Previously, we observed in a rat model that intravenous administration of intramuscular immunoglobulin preparations induced a long-lasting hypotension, which appeared to be associated with the presence of IgG polymers and dimers in the preparations, but unrelated to complement activation. We found evidence that this hypotensive response is mediated by platelet-activating factor (PAF) produced by macrophages. In this study, we compared the vasoactive effects of 16 intravenous immunoglobulin (IVIG) products from 10 different manufacturers, in anesthetized rats. Eight of the IVIG preparations showed no hypotensive effects (less than 15% decrease), whereas the other 8 had relatively strong effects (15%-50% decrease). The hypotensive effects correlated with the IgG dimer content of the preparations. Pretreatment of the rats with recombinant PAF acetylhydrolase completely prevented the hypotensive reaction on IVIG infusion, and administration after the onset of hypotension resulted in normalization of the blood pressure. We also observed PAF production on in vitro incubation of human neutrophils with IVIG, which could be blocked by anti-Fcγ receptor antibodies. This indicates that induction of PAF generation may also occur in a human system. Our findings support the hypothesis that the clinical side effects of IVIG in patients may be caused by macrophage and neutrophil activation through interaction of IgG dimers with Fcγ receptors. Because phagocyte activation may also lead to the release of other inflammatory mediators, recombinant PAF acetylhydrolase (rPAF-AH) provides a useful tool to determine whether PAF plays a role in the clinical side effects of IVIG. If so, rPAF-AH can be used for the treatment of those adverse reactions.
Intravenous administration of immunoglobulin preparations may cause anaphylactoid adverse reactions, including headache, facial flushing, back pain, nausea, chills, fever, dyspnea, hypotension, and circulatory shock. The incidence of these adverse reactions is reported by the manufacturers to be in the range of 1% to 15%, usually below 5%.1 2 The reactions are generally mild and self-limited and associated with a too high infusion rate. Nevertheless, adverse reactions may pose a serious problem in the treatment of some immunodeficient patients.
The side effects of immunoglobulin preparations are generally ascribed to complement activation in the recipient by IgG aggregates in the preparations. However, so-called intravenous immunoglobulin preparations (IVIG), which are made to be free of complement-activating activity in vitro, have been found to still cause clinical side effects, suggesting that other effector mechanisms are involved. Previously, we developed a rat model for the safety evaluation of immunoglobulin preparations.3 We observed that standard immunoglobulin preparations (= Cohn Fraction II without additional treatment) induced a long-lasting hypotension that appeared to be associated with the presence of IgG-polymers and dimers in the preparations, but unrelated to complement activation. We found evidence that this hypotensive response is mediated by platelet-activating factor (PAF) produced by macrophages.4 Others have confirmed our findings5 and several investigators have suggested the usefulness of the blood pressure response in animals for predicting clinical tolerability of an IVIG product in patients.6 7
In this study, we compared the hypotensive effects of 16 IVIG preparations from different manufacturers in rats to see whether the standard release criteria can guarantee the absence of vasoactive effects. Furthermore, we investigated the role of PAF in IVIG-induced hypotension by using a recombinant PAF acetylhydrolase (rPAF-AH).8 Recombinant PAF-AH has the substrate specificity and lipoprotein association of the native plasma PAF acetylhydrolase and blocks PAF-mediated inflammatory reactions in vivo. Therefore, we investigated whether rPAF-AH could provide a useful tool to evaluate the role of PAF in clinical adverse reactions and whether it might be used for their treatment.
Materials and methods
Animal model
Female Wistar rats (Harlan: HsdCpb:WU), weighing 180 to 250 g, were anesthetized by intraperitoneal injection of pentobarbital, 60 mg/kg body weight. Cannulas (Silastic®) were introduced into the right jugular vein and left carotid artery. Saline was infused at a rate of 2 mL/h in each cannula to ensure patency. The arterial cannula was connected to a pressure transducer for continuous recording of the mean arterial blood pressure (MABP) and mean heart rate, both averaged over 10-second time intervals. Human IVIG preparations were given intravenously in 10 seconds at a dose of 250 mg/kg, unless stated otherwise. The test solutions were administered only if a stable MABP (± 10 mm Hg) had been observed for a period of at least 10 minutes. Each rat received only 1 dose of immunoglobulins. The whole procedure took about 80 minutes, during which the rat remained anesthetized without the requirement of additional pentobarbital. The experiments were terminated 30 minutes after administration of the IgG test solution by administration of a pentobarbital overdose.
Human neutrophil activation in vitro
Neutrophils were purified from peripheral human blood anticoagulated with 0.4% (w/v) trisodium citrate, as described before.9Briefly, mononuclear cells and platelets were removed by density gradient centrifugation over Percoll (density 1.077 g/mL). Contaminating red blood cells were lysed by isotonic NH4Cl/HCO3 treatment on ice. After isolation, neutrophils were washed and resuspended in incubation medium (4.106/mL), containing 132 mmol/L NaCl, 20 mmol/L HEPES, 6 mmol/L KCl, 1 mmol/L MgSO4, 1 mmol/L CaCl2, 1.2 mmol/L KPi, 5 mmol/L glucose, and 0.5% (w/v) human serum albumin (pH 7.4). The neutrophils had a more than 95% purity.
Neutrophil activation was assessed at 37°C by mixing 1:1 with prewarmed IVIG solutions (5 mg/mL). After 20 minutes of incubation, 0.8 mL samples (2.106 neutrophils/mL) were taken for the measurement of the synthesis of PAF. PAF was measured with a commercially available competitive radioimmunoassay (New England Nuclear, Boston, MA) as previously described for eosinophils.10 In short, the activation process of the neutrophils was stopped by adding 3 mL methanol/chloroform 2:1 with 2% (v/v) acetic acid added to the methanol. After separation of the monophase with 1 mL chloroform and 1 mL H20, the lower chloroform phase was stored at −70°C under nitrogen. Subsequently, the chloroform phases were evaporated under a stream of nitrogen and processed for the radioimmunoassay according to the manufacturer's instructions. The amounts of PAF in the samples were determined from a standard curve constructed with known amounts of PAF. Recovery of PAF during the whole procedure was more than 90%.
Immunoglobulin preparations
Sixteen human IVIG products were obtained from 10 different manufacturers: Alpha Therapeutic Corporation (USA), Baxter Healthcare (USA), Biotest (Germany), BPL (UK), CLB (The Netherlands), FRC-BTS (Finland), Octapharma (Austria), Purissimus S.A. (Argentina), Sandoz (Switzerland), and SSI (Denmark). Ten of the preparations were licensed, whereas the others were still in a stage of preclinical or clinical evaluation. All preparations complied with the release criteria of the European and US Pharmacopeia, in particular regarding anticomplementary activity, IgG-aggregate content, and prekallikrein activator activity. IgG dimer and polymer contents were determined by size exclusion chromatography using a calibrated Superose 12 gel filtration column in a FPLC system (Pharmacia, Uppsala, Sweden) and computer analysis of the chromatograms (Ezchrom Chromatography Data System). Table 1 gives the product codes attributed to the preparations, the main characteristics of their production process, and their formulation. Products B and E were prepared at the CLB (The Netherlands). For all products IgG was isolated from human pool plasma using cold ethanol fractionation, and additional processing was applied to eliminate anticomplementary activity and to improve clinical tolerability. Various additional process steps were applied, including β-propiolactone treatment, incubation at low pH with or without a small amount of pepsin, diethylaminoethyl (DEAE) chromatography, PEG fractionation. The preparations contained various stabilizers, including glucose, sucrose, and albumin, and were freeze dried or liquid. Freeze-dried preparations were administered within 2 hours after reconstitution, unless noted otherwise. A human IgG preparation for intramuscular administration (IMIG), essentially consisting of Cohn fraction II in a glycin buffer, was obtained from the CLB (The Netherlands).
One freeze-dried IVIG (product E) was stored in liquid state at 4°C for more than 4 months after reconstitution to let occur IgG dimer formation (IVIG-E aged). For control, the same product was frozen in liquid nitrogen immediately after reconstitution and stored at −80°C (IVIG-E fresh). To dissociate the IgG dimers in IVIG-E aged, the preparation was incubated at 37°C for 24 hours after lowering the pH to 4.25 by dialysis against an acetate-containing formulation buffer. The preparation was then rapidly frozen and stored at −80°C.
Recombinant PAF-AH, generously provided by Dr G. Dietsch (from ICOS, Bothell, WA), was used as PAF antagonist. rPAF-AH was given intravenously, as a bolus at a dose of 5 mg/kg, either 15 minutes before, 5 minutes after, or 15 minutes after the immunoglobulin preparations.
Data analysis
Results are expressed as mean ± SD. Correlations between variables were evaluated by calculating Spearman rank correlation coefficient using GraphPad Prism.
Results
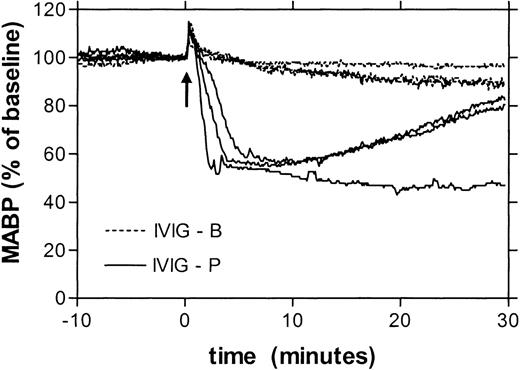
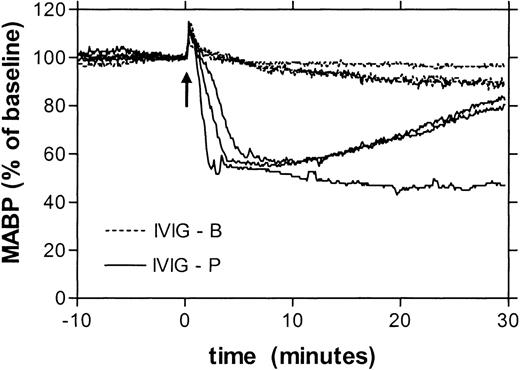
Table 1 gives the changes in blood pressure 10 minutes after administration of 16 different IVIG products, at a dose of 250 mg/kg in 10 seconds. Eight of these preparations induced no, or a minimal hypotensive reaction (less than 15% decrease), whereas the other 8 products had a substantial hypotensive effect (15%-50%). For 8 products, 2 or more lots were tested, in all cases with similar results. Hence, the effects on blood pressure were product-related rather than due to lot-to-lot variations. Figure1 gives as an example the blood pressure recordings after administration of either a product with minimal hypotensive effect (IVIG-B) or a product with strong effect (IVIG-P). The hypotensive reactions had the same time course as those previously described for IMIG preparations3 4: the blood pressure started to fall after 1 minute and reached a minimum level after 10 to 15 minutes. However, for all IVIG preparations, the hypotensive effects were smaller than those induced by an IMIG preparation that was tested for comparison (Table 1). By testing the IMIG preparation at different doses, the threshold bolus dose for hypotension (decrease in MABP > 15%) was found to be between 10 and 30 mg/kg. To investigate whether the rate of infusion was critical in this model, IMIG was also administered by continuous infusion at a rate of 1 or 4 mg/kg/min. This way of administration also induced severe hypotension, but the onset was delayed. The blood pressure started to fall after an interval ranging from 5 to 20 minutes, when a cumulative dose between 7 and 29 mg/kg was reached. This indicated that the hypotensive effect was dose dependent rather than rate dependent.
Superimposed recordings of mean arterial blood pressure (represented as percentage of baseline) of 3 rats receiving IVIG product B (dotted lines) and 3 rats receiving IVIG product P (solid lines).
Superimposed recordings of mean arterial blood pressure (represented as percentage of baseline) of 3 rats receiving IVIG product B (dotted lines) and 3 rats receiving IVIG product P (solid lines).
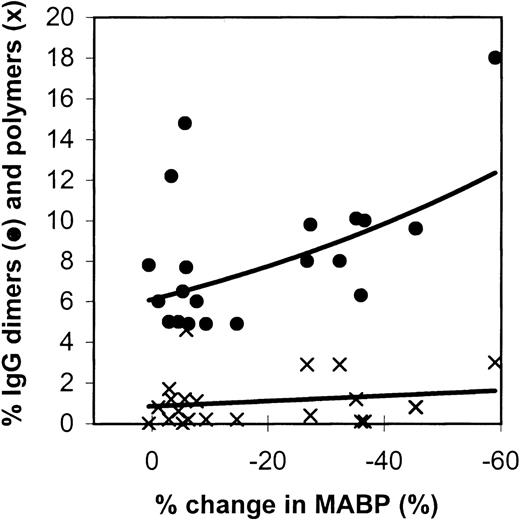
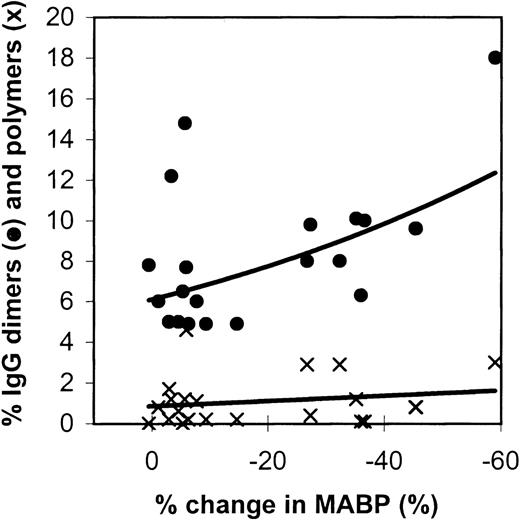
Figure 2 gives the relation between IgG dimers and polymers in the preparations tested and the effects on the blood pressure in rats. The IMIG preparation contained 18% dimers and 3% polymers. The changes in blood pressure correlated significantly with the dimer contents (Spearman rank correlationRs = −0.4484, P = .016), but not with the polymer contents (P = .20). It was remarkable that 1 IVIG product (product-A, β-propiolactone-treated) had a high dimer content (2 lots: 12% and 14%), but did not induce hypotension.
Relation between the changes in blood pressure in rats (10 minutes after administration) and the IgG dimer (•) and polymer (x) contents of the IVIG preparations tested.
Each data point gives the mean percentage change in MABP of 3 experiments.
Relation between the changes in blood pressure in rats (10 minutes after administration) and the IgG dimer (•) and polymer (x) contents of the IVIG preparations tested.
Each data point gives the mean percentage change in MABP of 3 experiments.
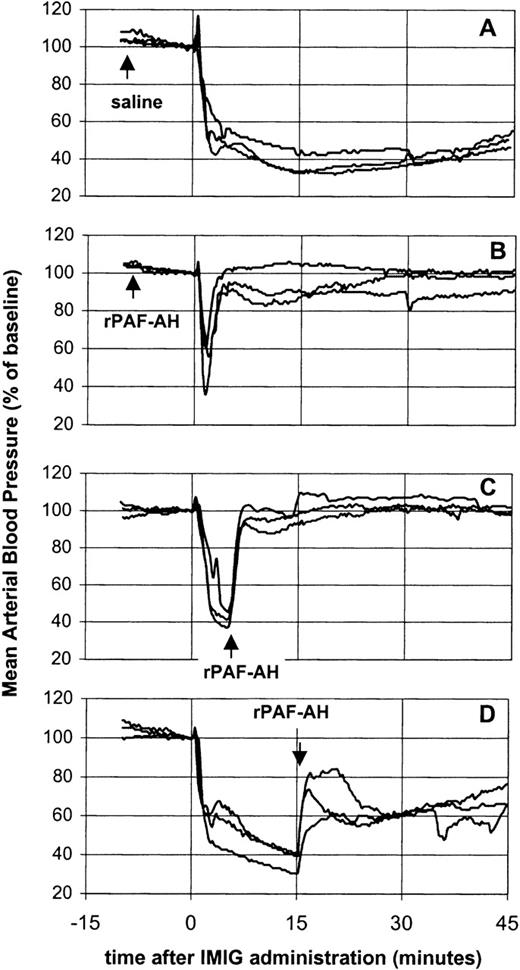
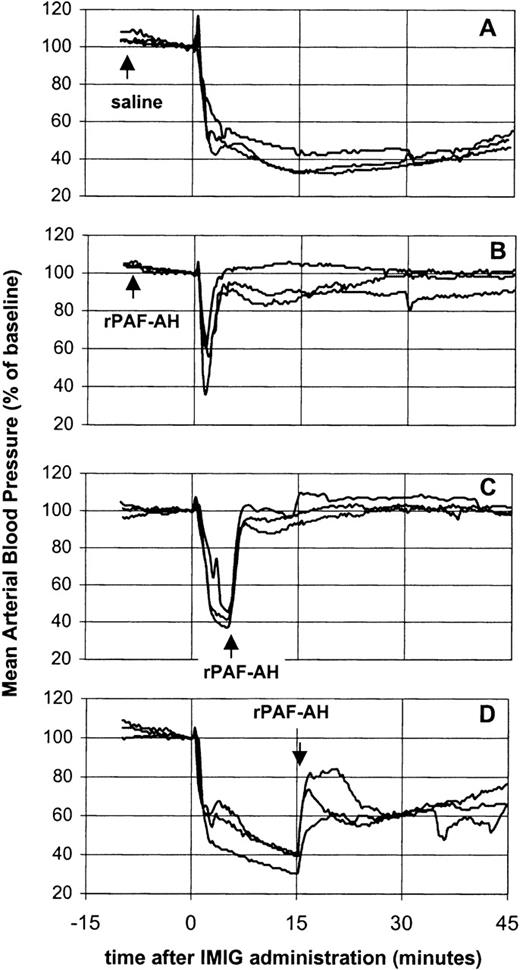
The potency of rPAF-AH to prevent or treat of IVIG-induced vasoactive reactions was investigated using IMIG as a model preparation for a “bad” IVIG (Figure 3). Pretreatment of rats with rPAF-AH (5 mg/kg) prevented the prolonged hypotensive response, only a short-lasting transient decrease remained (Figure 3B). Administration of rPAF-AH (5 mg/kg) 5 minutes after IMIG (250 mg/kg) resulted in a complete reversal of the blood pressure (Figure 3C). This indicated that the hypotension was maintained by a sustained PAF release. However, administration of rPAF-AH 15 minutes after IMIG caused only partial restoration of the blood pressure (Figure 3D), showing that also at this time point other mediators than PAF were involved in the hypotension.
Effect of rPAF-AH on IMIG-induced hypotension.
Saline, 15 minutes before IMIG, was used as control (A). rPAF-AH (5 mg/kg) was administered 15 minutes before (B), 5 minutes after (C) and 15 minutes after (D) IMIG (250 mg/kg). Each panel gives the superimposed mean arterial blood pressure recordings of 3 rats.
Effect of rPAF-AH on IMIG-induced hypotension.
Saline, 15 minutes before IMIG, was used as control (A). rPAF-AH (5 mg/kg) was administered 15 minutes before (B), 5 minutes after (C) and 15 minutes after (D) IMIG (250 mg/kg). Each panel gives the superimposed mean arterial blood pressure recordings of 3 rats.
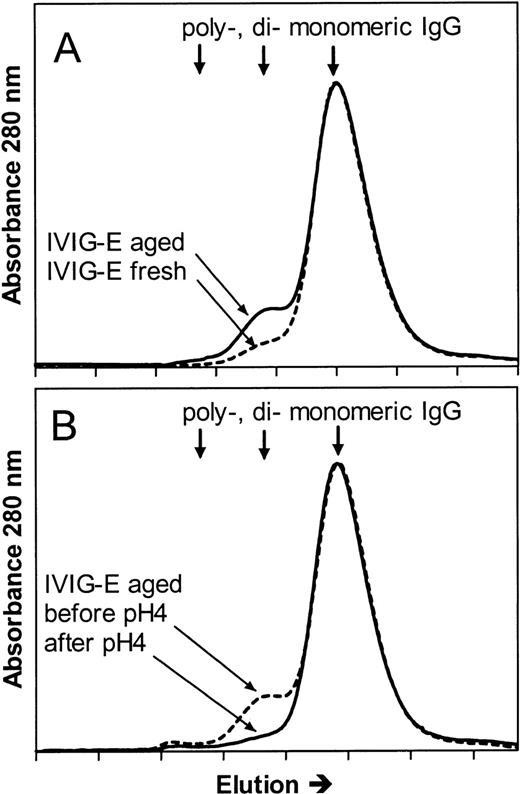
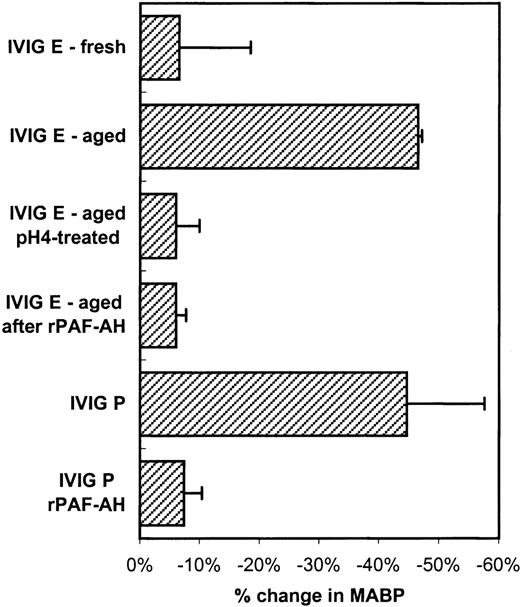
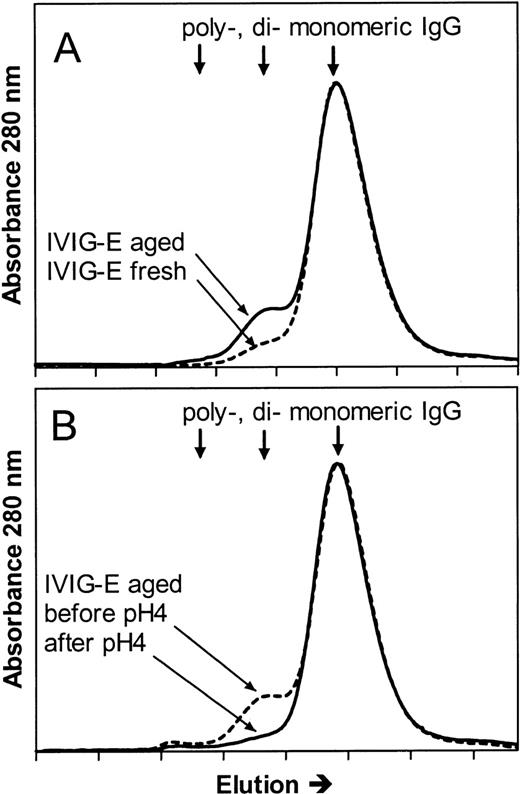
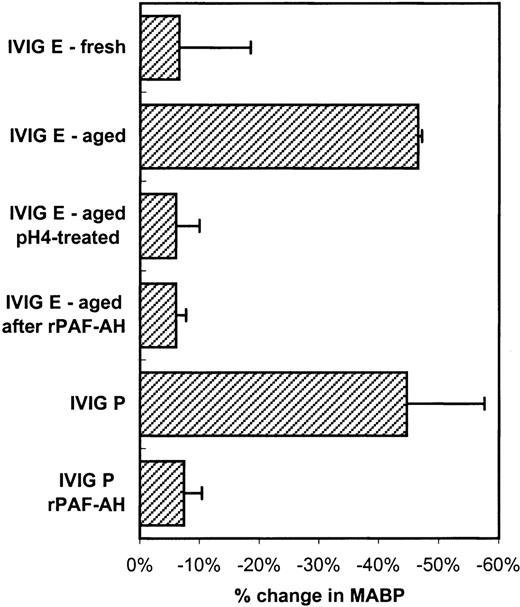
To further investigate whether the mechanisms of the hypotensive reactions after IVIG and IMIG administration were the same, we followed for several lots the change in molecular size distribution in freeze-dried IVIG (product-E) after reconstitution and liquid storage at 4°C. A gradual increase in dimer content was observed from less than 5% to values above 10% after 1 week, which coincided with the occurrence of hypotensive effects in rats. Figure4A shows the chromatogram of IVIG-E that was rapidly frozen after reconstitution and stored at −80°C (IVIG-E fresh), and that of the same product, kept in solution at 4°C for 1 year after reconstitution (IVIG-E aged). The dimer contents were 2.5% and 10%, respectively. The IgG dimers in IVIG-E aged were dissociated by incubating the preparation for 24 hours at 37°C, pH 4.25 (Figure 4B: before and after pH 4). Figure5 shows that dimer formation was accompanied by the occurrence of a hypotensive effect (IVIG-E aged) and that dissociation of the dimers by pH 4 treatment removed the hypotensive effect. Pretreatment of rats with rPAF-AH prevented the hypotension induced by IVIG-E aged and also that induced by another IVIG product (IVIG-P).
Size exclusion chromatograms of (A) an IVIG (product E, see Table 1) that had been stored at 4°C for 1 year after reconstitution of the freeze-dried product (IVIG-E aged), and that of IVIG-E that was stored at −80°C immediately after reconstitution (IVIG-E fresh) and (B) IVIG-E aged before (before pH 4) and after incubation for 24 hours at 37°C, pH 4.25 (after pH 4).
Size exclusion chromatograms of (A) an IVIG (product E, see Table 1) that had been stored at 4°C for 1 year after reconstitution of the freeze-dried product (IVIG-E aged), and that of IVIG-E that was stored at −80°C immediately after reconstitution (IVIG-E fresh) and (B) IVIG-E aged before (before pH 4) and after incubation for 24 hours at 37°C, pH 4.25 (after pH 4).
Changes in blood pressure (mean and SD, n = 3) induced by IVIG-E fresh (see Figure 4A), IVIG-E aged (increased dimer content, see Figure 4A), IVIG-E aged after pH 4 treatment (see Figure4B) and by IVIG-P.
Furthermore, the effect of rPAF-AH pretreatment of the rats on the hypotensive effect of IVIG-E and IVIG-P. The IVIG product codes correspond to that in Table 1.
Changes in blood pressure (mean and SD, n = 3) induced by IVIG-E fresh (see Figure 4A), IVIG-E aged (increased dimer content, see Figure 4A), IVIG-E aged after pH 4 treatment (see Figure4B) and by IVIG-P.
Furthermore, the effect of rPAF-AH pretreatment of the rats on the hypotensive effect of IVIG-E and IVIG-P. The IVIG product codes correspond to that in Table 1.
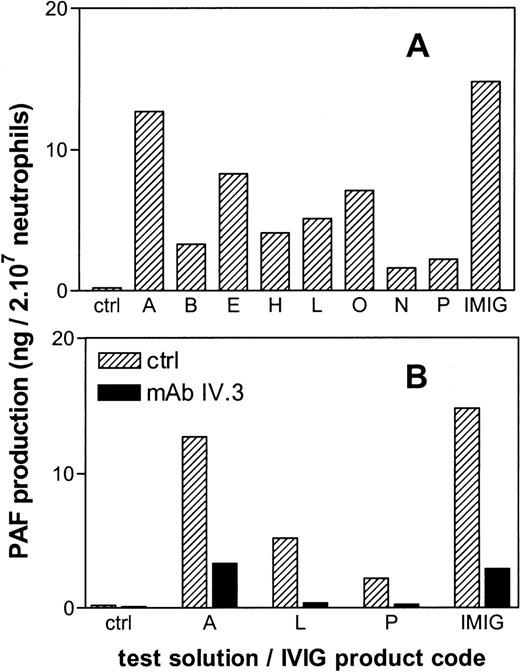
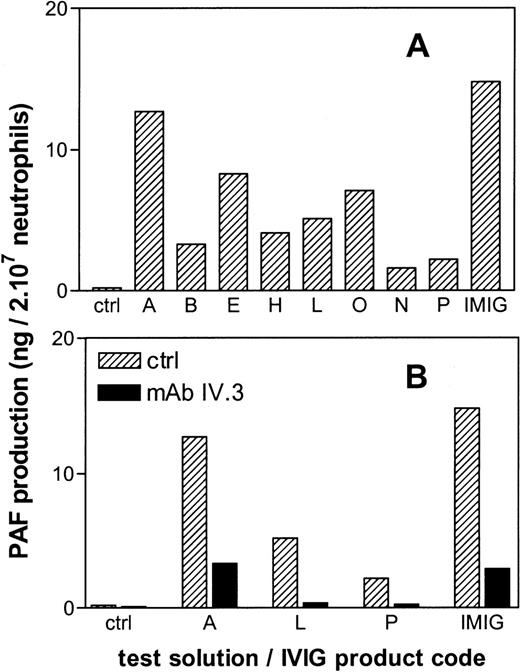
All IVIG products tested in vitro, induced PAF synthesis in human neutrophils, albeit to a different extent (Figure6A). There was no obvious correlation between the magnitude of the in vitro PAF synthesis and the in vivo hypotension for the different IVIG preparations. However, in both assays IMIG was the most potent activator. Preincubation of the neutrophils with a blocking monoclonal antibody against FcγR II (mAb IV.3) largely prevented the IVIG-induced PAF production (Figure 6B).
PAF production by human neutrophils in vitro after 20 minutes of incubation with IgG preparations.
The letters give the codes of the IVIG products as presented in Table1. Panel B gives the effect of preincubation of the neutrophils with a blocking anti-FcγRII antibody (mAb IV.3). Results are the mean of duplicate incubations.
PAF production by human neutrophils in vitro after 20 minutes of incubation with IgG preparations.
The letters give the codes of the IVIG products as presented in Table1. Panel B gives the effect of preincubation of the neutrophils with a blocking anti-FcγRII antibody (mAb IV.3). Results are the mean of duplicate incubations.
Discussion
Our study revealed major differences between different IVIG products regarding their vasoactive properties in rats. About half of the preparations tested had a pronounced hypotensive effect. All preparations complied with the release criteria set by the European and US Pharmacopoeia, in particular those regarding limits for IgG aggregates and anticomplementary activity. These criteria came forth from the idea that systemic complement activation by IgG aggregates in the preparations is the major cause of clinical side effects in the recipient. This was based on the observation of Barandun and colleagues13 that immunoglobulin preparations could be safely administered intravenously after elimination of the anticomplementary activity. Therefore, manufacturers of IVIG have implemented production steps to remove or inactivate IgG aggregates, thereby eliminating the anticomplementary activity. However, our study made clear that eliminating the complement-activating properties of IVIG preparations does not necessarily eliminates all vasoactive potential of IVIG. The most likely explanation seems to be that IgG isolated from pooled plasma from numerous donors tends toward the formation of dimers.14 The IgG dimers have no anticomplementary activity but appeared to be almost as potent as IgG polymers in inducing hypotension in rats.3 The storage experiments with a reconstituted freeze-dried preparation, in which vasoactivity was found to parallel the occurrence of dimers and disappeared after their dissociation, confirm the role of dimers in IVIG-induced hypotension. All IVIG products tested contained a certain amount of IgG dimers, but vasoactivity seems to occur only when the dimer content raises above a certain limit. A clear exception was product D; the 2 batches tested had a relatively high dimer content (12% and 15%), but showed no hypotensive effect. This suggests that the chemical modification by β-propiolactone treatment eliminates the hypotensive effect by diminishing interaction with Fcγ receptors (see below). This also raises the question whether this modification also affects other Fc-effector functions of IgG in the preparation.
Previously, we found evidence that the IMIG-induced hypotension in rats is mediated by PAF release from macrophages, independently from complement activation.4 Therefore, we speculated that IgG dimers in IVIG may activate macrophages through FcγR triggering and speculated that this is the major cause of clinical side effects of the IVIG preparations currently available. This hypothesis finds support in a recent clinical study in volunteers, comparing different experimental IVIG preparations.15 In that study, a correlation was found between IgG dimer content in the preparations and the occurrence of clinical adverse reactions in humans and vasoactive effects in rats. In addition, the clinical side effects in the humans were found to be accompanied by a transient decrease in neutrophil and monocyte number in the peripheral blood that paralleled an increase in TNFa serum concentrations.15 Furthermore, we demonstrated the possibility of FcγR triggering by IVIG in a previous in vitro study, in which we found that IgG dimers indeed bind to FcγRII and FcγRIII on human neutrophils, and stimulate these cells via FcγRII triggering.16 This is consistent with the observation in the in vitro part of this study that a monoclonal antibody against FcγRII could block the induction of PAF production by human neutrophils. Taken together, these data point to a central role of the activation of macrophages, monocytes, and possibly neutrophils in the induction of clinical side effects.
In this study we evaluated the potential of rPAF-AH in treating vasoactive effects of IVIG. A standard IMIG preparation, which is basically Cohn fraction II dissolved in glycine-saline, was used as a model for a “bad” IVIG preparation, in the sense that in the early days of immunoglobulin therapy, side effects were frequently observed on intravenous administration of these kind of preparations. The experiments showed that pretreatment with rPAF-AH prevents hypotension after IMIG infusion in rats. These results are consistent with those of previous studies with PAF-receptor antagonists.4 5 Furthermore, administration of rPAF-AH shortly after the start of the hypotension resulted in a complete restoration of the blood pressure. This demonstrates that sustained PAF release from macrophages (or other cell types) plays a central role in the induction and maintenance of hypotension after intravenous administration of immunoglobulin preparations to anesthetized rats. In addition, we observed that IMIG and certain IVIG products induced PAF production in isolated human neutrophils. We have no explanation for the fact that there was no obvious correlation between the in vitro PAF production and in vivo hypotension induced by the different IVIGs. However, the in vitro results clearly demonstrated that activation of phagocytes by human IVIG is not a particularity of rats but also occurs in a human system. It also showed that PAF release results from direct interaction of IVIG with phagocytes.
In a recent study in rats, we observed that IVIG preparations with high IgG dimer content also induce activation of neutrophils in vivo (Teeling et al, manuscript submitted). However, neutrophil activation was not prevented by rPAF-AH pretreatment, showing that PAF plays no major role in this effect and that rPAF-AH cannot prevent all effects of FcγR triggering by IVIG.
It has been stated that the standard, polyvalent IVIG products are therapeutically equivalent and interchangeable.2 Our study, however, shows that IVIG products may differ substantially regarding their vasoactive side effects in rats, which points to differences in the interaction with FcγR. We expect therefore that the products differ regarding the incidence of side effects.
In conclusion, our findings support the hypothesis that the clinical side effects of the current IVIG product are caused by FcγR triggering on phagocytes by IgG dimers in the preparations. First, we observed that many IVIG products induced hypotensive effects in rats, which correlated with the dimer content of the product. Second, rPAF-AH could effectively prevent these vasoactive effects indicating that IVIG may induce significant PAF release in vivo and suggesting that macrophage activation occurred. Finally, we observed that IVIG may also induce PAF generation in a human system in vitro. Because phagocyte activation may also lead to the release of other inflammatory mediators, rPAF-AH provides a useful tool to determine the role of PAF in the clinical side effects of IVIG in humans. If PAF appears to play a role, rPAF-AH can be used for the treatment of those adverse reactions.
Reprints:W. K. Bleeker, CLB, Dept PDH, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: w_bleeker@clb.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.