6). Cultures containing no growth factors were included to favor the selection of quiescent leukemic cells. TPO alone has been previously shown to maintain the viability of primitive normal cells with minimal stimulation of their proliferation36 and was thus expected to maximize the detection of quiescent cells of either genotype. The 5 growth factor combination was included to identify quiescent cells that would be maximally resistant to activation, because these conditions have been found to mitotically stimulate most primitive adult normal hematopoietic cells within this time frame.21 23
Distribution of cells according to their CFSE fluorescence after being cultured for 4 days in the presence or absence of growth factors as described in the text. The unshaded areas indicate the fluorescence distribution of (still viable) cells that had been prevented from dividing by the addition of colcemid to the cultures. The black peaks indicate the cells that had undergone 0 to 7 divisions during the 4-day culture period.
Distribution of cells according to their CFSE fluorescence after being cultured for 4 days in the presence or absence of growth factors as described in the text. The unshaded areas indicate the fluorescence distribution of (still viable) cells that had been prevented from dividing by the addition of colcemid to the cultures. The black peaks indicate the cells that had undergone 0 to 7 divisions during the 4-day culture period.
The various populations of cells obtained from these cultures were then assayed for the presence of CFC and LTC-IC. As shown in Table 3, some persisting quiescent cells could be identified in all 8 CML samples studied, regardless of the growth factors to which the cells were exposed during the period they were cultured before analysis. Moreover, the CD34+ cells that remained quiescent for 4 days also always included a subset with CFC activity. As expected, the relative proportion of the persisting quiescent cells retrieved from the cultures (initiated with either normal or CML cells) decreased with increasing growth factor supplementation of the medium. This proportion was consistently lower in the cultures initiated with cells from the CML samples. Interestingly, the quiescent cells also showed lower forward light scattering characteristics indicative of a reduced cell size, regardless of their genotype (data not shown).
DISCUSSION
In designing the present study, we first sought a method that would allow the direct isolation from a CD34+ cell population of a viable G0 fraction of cells distinct from those in G1 as well as S/G2/M. For this, we used a nontoxic double staining procedure with Hoechst 33342 and Pyronin Y and multiparameter flow cytometry. This allowed the viable (PI−) CD34+ cells to be divided into 2 fractions: 1 containing cells with a 2n DNA content and a low RNA content (Hstlo/Pylo; ie, the G0cells) and 1 containing all of the remaining (G1/S/G2/M) cells.18-20 Analysis of quiescent (G0) cells in other systems has shown that they typically are smaller in size and have reduced numbers of mitochondria, a decreased protein content, and a higher degree of chromatin condensation.37 In addition, expression of certain genes (eg, growth-arrest–specific, gas, genes,38 and statin39) is turned on and expression of others (eg, proliferating cell nuclear antigen,40Ki-67,24,30 and the D cyclins31) may be silenced. Validation of the G0 status of the cells assigned a Hstlo/Pylo phenotype here was provided by demonstrating the specific absence of 2 of the above-noted markers (Ki-67 and the D cyclins) in these cells in addition to noting their reduced size.
Functional characterization of these directly isolated G0cells confirmed that a significant proportion of the CFC and LTC-IC in normal bone marrow are quiescent,10,20,41 indicative of multilevel regulatory mechanisms normally constraining the expansion of primitive hematopoietic cells. Parallel studies of CML cells showed most of the leukemic CFC and LTC-IC to be cycling, as expected.2 10 However, quiescent leukemic CFC and LTC-IC could also be consistently isolated. Both quiescent and cycling populations of leukemic cells were capable of generating leukemic progeny in irradiated immunodeficient mice. Thus, the quiescent status of Ph+ progenitors, like that of their normal counterparts, is reversible both in vitro and in vivo.
Recent studies suggest that growth factor activation may modulate the engraftment capacity of transplantable hematopoietic cells.42 Gothot et al43 have proposed that this may result from early growth factor-induced changes in the homing function(s) of these cells rather than in their differentiated state. If the majority of the leukemic stem cells in patients with CML are proliferating more rapidly than is typical of their normal counterparts, as suggested by LTC-IC analyses,2 then it might be anticipated that the ability of CML stem cells to be transplanted might also be compromised. This would be consistent with the widely encountered difficulty in rapidly obtaining high levels of leukemic (Ph+/BCR-ABL+) cells in NOD/SCID mice.44-46 In the present studies, we found that both G0 and, to a lesser extent, G1/S/G2/M cells were able to engraft mice with leukemic cells. However, a more immunodeficient strain of NOD/SCID mice (also lacking β2-microglobulin29) was used, and limiting dilution analyses of the frequency of engrafting cells were not feasible. Thus, the intriguing question of whether an engraftment endpoint may underestimate the leukemic stem cell population in CML by a bias in favor of those that are quiescent must await resolution from further studies. Similarly, the possibility that some cycling leukemic stem cells contaminating the G0fraction may have contributed to the engraftment seen here cannot be completely ruled out.
The presence in CML patients of a quiescent population of leukemic cells with progenitor activity was also demonstrated using a completely independent approach. In this second strategy, we used CFSE labeling to allow the specific isolation of cells that would remain viable but not divide when cultured under different conditions. These experiments confirmed that primitive CML cells can proliferate in the absence of added growth factors,6,8 9 although a small number will also remain quiescent for at least 4 days, even in the presence of a strong mitogenic stimulus. The fact that such cells subsequently formed colonies in vitro again indicates the transient nature of their arrest in G0.
The mechanisms underlying the control of normal human hematopoietic progenitor cell cycling are known to be complex and to involve the integration of signals from the environment that either promote or block cell cycle progression. Defects in a subset of these mechanisms have been shown to exist in CML progenitors.47-49 However, it is now clear that very primitive normal hematopoietic cells undergo early changes in the types of external stimuli they respond to as well as in the types of responses elicited by a given stimulus.15,50,51 It is thus interesting to note that the chemokines able to inhibit the proliferation of primitive normal but not CML clonogenic cells are not effective in inhibiting the proliferation of more primitive normal progenitors detected as LTC-IC.52 Thus, the increased turnover exhibited by the leukemic LTC-IC isolated from CML patients2 is likely to be explained by another mechanism. One possibility would be the autocrine production of IL-3 that we have recently described to occur in primitive CML cells.9
However, these findings are not readily reconciled with the existence of a subpopulation of primitive quiescent leukemic cells in patients with CML. As a first approach to investigating an underlying mechanism, we used single-cell RT-PCR to determine whether these cells might not express BCR-ABL. However, in all 3 patients studied, ≥50% of the freshly isolated G0 cells contained BCR-ABL mRNA. Thus, BCR-ABL expression alone is not sufficient to force the rapid transit through G1 of primitive hematopoietic cells. Interestingly, preliminary studies indicate that such G0BCR-ABL+ cells may not contain IL-3 or G-CSF mRNA. At least some of these leukemic G0 cells can, nevertheless, survive and subsequently proliferate even in the absence of further growth factor stimulation (Holyoake et al, manuscript submitted). This raises the possibility that quiescence and a non–growth factor producing phenotype may be linked properties. Investigation of this possibility is the subject of ongoing studies and could provide important clues to potential therapeutic targets.
ACKNOWLEDGMENT
The authors acknowledge the help of Dr Michael Barnett and the staff in the Leukemia and Bone Marrow Transplant Program as well as the Stem Cell Assay Service for the provision, storage, and preparation of normal bone marrow and CML samples. We also thank Pamela Austin, Chris Laird, and Margaret Hale for technical assistance; Gloria Shaw for the cytogenetic data; Maya Sinclaire for assistance with the animal procedures; Gayle Thornbury and Giovanna Cameron for help with the flow cytometry; Dr Peter Lansdorp (Terry Fox Laboratory); Amgen, Cangene, Genentech, Immunex, Novartis, and StemCell for generous gifts of reagents; and Bernadine Fox for typing the manuscript.
Supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society and the Terry Fox Run and a grant from Novartis Canada. T.H. holds a United Kingdom Leukaemia Research Fund Senior Lectureship and C.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Allen Eaves, MD, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, British Columbia, Canada V5Z 1L3; e-mail: allen@terryfox.ubc.ca.