Previously, we demonstrated that B-chronic lymphocytic leukemia (B-CLL) cells could be divided into 2 groups depending on the expression of CD38 by the malignant cells. The 2 groups differed in their signal-transducing capacities initiated by cross-linking of surface IgM; only in CD38-positive cells was an efficient signal delivered, invariably resulting in cell apoptosis. In this study, we investigated the effect of surface IgD cross-linking in 10 patients with CD38-positive B-CLL. Exposure of the malignant cells to goat antihuman δ-chain antibodies (Gaδ-ab) caused [Ca++]i mobilization and tyrosine kinase phosphorylation in a manner not different from that observed after goat antihuman μ-chain antibody (Gaμ-ab) treatment in vitro. However, Gaδ-ab-treated cells failed to undergo apoptosis and instead displayed prolonged survival in culture and differentiated into plasma cells when rIL2 was concomitantly present. Cross-linking of surface IgD failed to induce proliferation of the malignant cells in vitro. Moreover, treatment with Gaδ-ab did not prevent apoptosis of B-CLL cells induced by Gaμ-ab. Collectively, these experiments demonstrated that IgM and IgD expressed by the same cell may deliver opposite signals under particular circumstances and provide some clues for the understanding of the pathophysiology of B-CLL.
IgD is expressed together with IgM on the surface of most mature B-lymphocytes in humans and other animal species.1 When expressed on the surface of the same cell, the 2 isotypes share the same light chain type, idiotype, and antibody specificity. Because of the description of IgD as a major B-cell antigen-receptor (BCR), several questions have been raised concerning its physiological function and its relationship with surface IgM. The different constant region structures of the 2 immunoglobulins,2 the differential expression of IgM and IgD during B cell development,1 and the identification of proteins associated specifically with surface IgM or IgD3-5all suggest that each membrane immunoglobulin isotype has a specialized function. Indeed, a number of studies indicate that surface IgM, but not IgD, can induce negative responses in B cells, such as growth arrest, anergy, or apoptosis,6-8 and they suggest that the negative selection of immature B cells may result from a relatively low expression of surface IgD.9 10 A correlation between IgD expression level and decreased sensitivity to tolerance was observed in those studies.
There are, however, observations that contradict this notion. For example, it has been observed that in irradiated mice reconstituted with immature B cells11 or in certain transgenic mice,12 both surface IgM and surface IgD transduce signals that lead to the apoptosis of immature murine B-lymphocytes. Furthermore, independent engagement of either IgM or IgD of mature B-lymphocytes results in their proliferation in vitro.13 14
In this study, performed on a particular subset of B-chronic lymphocytic leukemia (B-CLL) cells characterized by the presence of CD38 surface marker, we show that sIgM and sIgD can deliver different signals. Specifically, though cross-linking of IgM was invariably followed by cell apoptosis, IgD cross-linking resulted in improved cell survival and the promotion of plasma cell differentiation in vitro. The subset of CD38-positive B-CLL cells studied was selected because we had previously demonstrated that their malignant cells have a functional signal transduction pathway initiated by BCR cross-linking, whereas the malignant cells of CD38-negative B-CLL cells do not respond to signals delivered by surface immunoglobulin.15Collectively, the current data demonstrated that in particular circumstances, surface IgM and IgD can deliver signals that activate different physiological functions of B cells. Moreover, because B-CLL results from the expansion of malignant cells frozen at a particular differentiation stage,16 17these studies may also help trace the stages at which normal cells display different responses to the stimulation of surface IgM or IgD. This finding may contribute to the definition of the role of isotypes in fine-tuning the humoral response. Finally, CD38-positive B-CLL may constitute a suitable ex vivo source of B cells for investigating the differences in the biochemical events initiated by cross-linking of surface IgM or surface IgD, respectively.
Patients and methods
Cell preparation
Ten patients (A-L) with B-CLL at Rai classification stages 0, 1, and 2 were studied at the time of diagnosis. These patients were selected because of the high expression of CD38 on malignant B cells, as determined by flow cytometry analysis. Purified B cells from the peripheral blood of CD38-positive patients with CLL were obtained by Ficoll-Hypaque density gradient centrifugation and by the depletion of monocytes or T cells through adherence and erythrocyte rosette formation methods, as described.15
Flow cytometry analysis performed on purified B-CLL cells showed that these cells comprised more than 95% CD19+, CD5+ B cells and less than 3% CD3+ T cells and CD68+ monocytes. In each patient selected for this study, comparable levels of membrane IgM and IgD were coexpressed, as demonstrated by the similar relative fluorescent intensity values of the IgM and IgD. In selected patients (A, I, L), B-CLL cells were fractionated into CD38bright and CD38low by magnetic cell separation. Briefly, cells were incubated with CD38 monoclonal antibody (mAb) for 30′ at 4°C and then with goat antimouse immunoglobulin micro beads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 10′ at 4° C, and the cells were separated on magnetic columns (Miltenyi Biotec).
Peripheral blood B cells from normal donors were purified as previously described.15 Briefly, mononuclear cells were obtained by F-H centrifugation and were depleted of monocytes and T cells by leucine-methyl-ester treatment and erythrocyte rosette formation, respectively. The obtained cell suspensions were enriched in B cells (80% CD19+) as demonstrated by flow cytometry analysis.
Immunofluorescence
The following mAbs were used: anti-IgM, anti-CD5, anti-CD23, anti-CD38, anti-CD10, anti-CD95, anti-CD69, anti-CD71 (Becton Dickinson, San Jose, CA); anti-IgD, anti-Bcl-2, anti-Ki-67 (Dakopatts, Glostrup, Denmark); anti-CD79b, anti-CD86 (Ancell Europe, Laüfelfingen, Switzerland); anti-CD44 (Janssen Chimica, Beerse, Belgium); and anti-CD138 (Caltag Laboratories, Burlingame, CA); and rabbit anti-CD77 (Immunotech, Marseilles, France). Indirect immunofluorescence was performed with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated goat antimouse immunoglobulin isotype antibodies (Southern Biotechnology, Birmingham, AL) as the second reagent. CD38 staining was also performed using PE-conjugated CD38 mAb (Becton Dickinson). Staining for intracellular molecules (Ki67, Bcl-2) was performed on permeabilized cells as described previously.18 For control staining, the cells were treated with murine immunoglobulin followed by a second reagent conjugated with FITC or PE. Stained cells were analyzed with a FACSort flow cytometer (Becton Dickinson). Data were expressed as histograms of the fluorescence intensity versus cell number or as relative fluorescence intensity calculated according to the following formula: mean fluorescence intensity of cells stained with the mAb/mean fluorescence intensity of control cells. The mean fluorescence intensities were calculated by CELL QUEST software.
Cell cultures
Cells were cultured at the concentration of 1 × 106 cells/mL in RPMI 1640 medium supplemented with 10% fetal calf serum (both from GIBCO, Paisley, UK) for various time intervals. In the different experiments, the cells were exposed to goat antihuman μ-chain antibodies (Gaμ-ab) (Southern Biotechnology), goat antihuman δ-chain antibodies (Gaδ-ab) (Sigma, Saint Louis, MO) at the indicated concentrations, rabbit (Fab')2antihuman μ-chain antibodies (Sigma), rabbit (Fab')2antihuman δ-chain antibodies (Caltag Laboratories), normal goat immunoglobulin (NGI), phorbol myristate acetate (10 ng/mL) (Sigma) in the presence of A23 187 (Sigma) at the concentration of 5 ng/mL, SAC (1:10 000 vol/vol) (Calbiochem, Novabiochem, San Diego, CA). In selected experiments, the cells were cultured with the stimuli listed above in the presence of recombinant lymphokines in various combinations: rIL2 (100 U/mL), rIL4 (100 U/mL), rIL6 (70 U/mL), and rIL10 (100 U/mL) (Genzyme Diagnostics, Cambridge, MA). Morphology of the cells was studied by either of 2 methods. Cytospin preparations were stained with GIEMSA and analyzed by light microscopy or they were stained with FITC-conjugated anti-IgM mAb (Becton Dickinson) and analyzed by fluorescence microscopy. Immunoglobulin released by B-CLL cells in the culture supernatants were measured by ELISA using anti-κ or anti-λ chain-specific reagents (Southern).
Apoptosis
Apoptosis was measured by 2 different methods. Permeabilized cells were stained with propidium iodide (PI, 50 mg/mL; Sigma), and the amount of DNA fragmentation was calculated by flow cytometry analysis as previously described.15 Results were expressed as the percentage of fragmented DNA compared to total DNA. Alternatively, cultured cells were double stained with annexin V-FITC conjugate (ApoAlert Annexin V-FITC) (Clontech, Palo Alto, CA) and PI and then analyzed by flow cytometry. Early apoptotic cells were positive for annexin V-FITC conjugate but did not stain with PI because their membranes were still intact. Late-stage apoptotic cells or dead cells that had damaged permeable plasma membranes stained concurrently with annexin V-FITC conjugate and PI.19 In selected experiments, cell viability was determined by PI exclusion tests and flow cytometry analysis.
Cell cycle analysis and thymidine incorporation assay
Cell proliferation was measured by 2 methods. Cells were permeabilized by exposure to hypotonic solutions, stained with PI, and analyzed by flow cytofluorimetry.15 The percentage of cells present in each cell-cycle phase was calculated by the Modfit program (Becton Dickinson). Alternatively, 3H-thymidine (Amersham International, Buckinghamshire, UK) was added to the culture for 8 hours at various time intervals. Then the cells were harvested, and the3H-thymidine incorporated by the cells was measured by a β-counter.
Protein tyrosine phosphorylation assay
With the appropriate stimuli for the indicated times, 1 × 107 cells/mL were incubated, washed twice in cold phosphate-buffered saline, pelleted by centrifugation, and lysed in ice-cold lysis buffer (200 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 1% NP40, 2 mmol/L EDTA, 100 mmol/L phenylmethylsulfonyl fluoride, 10 μmol/L leupeptin, 10 μmol/L apoprotein, and 1 mmol/L sodium orthovanadate). Protein concentration of the cell lysates was measured with high precision using the Micro BCA protein assay reagent kit (Pierce, Milan, Italy). Equal amounts of protein (10 μg/lane) from each sample were separated by SDS-PAGE (10% acrylamide) under reducing conditions and were transferred electrophoretically to nitrocellulose (Hybond-C nitrocellulose membrane; Amersham). Tyrosine-phosphorylated proteins were detected by probing the filters with antiphosphotyrosine mAb 4G10 (UBI, Lake Placid, NY) followed by horseradish peroxidase-conjugated goat antimouse immunoglobulin, used as a developing reagent. The reaction was detected by enhanced chemiluminescence detection reagents (Amersham) and exposure to Hyperfilm-MP. In the immunoprecipitation experiments, the lysates were pre-cleared with protein A-Sepharose (Pharmacia, Uppsala, Sweden) and an irrelevant mAb and subsequently incubated with antiphosphotyrosine mAb followed by protein A-Sepharose. Specific immunoprecipitates were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE; 10%), transferred to nitrocellulose, and probed with anti-syk or anti-PLCγ1 mAb (UBI). The reaction was detected as above.
Calcium mobilization
Measurements of intracellular free Ca++ concentrations ([Ca++]i) were performed as described previously.15 Briefly, 1 × 107 cells were loaded with FURA2/AM (Calbiochem, Novabiochem) and exposed to the indicated stimuli. [Ca++]i was detected by excitation of the probe at 2 alternative wavelengths (340 and 380 nm) and by measurement of the emitted fluorescence at 510 nm with a spectrofluorometer (Perkin-Elmer Cetus, Norwalk, CT). A separate calibration for each measurement was performed.
Results
Cell surface phenotype of CD38-positive B-CLL cells
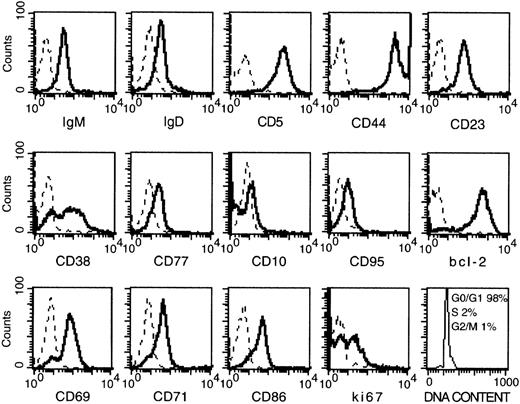
Malignant cells of the 10 patients with B-CLL (A-L) selected for this study were characterized by high levels of CD38 expression (relative fluorescent intensity, > 4) as assessed by flow cytometry. The cells expressed markers typical of B-CLL cells, including CD5, CD23, CD44, and low levels of IgM and IgD (Figure1). Bcl-2 was expressed at high levels, in agreement with previous observations.15 20 Molecules usually found on germinal center (GC) B cells were consistently low or absent (CD95, CD77, and CD10), with the obvious exception of CD38. Activation markers such as CD69, CD71, and CD86 were present, though variations in the staining intensity existed among the patients (not shown). Cell-cycle analysis demonstrated that virtually all the cells were constantly in G0 or G1 phase (see Figure 1). Because most of the cells expressed Ki67, it is likely that they were activated and probably arrested in the G1 phase of the cell cycle.
Phenotypical analysis of CD38-positive B-CLL cells.
Solid lines: Freshly prepared B-CLL cells were stained with the indicated mAb by indirect immunofluorescence. Dashes: Control stainings with an irrelevant mAb. Cells were permeabilized before the staining for Bcl-2, Ki-67, and cell-cycle analysis. Data are the results of 1 representative test (patient D) of the 10 performed (A-L) on patients with CD38-positive B-CLL.
Phenotypical analysis of CD38-positive B-CLL cells.
Solid lines: Freshly prepared B-CLL cells were stained with the indicated mAb by indirect immunofluorescence. Dashes: Control stainings with an irrelevant mAb. Cells were permeabilized before the staining for Bcl-2, Ki-67, and cell-cycle analysis. Data are the results of 1 representative test (patient D) of the 10 performed (A-L) on patients with CD38-positive B-CLL.
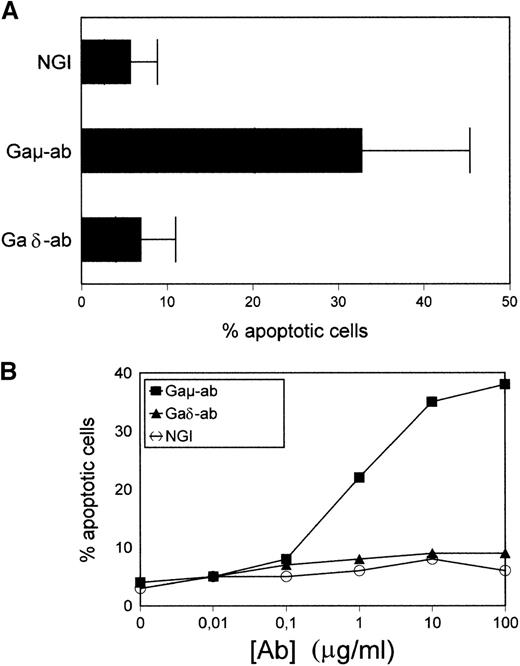
Apoptosis of CD38-positive B-CLL cells induced by Gaμ-ab and not by Gaδ-ab
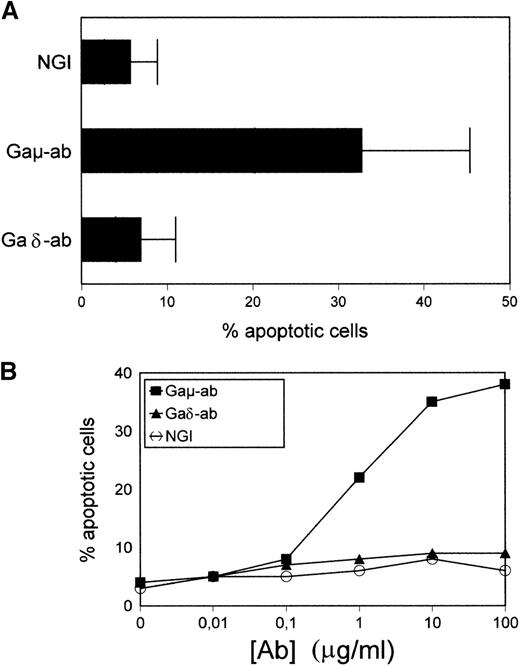
Purified B-CLL cells were exposed to Gaμ-ab, Gaδ-ab, or NGI for 18 hours in vitro and tested for apoptosis by PI staining of permeabilized cells. As shown in Figure 2A, in the presence of Gaμ-ab, the percentages of apoptotic cells increased compared to control cells treated with NGI (33% ± 15% versus 8% ± 3%, mean ± SD of 10 patients;P < .05). In contrast, exposure to Gaδ-ab did not induce the apoptosis of B-CLL cells even when Gaδ-ab was used for a wide range of concentrations (Figure 2B). Similar results were obtained when Annexin-V FITC-conjugated staining was substituted for PI staining to detect apoptosis (see below). Substituting Gaμ-ab and Gaδ-ab with rabbit F(ab')2 antihuman μ-chain or δ-chain antibodies yielded comparable results (not shown), ruling out the possibility that signals potentially delivered through the Fc portion of the stimulating antibodies could prevent or even induce apoptosis.21 22
Apoptosis in CD38-positive B-CLL cells exposed to Gaμ-ab or Gaδ-ab.
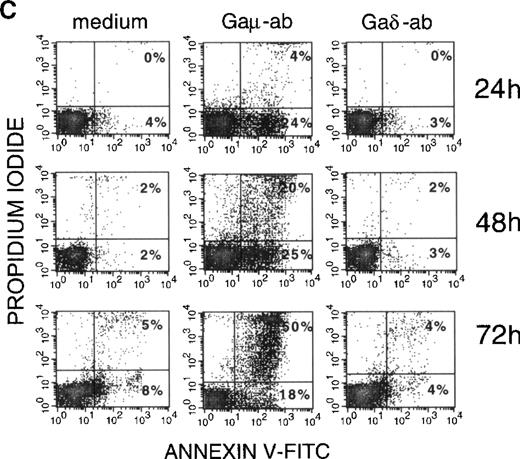
(A) B-CLL cells were cultured in the presence of Gaμ-ab, Gaδ-ab, or NGI at the concentration of 10 μg/mL. After 18 hours, apoptotic cells in the cultures were measured by PI staining of permeabilized cells. Data represent the mean ± SD of the values obtained in the 10 patients with B-CLL (A-L). (B) B-CLL cells from patient G were incubated with the indicated concentrations of Gaμ-ab (▪), Gaδ-ab (▴), or NGI (○) for 18 hours, after which apoptosis was determined as above. (C) B-CLL cells from patient D were cultured in the presence of Gaμ-ab, Gaδ-ab, or NGI for the indicated time. At the end of the cultures, cells were double stained in suspension with Annexin-V FITC (to detect apoptotic cells) and PI (to detect late apoptotic or nonviable cells) and then analyzed by flow cytometry. Data in B and C represent 1 typical experiment of the 6 performed (patients B, C, G, D, F, I).
Apoptosis in CD38-positive B-CLL cells exposed to Gaμ-ab or Gaδ-ab.
(A) B-CLL cells were cultured in the presence of Gaμ-ab, Gaδ-ab, or NGI at the concentration of 10 μg/mL. After 18 hours, apoptotic cells in the cultures were measured by PI staining of permeabilized cells. Data represent the mean ± SD of the values obtained in the 10 patients with B-CLL (A-L). (B) B-CLL cells from patient G were incubated with the indicated concentrations of Gaμ-ab (▪), Gaδ-ab (▴), or NGI (○) for 18 hours, after which apoptosis was determined as above. (C) B-CLL cells from patient D were cultured in the presence of Gaμ-ab, Gaδ-ab, or NGI for the indicated time. At the end of the cultures, cells were double stained in suspension with Annexin-V FITC (to detect apoptotic cells) and PI (to detect late apoptotic or nonviable cells) and then analyzed by flow cytometry. Data in B and C represent 1 typical experiment of the 6 performed (patients B, C, G, D, F, I).
Time course experiments were carried out by exposing the cells to Gaμ-ab or Gaδ-ab for different periods of time. The cells were concomitantly stained with Annexin-V FITC-conjugate to measure apoptosis, and they were stained with PI to assess cell viability by dye exclusion (Figure 2C). Gaμ-ab-induced apoptosis had already occurred in 25% or more of the cells after 24 hours and increased progressively. Later there was an accumulation of PI-adsorbing cells, possibly cells that underwent lysis after apoptosis. As expected, exposure to Gaδ-ab caused very low amounts of apoptosis at 48 hours and a correspondingly small amount of cell lysis at 72 hours of culture. Values observed with this reagent were similar to those observed with NGI (Figure 2C).
Cells from 3 patients (A, I, L) were fractionated into CD38high and CD38dim cells using a magnetic cell separation method. Both fractions were exposed to Gaμ-ab or Gaδ-ab and tested for apoptosis. Gaμ-ab induced apoptosis in both cell fractions, though it was consistently more marked in the fraction enriched for CD38high, perhaps suggesting a close correlation between CD38 levels on the cell surface and a propensity to apoptosis as described previously18 and in other cell types.15 23 Exposure to Gaδ-ab had no effect on either cell fraction (data not shown).
In another experiment, B-CLL cells from 4 different patients (C, G, D, F) were exposed to Gaμ-ab in the presence or absence of increasing concentrations of Gaδ-ab with the aim of investigating the potential ability of Gaδ-ab to prevent Gaμ-ab-induced apoptosis. Gaδ-ab treatment did not prevent Gaμ-ab-induced apoptosis (Figure3). Some inhibition of apoptosis was observed with high concentrations of Gaδ-ab (80 μg/mL) in the cultures exposed to 50 μg/mL Gaμ-ab, but this was not significant.
Failure of Gaδ-ab to prevent Gaμ-ab-induced apoptosis.
B-CLL cells from patient C were exposed to Gaμ-ab at 3 different concentrations in the presence of Gaδ-ab or NGI at the indicated concentrations. Apoptotic cells were measured by Annexin-V FITC staining after 18 hours in vitro. The experiment is representative of the 4 carried out on patients with B-CLL (patients C, G, D, F).
Failure of Gaδ-ab to prevent Gaμ-ab-induced apoptosis.
B-CLL cells from patient C were exposed to Gaμ-ab at 3 different concentrations in the presence of Gaδ-ab or NGI at the indicated concentrations. Apoptotic cells were measured by Annexin-V FITC staining after 18 hours in vitro. The experiment is representative of the 4 carried out on patients with B-CLL (patients C, G, D, F).
Activation of the early events in the signal transducing pathway by Gaδ-ab and Gaμ-ab
In these experiments, we compared the ability of Gaδ-ab and Gaμ-ab to activate tyrosine kinases and to induce changes in intracellular free-calcium concentration ([Ca++]i). Cells were stimulated with Gaδ-ab or Gaμ-ab for different times, lysed, and analyzed for the induction of protein tyrosine phosphorylation. As shown in Figure 4A, which reports 1 representative experiment (patient G) of the 3 performed on different patients with B-CLL (G, H, F), Gaμ-ab and Gaδ-ab were able to induce tyrosine phosphorylation of several proteins, the most prominent of which were 2 proteins of 145 and 75 kd (see below).
Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells.
(A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).
Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells.
(A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).
Although exposure of the cells to Gaδ-ab resulted in the rapid (2-minute) phosphorylation of tyrosine followed by a sharp decline, treatment with Gaμ-ab caused a progressive increase in phosphotyrosine that reached its maximum by 5 minutes and slowly decreased thereafter (Figure 4A).
Further studies were carried out to identify the 2 proteins of 145 and 75 kd. Because their molecular weights were similar to those of syk and PLCγ1 proteins, respectively, B-CLL cells from 2 patients (G, H) were stimulated as above, lysed, immunoprecipitated with antiphosphotyrosine mAb, electrophoresed, and blotted. The blots were subsequently stained with specific antibodies against syk and PLCγ1 proteins. As shown in Figure 4B the 2 proteins, which were phosphorylated after the 2 stimuli, indeed were identified as syk and PLCγ1.
Next we investigated the ability of Gaδ-ab and Gaμ-ab to induce [Ca++]i mobilization. Results of 10 experiments on different B-CLL patients (A-L) are summarized in Figure 4C, which also shows typical profiles observed in 1 experiment (patient A). Again, though the observed increments in [Ca++]i induced by surface IgM cross-linking were similar in amplitude to those obtained with IgD cross-linking, differences were noted in the kinetics and in the shape of the profiles of the [Ca++]i responses. Thus, after treatment with Gaδ-ab, the observed peak in [Ca++]i was followed by a sharp decrease. In contrast, a slower decline in [Ca++]i was noticed in the cells exposed to Gaμ-ab.
Ability of Gaδ-ab to prolong cell survival
The above experiments showing that surface IgD cross-linking induced tyrosine phosphorylation of the cytoplasmic proteins and [Ca++]i mobilization suggested that Gaδ-ab was perhaps capable of delivering signals to the cells that resulted in physiological functions other than apoptosis. We investigated whether Gaδ-ab caused cell proliferation, increased cell survival, or both. As shown in Table 1, no changes in the cell cycle were observed in cells exposed to Gaμ-ab or to Gaδ-ab for 48 hours, even when different cytokines (rIL2, rIL4, rIL6, rIL10) were added to the culture individually or in combination (data not shown). However, the cells were recruited into the cell cycle on treatment with phorbol 12-myristate-13-acetate (PMA) and A23187. Similarly, no thymidine incorporation above control values was observed by exposing the cells to Gaδ-ab for 24, 48, or 72 hours. Table 1shows the results of 1 representative experiment out of the 3 performed on different patients with B-CLL (A, E, L). In contrast, exposure of normal peripheral blood B cells to Gaμ-ab and Gaδ-ab caused their recruitment into the S-phase of the cell cycle with little or no apoptosis (Table 2).
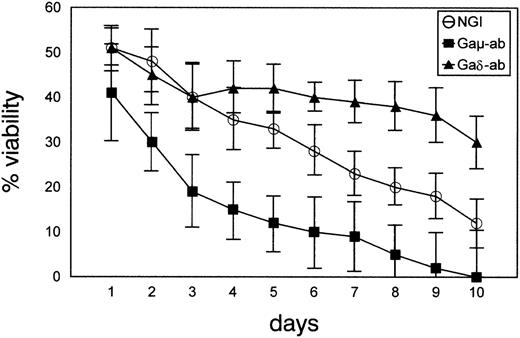
Next, the cells from 7 CD38-positive patients with B-CLL (patients B, C, D, E, F, G, L) were cultured in the presence of Gaδ-ab, Gaμ-ab, or NGI for different times, and their viability was measured by PI dye exclusion and flow cytometry analysis. Exposure to Gaδ-ab resulted in a substantial improvement in cell viability that became most apparent after 4 to 5 days of culture (Figure 5). After 10 days of culture, the mean of the cell viability values in Gaδ-treated cells (30 ± 6) was more than 2-fold that of cells treated with NGI (12 ± 6). As expected, incubation with Gaμ-ab lowered cell viability, and no viable cells were detected after 6 days of culture. The addition of increasing concentrations of Gaδ-ab to the cultures exposed to Gaμ-ab did not modify their poor viability (data not shown).
Prolonged survival of CD38-positive B-CLL cells in vitro on exposure to Gaδ-ab.
B-CLL cells were cultured with Gaμ-ab (▪), Gaδ-ab (▴), or NGI (○) at 10 μg/mL for various time intervals. Viability was determined by PI exclusion and flow cytometry analysis. Results are shown as mean ± SD of the experiments carried out on 7 patients with B-CLL (patients B, C, D, E, F, G, L).
Prolonged survival of CD38-positive B-CLL cells in vitro on exposure to Gaδ-ab.
B-CLL cells were cultured with Gaμ-ab (▪), Gaδ-ab (▴), or NGI (○) at 10 μg/mL for various time intervals. Viability was determined by PI exclusion and flow cytometry analysis. Results are shown as mean ± SD of the experiments carried out on 7 patients with B-CLL (patients B, C, D, E, F, G, L).
Ability of Gaδ-ab to induce plasma cell differentiation
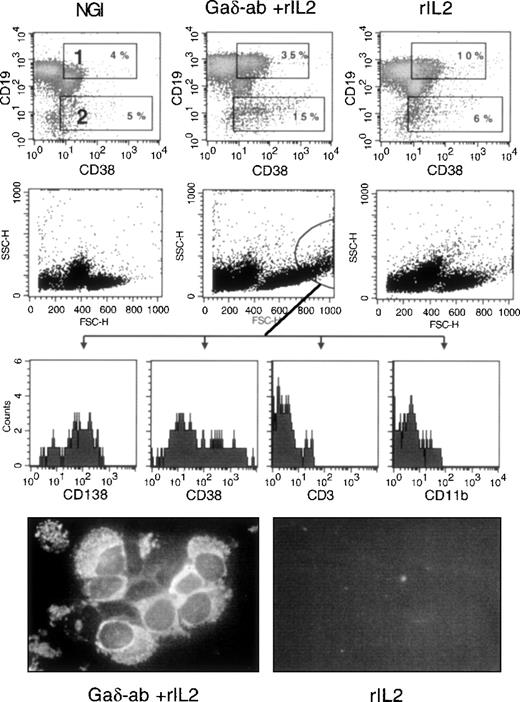
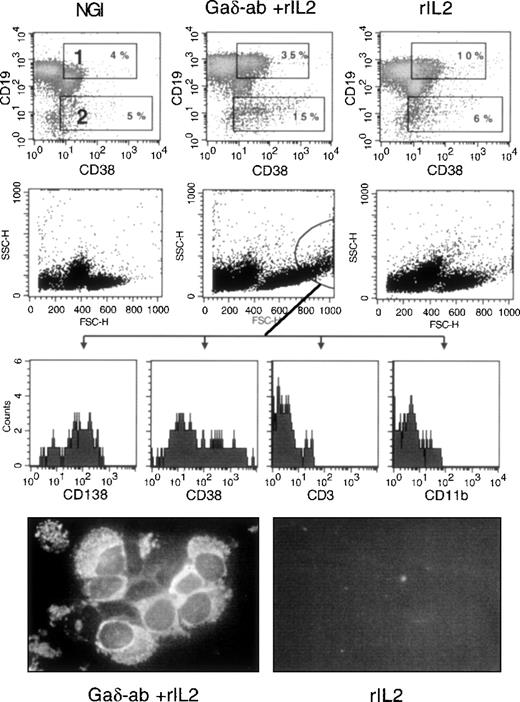
We investigated whether exposure of CD38-positive B-CLL cells to Gaδ-ab could induce their differentiation into immunoglobulin-secreting plasma cells. Purified B-CLL cells from 3 different patients (B, F, E) were incubated with Gaδ-ab in the absence or presence of rIL2 and harvested after 3, 5, and 7 days. They were stained by 2-color immunofluorescence with anti-CD19 and anti-CD38 mAb to detect cells with the CD19-low/CD38-high surface phenotype typical of plasma cells.24 Figure6 illustrates the results of 1 representative experiment (patient B) in which the cells were stained after 5 days of culture, the optimal incubation time for observing plasma cell differentiation. As shown in Figure 6, cells incubated with Gaδ-ab and rIL2 displayed an increased percentage of CD19+/CD38+ (gate 1) and the appearance of CD19-dim/CD38+ bright cells (gate 2). These cells, likely to represent cells undergoing plasma cell differentiation, were virtually absent from the cultures treated with NGI, rIL2 (Figure 6), or Gaδ-ab alone (data not shown). In these experiments, the analysis of FSC and SSC parameters (Figure 6) showed that in the cultures exposed to Gaδ-ab and rIL2, there were large cells with substantial granularity (circle in Figure 6). These cells stained with the plasma cell-specific mAb (CD138)25 and were brightly stained by the CD38 mAb. Moreover, they were consistently negative for CD3 and CD11b, thus excluding that they represented contaminant T cells or monocytes. Collectively, these data favor the accumulation of newly formed plasma cells in culture (Figure 6). The expression of CD138 was very low to absent in the cells outside the indicated gate and in the cells cultured with NGI or rIL2 alone.
Differentiation into plasma cells of CD38-positive B-CLL cells after treatment with Gaδ-ab and rIL2.
CD38-positive B-CLL cells were cultured in the presence of NGI, Gaδ-ab (10 μg/mL) plus rIL2 (100 U/mL), or rIL2 alone for 5 days. Cells were recovered, stained, and analyzed by flow cytometry. (top) Double staining for CD19 and CD38 of the cells cultured with the indicated stimuli. Gates 1 and 2 show CD19-bright, CD38-bright, and CD19-dim/CD38-bright cells, respectively, together with their percentages. (middle) FSC and SSC parameters of the cultured cells. Large cells with substantial cytoplasmic granularity were gated, and their fluorescence relative to the CD138, CD38, CD3, and CD11b stainings was recorded (bottom histograms). These cells were present only in Gaδ-ab-stimulated cultures. Cytospin preparations from Gaδ-ab rIL2–cultured cells (left) or rIL2 cultured cells (right) were fixed and stained with FITC-conjugated anti-IgM mAb. Results are from 1 representative experiment (patient B) of the 3 performed (patients B, E, F).
Differentiation into plasma cells of CD38-positive B-CLL cells after treatment with Gaδ-ab and rIL2.
CD38-positive B-CLL cells were cultured in the presence of NGI, Gaδ-ab (10 μg/mL) plus rIL2 (100 U/mL), or rIL2 alone for 5 days. Cells were recovered, stained, and analyzed by flow cytometry. (top) Double staining for CD19 and CD38 of the cells cultured with the indicated stimuli. Gates 1 and 2 show CD19-bright, CD38-bright, and CD19-dim/CD38-bright cells, respectively, together with their percentages. (middle) FSC and SSC parameters of the cultured cells. Large cells with substantial cytoplasmic granularity were gated, and their fluorescence relative to the CD138, CD38, CD3, and CD11b stainings was recorded (bottom histograms). These cells were present only in Gaδ-ab-stimulated cultures. Cytospin preparations from Gaδ-ab rIL2–cultured cells (left) or rIL2 cultured cells (right) were fixed and stained with FITC-conjugated anti-IgM mAb. Results are from 1 representative experiment (patient B) of the 3 performed (patients B, E, F).
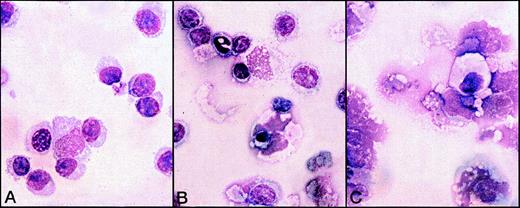
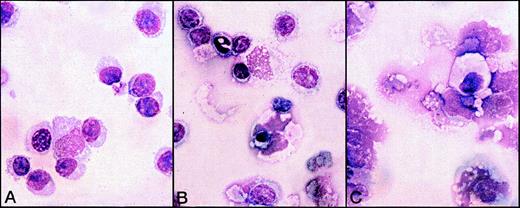
The presence of plasma cells was confirmed by morphologic analysis of the 5-day cultures carried out by immunofluorescence with anti-IgM mAb (Figure 6) and Giemsa staining (Figure 7). In the cultures treated with Gaδ-ab plus rIL2, we observed many cells with the morphology of plasma cells or plasmablasts (Figure 7A) that containing intracytoplasmic IgM (Figure 6). They were absent from the cultures in medium (not shown) or with rIL2 alone, in which most small lymphocytes and a few apoptotic cells were observed (Figure 7B). As expected, apoptotic and necrotic cells were present in the culture exposed to Gaμ-ab (Figure 7C).
Morphological analysis of B-CLL cells exposed to various stimuli for 5 days in vitro.
B-CLL cells (patient B) were exposed to Gaδ-ab + rIL2 (A), rIL2 alone (B), or Gaμ-ab + rIL2 (C). Plasmablasts and plasma cells were observed after culture with Gaδ-ab + rIL2. In contrast, the cells treated with Gaμ-ab were primarily apoptotic or necrotic. Necrosis occurred after apoptosis probably because of the prolonged time in culture (see also Figure 2C). In the preparations exposed to rIL2 only, there were a few surviving small lymphocytes.
Morphological analysis of B-CLL cells exposed to various stimuli for 5 days in vitro.
B-CLL cells (patient B) were exposed to Gaδ-ab + rIL2 (A), rIL2 alone (B), or Gaμ-ab + rIL2 (C). Plasmablasts and plasma cells were observed after culture with Gaδ-ab + rIL2. In contrast, the cells treated with Gaμ-ab were primarily apoptotic or necrotic. Necrosis occurred after apoptosis probably because of the prolonged time in culture (see also Figure 2C). In the preparations exposed to rIL2 only, there were a few surviving small lymphocytes.
The ability to Gaδ-ab to induce the differentiation of CD38-positive BLL cells to immunoglobulin-secreting plasma cells in vitro was confirmed by measuring immunoglobulin molecules in the culture supernatants. As shown in Table 3, substantial amounts of immunoglobulin molecules were measured virtually only after exposure of the cells to Gaδ-ab in the presence of rIL2. Moreover, these immunoglobulins expressed the same κ- or λ-type found at the surfaces of the malignant B cells.
Discussion
This study demonstrated that cross-linking the surface IgM or IgD of CD38-positive B-CLL cells has different physiological outcomes. Signals delivered by IgM activate programmed cell death, whereas those released by IgD promote cell survival and differentiation. Furthermore, signals received through the IgM receptor appear to predominate over those released by IgD because IgD cross-linking does not prevent Gaμ-ab-induced apoptosis.
It is unlikely that these data can be explained on the basis of an inferior ability of Gaδ-ab to trigger the appropriate signal transduction pathway. Gaδ-ab was able to induce [Ca++]i mobilization and tyrosine kinase activation in malignant cells as efficiently as Gaμ-ab. The different responses to Gaμ-ab and Gaδ-ab cannot be explained by any of several technicalities related to the anti-immunoglobulin reagents used. For example, differences in the binding affinities do not seem to be involved because Gaμ-ab and Gaδ-ab equally stimulated normal peripheral blood B cells to proliferate in vitro (see Table 2). Moreover, Gaμ-ab or Gaδ-ab did not have different capacities for modulating surface IgM or IgD expression, at least as determined by the equal ability of the leukemic cells to re-express both surface isotypes in vitro after their modulation by capping with Gaμ-ab or Gaδ-ab (not shown). Finally, neither IgD nor IgM expression was selectively lost in culture during these experiments (not shown).
Previous studies using special cell lines in vitro demonstrated that IgM, but not IgD, could deliver apoptotic signals.6,7,26,27Our results were in line with those observations and demonstrated that surface IgD was capable of delivering a signal that improved cell survival and promoted differentiation. The latter phenomenon was not seen in the cell lines used for the experiments cited above, probably because of the elevated proliferative activity of the malignant cells in vitro, which might have prevented maturation into terminally differentiated cells. It should be stressed that in our experiments, the capacity to differentiate immunoglobulin-secreting cells was a feature of a relatively small proportion of the malignant B-CLL cells. Whether this resulted from an ongoing process of intraclonal maturation that rendered only a proportion of the cells susceptible to the signal delivered by IgD remains to be established. Similarly, the requirement for additional signals to improve cell differentiation or even to promote cell proliferation, such as that delivered by CD40L, has to be investigated.28
The choice of CD38-positive B-CLL for this study was indicated by the observation that in CD38-positive B-CLL cells, the signal transduction pathway initiated by cross-linking of IgM is generally preserved.15 It is of interest that CD38 per se does not seem to be involved in the signal-transducing pathway of the B-CLL cells, at least based on the observation that its cross-linking is not followed by [Ca++]i mobilization.15 Thus, although studies in murine and human experimental systems have demonstrated an involvement of CD38 in BCR signaling,29-33in B-CLL cells CD38 should be considered a marker of a particular physiological stage of the cell whose precise function has yet to be determined.
A crucial problem related to the current observations is to determine why CD38-positive B-CLL present different IgM- and IgD-mediated responses. In principle, 2 hypotheses can be formulated. One implies that B-CLL cells are different from normal B cells in that neoplastic transformation causes a number of changes, including differential responses through IgM or IgD receptors. The other and, in our opinion, more likely possibility is that, because of the malignant transformation, CD38-positive B-CLL cells remain frozen at a particular stage of differentiation in which normal B cells also have the same physiological properties. A similar condition has been observed in transgenic mice: B cells expressed BCR with anti-TNP activity.9 These mice are characterized by a subpopulation of B cells that remains frozen at a particular stage of maturation in which surface IgM engagement causes clonal elimination, whereas IgD engagement results in cell protection and activation. Cells sharing the same features were not observed, however, in transgenic autoimmune mice12 or in irradiated mice reconstituted with normal splenic B cells.11 This suggests that in anti-TNP transgenic mice, conditions favored the accumulation of B cells at a stage that might have been physiologically transient.
Studies are in progress to identify the normal counterpart of the CD38-positive B-CLL cells in lymphoid tissues from patients without neoplasia. The CD38-positive B-CLL cells described in this study did not seem to correspond to either the normal germinal center B cells34 or the germinal center founder cells.35Both B-cell subsets share CD38 expression but differ in several aspects from CD38-positive B-CLL cells. For example, unlike B-CLL cells, both of these cell types express CD10, CD77, and CD95, both are prone to spontaneous apoptosis,34,35 and both have substantial somatic mutations in their VH and VL immunoglobulin genes.35,36 In contrast, though many B-CLL cells display somatic mutations in their VH and VL genes,37 CD38-positive B-CLL cells seem to represent a remarkable exception in that they express VH and VL genes that have not undergone mutation.38
The finding that opposing signals can be delivered by 2 receptors with similar cytoplasmic tails and the same ability to associate with Ig-α and Ig-β molecules raises a number of as yet unanswered questions related to the mode of their signal transduction. Preliminary evidence indicates an involvement of caspase 3 in the Gaμ-ab-induced programmed cell death of B-CLL cells. In contrast, caspase 8 does not seem to be activated during this process, suggesting that apoptosis is directly induced by signals delivered through the BCR and is not mediated by Fas and FasL surfacing, as has been proposed to explain apoptosis of several cell types.39 Consistent with this observation is the failure to induce Fas expression of B-CLL cells by Gaμ-ab. It is possible that IgM and IgD activate basically the same signal-transducing pathway to the level of [Ca++]i mobilization and PLCγ1 activation. From this point on, the 2 pathways may diverge, as has been described for certain cell lines.40
Recent evidence indicates that surface IgM and IgD are associated with different connecting molecules3,4 41 and that, though not formally proven, the presence of these specific proteins could explain the functional difference between the IgM and IgD BCR.
CD38-positive B-CLL cells may represent a useful model to clarify the role of a number of apoptotic and antiapoptotic genes. For example, we have preliminarily determined that c-myc, which predisposes cells to apoptosis,23,42 is elevated in B-CLL. Moreover, the antiapoptotic gene bcl-2 is up-regulated, and the induction of apoptosis by Gaμ-ab is not followed by its prompt down-regulation (preliminary data). The latter finding probably emphasizes the importance of other apoptotic genes such as Bax, BcL-X, BAG1.43-46 Studies along this line are in progress.
Finally, a crucial problem is represented by the relative functions in cell physiology of 2 surface isotypes that deliver opposite signals. Although the current in vitro studies indicated that the signals delivered by IgM always predominated and hence that a cell in the physiological state, corresponding to a leukemic CD38-positive B cell, would invariably be destined to apoptosis, this may not necessarily be the case in vivo after antigen interactions. Under these conditions, the relative ratios of IgM and IgD, the antigen-binding capacity of the 2 isotypes, and their different abilities to elicit help from T cells may play a role in determining the outcome of the response to apoptosis induction or antibody production. Therefore, different signaling by IgM or IgD may contribute to the fine-tuning of responses under particular circumstances.47 48
Acknowledgments
The authors thank Drs Nicholas Chiorazzi and John Monroe for helpful discussions, Nicholas Chiorazzi for critical review of the manuscript, Massimo Ulivi for technical advice, and Ms Teresa Tavilla for skillful secretarial assistance.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro, Progetto di Ricerca di Ateneo, and Cofinanziamento Murst 1999.
Reprints:Simona Zupo, Servizio di Immunologia Clinica, Istituto Nazionale per la Ricerca sul Cancro, Largo Rosanna Benzi N. 10, 16132 Genoa, Italy; e-mail: szupo@hp380.ist.unige.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004aw.jpeg?Expires=1767901158&Signature=ZlBOCTbfIrk7l0XY9AzHoWg6YQx2d9IIh-Ys97r3gTzIA9UlZS2WirPCMh5F0KHHD8NFJO2GsnqB80B5SbNoAPiLMs6eIx3FGH2lMySspQvIOhgQo8MYXOT6060sE1LlUE-0H1YSwIYxa3lb8Df0hS4re92X4hUzF9Fo4UEoXE0x4JI5UxsPpHVVgtSE0zCuVPCdQ-dB4ay2-TMWkz-ENDueqn5xkq3aPnJOZzrjQwKjU4DukT6rftuq-3x6GQUghA~OCuclGL~79VppfN2fc2UdCPiQuRetNeoXwmYTJ7MT4OHQ-yFO6p2qG65DNSoUhSsauNaLrwz9od2IHDhlsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004bw.jpeg?Expires=1767901158&Signature=FZJdZblDSpvfnVHfZ9EwJedOp0IgK7qC9W9ZiW07wbSaZQO-kWJnv6hRebIZxiY0sPdkGce-Ri40d2dJD4y9A7ZX8C4-olicuJopFoAgUUSzq8rHRSlrFGdny3kRTx5tkv7Hu9zgtCs~TPlzRtKmC9JakFaIOx0nZbpDzWwKobC9BjdudVy8ugzumeqcLtqbktF-BxBi17KdzY8IjY0kIIjM-z-EBk8SIp2sdIkYzACCb7yiPnClL4PcaMaMdGaEwp-JOcXXiuoI2wqWADjDbxX3rosIFDFHF-A4Wxyay~uhbx9SGPdrtdPlC8ufcDaR4NQMovHlQG9Bcz121nK5jQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004cx.jpeg?Expires=1767901158&Signature=cvQ~4Xq3qbCnpTa6-WzBdbACfQ34XkxlE9QMBopCq527hRvLEud3Cd0LK3S8zflTJsW~TvAPTrvaXFJwQuklkE51IYncColUlXMhnw7wI3iwl1aTHRgn1d8d8SeKXqWePLUuCRlUX1-qLHCg1rXUTtpy~SBTeLbBufOIvR8Y19CaUNilp8WyTKS7m4soaszmcTbWhFvoygeiIBJzJqrQyX~kCY7j3b~4qnLrHOjs-DVgOCFLoPVFeCXjCRrNfBfcay43eA9uDH4U987bXwsPvN2JGZ5uxwg0o96IxeYPDAzqdQcEjh-ZomHEA-ClKbuph9k~FjtoYsf-WlS0VV2P3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004aw.jpeg?Expires=1768832819&Signature=zJidRz8V6u1KUoy~gzsDbeeZPngo5xyiC019761cGGrSWdjOqi8Apu~ttY7-8KNGFTjcuSzpZu-VpopTJIPdfT7Vpj-76zxPJ~VdhyfE~cdpJHp-aMNBmIPbFgqZiKWaF8sS8JsmVw3HPYUhWMOf4NMTKwsS0dWmnt3YGOMUvVqwFiyycnoymWefW0uuSBgccuTnpABKFLDluSYcg48IzQOPLLVrfF7rIcd8JBhfKBx4sZ7daXpz6lvcTUhfgcFOtTsaQ2pbOsNhXZcS4Z91x7YZMA7S-mAuBaIdK5lRtiTE~JrEn5FbtPfzthCuaczdDvm8G7c-DmesALSRWzZafA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004bw.jpeg?Expires=1768832819&Signature=PJvKyG72FIcO2lcW9xqUSPzQ0k81-fZnsBX2WYzQAF210dg3eJoVKl9R4eiIIMJKwTkrDk4Xn711MYMRbcXqA0oWiU7CCS615lX6VuVl9rwXgE61NQlIJz9NuDwgZAgg0z8GbJlv3Bz7HWOTb67gjyv8S-8j64LNLbnG6GiNDB7L8HCJLR~LOPKQUHGzLA2mQ3idSh9qtIWs9ZaGAfDA72WOsVZKsyGhFOodKytP~0qghJAMQhYLZsPBI73f8rzFbCYn19q5ORHYx-ZVCULVNP46K9DHdMwwVKO9nt4cBHN4KZzvK~XPbAkVzPQqs5cz1082wOZIT-YxpVCQw1Fprw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Ability of Gaμ-ab and Gaδ-ab to activate the signal transducing pathway in CD38-positive B-CLL cells. / (A) B-CLL cells (patient G) were stimulated with NGI, Gaμ-ab, or Gaδ-ab at the optimal concentration (10 μg/mL) for the indicated time. Cell lysates (20 μg/lane) were separated on 10% reducing SDS-PAGE gels and analyzed by immunoblotting with the antiphosphotyrosine mAb, 4G10. Molecular weight in kilodalton is indicated on the right. (B) B-CLL cells (patient H) were stimulated for 3 or 5 minutes with the indicated stimuli. Cell lysates were immunoprecipitated with antiphosphotyrosine mAb (4 G10). The immunoprecipitates were separated on 10% reducing SDS-PAGE gels and probed with an anti-syk mAb (top) or anti-PLCγ1 mAb (bottom). (C) Fura2/AM loaded B-CLL cells were stimulated with Gaμ-ab or Gaδ-ab and studied for [Ca++]i mobilization. Means ± SD of the experiments on the 10 different patients with B-CLL (patients A-L) examined. Panels on the right show the typical profiles of [Ca++]i response to the indicated stimuli (patient A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1199.004k21_1199_1206/6/m_bloo00421004cx.jpeg?Expires=1768832819&Signature=xQ8SU1cD5kBnIIzketNb-Cy0B55uPbfNCXOTDmRwwCzCW8qdZ6NcTPFkS-TaYqhUpzNRcthmtAHZNpCp8YwMpCg-c6o9yaGTQrPqfmeyCd0KdlkbaXtI3jTQPVURQHGHuftgyPpdOKshqSZhsdp5~Zb1hRadxZf2IKXJk7Yx5IvzhQMIcZnzjCPECund4o1UEv3ZXbYJoztfAOOwxiozOjTWe3KyIv1D9FtPoK8w1zLUG4LOudsIEPizdd9Vooo5QmgqHZqFoEFdIQUrcyD5xPAMgazPqhuGjyVbwN9Lo9dto9MkZ6uVFK6I~6Ava0ytcDwblROn8YXo3md0XkXu1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)