Abstract
Fifty children who had symptomatic sickle cell disease received matched sibling marrow allografts between September 1991 and March 1999, with Kaplan-Meier probabilities of survival and event-free survival of 94% and 84%, respectively. Twenty-six patients (16 male, 10 female) had at least 2 years of follow-up after transplantation and were evaluated for late effects of transplantation and for its impact on sickle cell-related central nervous system (CNS) and pulmonary disease. Patients ranged between 3.3 and 14.0 (median, 9.4) years of age and had a median follow-up of 57.9 (range 38-95) months after transplantation. Among 22 of 26 patients who had stable donor engraftment, complications related to sickle cell disease resolved, and none experienced further episodes of pain, stroke, or acute chest syndrome. All 10 engrafted patients with a prior history of stroke had stable or improved cerebral magnetic resonance imaging results. Pulmonary function tests were stable in 22 of the 26 patients, worse in two, and not studied in two. Seven of eight patients transplanted for recurrent acute chest syndrome had stable pulmonary function. Linear growth measured by median height standard deviation score improved from −0.7 before transplantation to −0.2 after transplantation. An adverse effect of busulfan conditioning on ovarian function was demonstrated in five of seven evaluable females who are currently at least 13 years of age. None of the four males tested had elevated serum gonadotropin levels. These data confirm that allogenic bone marrow transplantation establishes normal erythropoiesis and is associated with improved growth and stable CNS imaging and pulmonary function in most patients.
Hematopoietic cell transplantation has curative potential in the treatment of hereditary anemias like sickle cell disease and β-thalassemia major.1-5 Currently, more than 800 patients with β-thalassemia major have proceeded to allogenic transplantation; however, many fewer patients with sickle cell disease have done so, due in part to the more variable clinical course of sickle cell disease and limitation of patient eligibility to individuals with advanced stage symptomatic disease often involving neurological and pulmonary vasculopathy.6-8 Although most sickle cell disease patients who received HLA-identical marrow allografts survive free of sickle cell disease, there are several follow-up evaluations that are required to assess the long-term outcome. The first has to do with sickle cell-related clinical events, and among patients who have stable engraftment of donor cells, all evidence points to cessation of clinical vaso-occlusive events.9-11 Other important factors to consider are the late effects of transplantation related to the administration of myeloablative chemotherapy in children and the beneficial effect of donor erythropoiesis on sickle cell-related organ damage.
This report summarizes our initial evaluation of the late effects of transplantation in a cohort of children with symptomatic sickle cell disease enrolled in a multicenter collaborative investigation of bone marrow transplantation for sickle cell disease.
Patients and methods
Patients
Patients less than 16 years of age with symptomatic sickle cell disease (SS, SC, or SB°-thalassemia) who had an HLA-identical family member donor (Hb AA or AS) were considered for marrow transplantation. All individuals were required to meet eligibility criteria as reported earlier.3 Patients with extensive end-organ dysfunction were excluded from enrollment. These conditions included significant functional impairment (Lansky Play Performance Scale or Karnofsky score <70%), hepatic disease (active hepatitis or cirrhosis), severe renal impairment (glomerular filtration rate <30% predicted normal for age), severe residual functional neurologic impairment (hemiplegia alone was not an exclusion) or stage III-IV sickle cell lung disease.12 Patients were enrolled from 24 centers in the United States and Europe (see the for collaborating centers). The study was approved by the institutional review board of the Fred Hutchinson Cancer Research Center and by institutional review boards or their equivalents at each of the collaborating sites. All patients and/or their parents or guardians gave written informed consent for their participation.
The National Heart, Lung, and Blood Institute appointed a data safety and monitoring board for the purpose of monitoring patient safety and the ethical conduct and progress of this investigation. The board consisted of five hematologists, a clinical statistician, and a patient advocate. The principal investigators (M.C.W. and K.M.S.) submitted quarterly reports to the data safety and monitoring board.
Treatment regimen
Patients were prepared for transplantation with a combination of busulfan (BU, 14 mg/kg), cyclophosphamide (CY, 200 mg/kg), and horse anti-thymocyte globulin (ATGAM®, 90 mg/kg) or CAMPATH IG (10 mg/kg for 5 days) in lieu of ATG.13 Three patients (patients 9-11) received BU (500 mg/m2), CY (200 mg/kg), and rabbit ATG (20 mg/kg) and another (patient 6) received BU (16 mg/kg), CY (200 mg/kg), and ATG (80 mg/kg). Since November 1994, all North American patients had BU pharmacokinetics performed with targeted steady-state concentrations adjusted to 400-600 ng/mL. Patients received a combination of methotrexate and cyclosporine (CSP), CSP and prednisone, or CSP alone for the prevention of acute graft-versus-host disease (GVHD) as previously reported.13,14 Prophylaxis with CSP was given for 6 months following transplantation. Definition and grading of acute and chronic GVHD have been described.13 14
Prior to transplantation, patients not receiving chronic transfusion therapy underwent a partial exchange transfusion to achieve a fraction of Hb S = 30%. All patients who were seronegative for cytomegalovirus received cytomegalovirus-antibody negative screened blood products. Patients also received prophylactic intravenous broad-spectrum antibiotics after transplantation and oral penicillin for at least 2 years or longer until splenic recovery was documented by liver-spleen radionuclide scan after transplantation.
In response to an apparent increased incidence of neurologic complications after transplantation,15 16 the following guidelines were employed since June 1993: anticonvulsant prophylaxis with phenytoin initiated with BU dosing and continued for 6 months following transplantation (or until CSP was discontinued), strict control of hypertension, prompt repletion of magnesium deficiency, and maintenance of hemoglobin concentrations between 9-11 gm/dL and platelet counts more than 50 000/μL.
Late effects evaluation
All 26 long-term survivors had late effects evaluations performed. This cohort included 4 patients who had graft rejection accompanied by return of sickle cell disease and 2 patients with stable mixed chimerism. Engrafted patients were defined as having full donor chimerism or having stable persistence of donor cells sufficient to eliminate complications of sickle cell disease. As reported earlier, a minority of donor cells was sufficient to eliminate the anemia and clinical complications of sickle cell disease in one patient who had stable mixed chimerism.3 10 Cerebral magnetic resonance imaging (MRI) and magnetic resonance angiography examinations were requested in all patients before transplantation as well as at 1 and 2 years after transplantation. Similarly, pulmonary function tests (total lung capacity, forced vital capacity, residual volume, and the ratio of forced expiratory volume to forced vital capacity), endocrine function tests (thyroid function tests and luteinizing hormone [LH], follicle stimulating hormone [FSH], and estradiol or testosterone assays in serum) were measured before and at annual intervals after transplantation.
Pulmonary function testing was based on common methods for comparison of reference values.17 Actual lung volumes were compared with predicted values from age- and sex-matched controls to generate a percentage of the predicted value for each subject. The staging criteria for sickle pulmonary disease described by Powars et al12 was used for grading restrictive pulmonary disease. Mild restrictive pulmonary disease was defined as 80% of predicted normal, or 1 standard deviation (SD) below normal, moderate was defined as 60% of predicted normal, or 2 SD below normal, and severe was defined as 40% of predicted normal, or 3 SD below normal.
Height was measured before transplantation and annually. Height determinations were converted to the number of standard deviations from the age- and sex-adjusted norms to give height standard deviation scores (SDS). These were calculated as the difference between the 90th and 10th percentiles for a given age and gender, divided by 2.56 (GrowTrak; Genentech, Inc, South San Francisco, CA).18Quality of life was evaluated by using the Lansky Play Performance Scale or the Karnofsky performance score results reported by parent/guardians or physicians on an annual basis.19
Statistical analysis
Statistical analyses were performed to summarize results. The method of Kaplan and Meier was used to estimate survival and event-free survival (where an event was defined as death, graft rejection, or return of sickle cell disease).20 A cumulative incidence curve for graft rejection was also calculated.21 Event-free survival was defined as survival in the absence of clinical vaso-occlusive complications typical of sickle cell disease.
Results
Outcome after transplantation
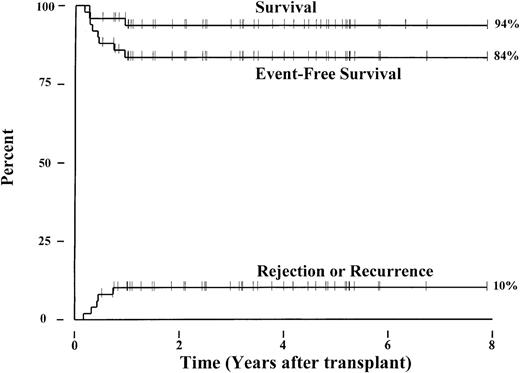
Forty-eight patients with sickle cell anemia (hemoglobin S/S), one with sickle β+-thalassemia, and one with sickle/O-Arab disease from 24 transplant centers in the United States and Europe have been enrolled in this collaborative study. The patients received matched sibling marrow allografts between September 1991 and March 1999 and ranged in age from 3.3 to 15.9 (median, 9.9) years. Currently, 47 of the 50 patients survive a median of 38.6 (range, 6.1-94.8) months following transplantation. Three patients died of intracranial hemorrhage (n = 1) or of complications from chronic GVHD (n = 2). Five patients experienced graft rejection and recurrent sickle cell disease a median of 5.1 (range, 2.1-8.9) months after transplantation. The 6-year Kaplan-Meier probabilities of survival and event-free survival for the 50 patients were 94% and 84%, respectively (Figure1). The cumulative incidence of graft rejection or sickle cell disease recurrence was 10%. Four patients developed persistent stable mixed donor-host chimerism after transplantation that correlated with resolution of sickle cell disease-related symptoms and anemia. Thus, among the 47 surviving patients, 5 have recurrent sickle cell disease, 4 have stable mixed chimerism, and 38 have full donor chimerism.
Outcome after transplantation for 50 children with advanced, symptomatic sickle cell disease.
Kaplan-Meier estimates for survival and event-free survival following marrow transplantation are shown. An event is defined as death, graft rejection, or recurrence of sickle cell disease. A cumulative incidence curve for graft rejection and return of sickle cell disease is also depicted.
Outcome after transplantation for 50 children with advanced, symptomatic sickle cell disease.
Kaplan-Meier estimates for survival and event-free survival following marrow transplantation are shown. An event is defined as death, graft rejection, or recurrence of sickle cell disease. A cumulative incidence curve for graft rejection and return of sickle cell disease is also depicted.
Growth and development after transplantation.
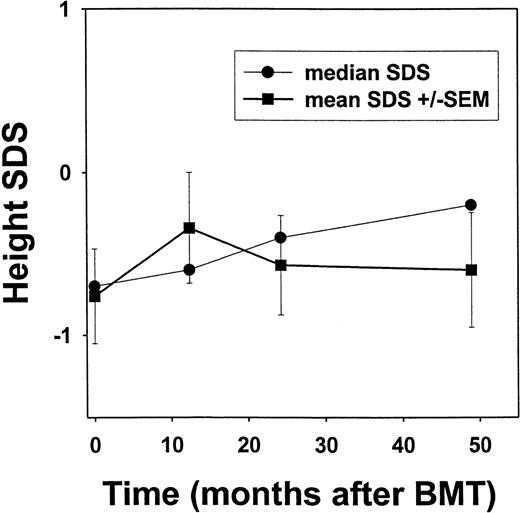
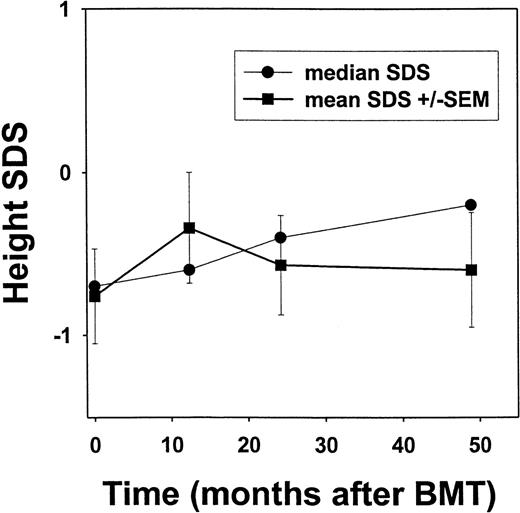
Of the 50 patients enrolled in this investigation, 26 surviving children who were treated between September 1991 and May 1996 and had a median follow-up of 56.6 (range, 32-80) months were evaluated for endocrine function and growth after myeloablative transplant conditioning. Currently, this group of patients is 6.9-19.2 (median, 14.7) years of age. All had normal thyroid function after transplantation (data not shown). Mean and median SDS scores were calculated at annual time points after transplantation and are shown in Figure 2.18 The median SDS score was −0.7 before transplantation, improved to −0.4 by 24 months after transplantation, and was −0.2 after 48 months, although there was no significant change in the mean SDS scores for the cohort.
Height standard deviation scores (SDS) after bone marrow transplantation for sickle cell disease.
Linear growth among individuals after transplantation for sickle cell disease is depicted. Height determinations were converted to the corresponding number of standard deviations from age- and sex-adjusted norms.18 By using such SDS, a child with average height would have a SDS of zero. Error bars represent standard error of the mean (SEM).
Height standard deviation scores (SDS) after bone marrow transplantation for sickle cell disease.
Linear growth among individuals after transplantation for sickle cell disease is depicted. Height determinations were converted to the corresponding number of standard deviations from age- and sex-adjusted norms.18 By using such SDS, a child with average height would have a SDS of zero. Error bars represent standard error of the mean (SEM).
Gonadotropin and sex hormone levels of patients who are currently more than 13 years of age are presented in Table1. Among seven females, five had primary amenorrhea and five had elevated LH and FSH levels that were associated with decreased serum estradiol levels in four. One individual receiving hormonal replacement therapy had elevated LH and FSH levels and a normal serum estradiol (patient 1). One postpubertal female (patient 12) had normal serum FSH and estradiol levels. Of seven males who are currently more than 13 years of age, none of four tested had elevated serum LH/FSH levels. However, one male who was 16 years of age had a low testosterone level that was correlated with gonadotropin levels in the prepubertal range.
Evaluation of central nervous system (CNS) disease after transplantation.
All 26 evaluable survivors in the late effects cohort had CNS assessments performed. This group included 22 patients with stable engraftment of donor cells and 4 patients who had graft rejection with return of sickle cell disease after transplantation. Before transplantation, 19 patients of the 26 had evidence of CNS abnormalities that were related to sickle cell disease. Thirteen had stroke and were receiving regular red blood cell transfusions, one had a transient ischemic attack, four had evidence of cerebral infarction by cerebral MRI that was clinically silent, and one patient had an elevated cerebral arterial velocity, as measured by transcranial ultrasonography (see Table 2). After transplantation, 3 of 13 patients with prior stroke developed graft rejection accompanied by return of sickle cell disease (patients 3, 19, 20). One patient with graft rejection had a second stroke when the sickle hemoglobin fraction reached 60%, as described earlier.3 Two of the three patients resumed regular red blood cell transfusions after allograft rejection.
After transplantation, most patients had cerebral MRI scans performed, and all patients were followed for significant CNS events (Table 2). Of 10 engrafted patients with prior stroke, none had a stroke after transplantation, and all had stable or improved MRI scans. This included one patient with persistent mixed donor-host hematopoietic chimerism (patient 13) who had old lesions that were more evident on a MRI scan obtained more than 5 years after transplantation. Of four patients with silent cerebral infarction, none had a clinical stroke after transplantation. Three of these patients were studied after transplantation, and two had a stable cerebral MRI scan. A third patient (patient 18), who had been studied 3 months before transplantation, developed new MRI lesions 1 month after transplantation, but subsequent annual studies have remained stable. It is likely that this patient had progression of CNS disease before transplantation. The remaining eight engrafted patients, which included five who had normal pretransplant cerebral MRI scans, did not have strokes after transplantation. In addition, all the patients who were studied after transplantation (3 of 8) had normal cerebral MRI scans. Thus, none of the 22 engrafted patients had significant sickle cell-related neurologic events after transplantation, and most had stabilization of underlying cerebral vasculopathy.
As reported earlier, patients with prior stroke were at increased risk of transplant-related neurologic events.15,16 22 Four of seven initial patients had events including two episodes of intracranial hemorrhage, which led to incorporation of measures to prevent CNS complications after transplantation. Since implementing these measures, no patient suffered intracranial hemorrhage after transplantation; however, 9 of 43 subsequent patients (21%) developed seizures. Seven of the nine patients with seizures had evidence of underlying cerebral vasculopathy before transplantation, and the seizures often occurred while patients were hypertensive and receiving CSP. In two of these nine patients, seizures were associated with transient cerebral cortical changes from CSP or foscarnet toxicities.
Pulmonary function after transplantation.
The results of pulmonary function testing are summarized in Table3. Patient 9 who died of obliterative bronchiolitis had changes consistent with obstructive pulmonary disease after transplantation (not shown). Twenty-one of 26 surviving patients had stable or normal pulmonary function documented after transplantation. Two patients (patients 5 and 16) who did not have chronic GVHD had worsening restrictive pulmonary disease documented after transplantation (see Table 4), and one patient, who had no baseline examination, also had restrictive pulmonary disease after transplantation. Two patients had no posttransplant data submitted for analysis. We found that among eight patients transplanted for recurrent episodes of acute chest syndrome, seven had stable pulmonary function after transplantation. None of the patients experienced new episodes of acute chest syndrome. Together, these data show that engrafted patients no longer experience acute chest syndrome, and, in the majority of patients, pulmonary function remained stable during this period of follow-up.
Quality of life
One patient with grade III acute GVHD died from complications of chronic GVHD. Three of the remaining 26 patients in the late effects cohort developed grade I to III acute GVHD after transplant, and two had chronic GVHD. Among individuals with engraftment of donor cells, all but one have Karnofsy or Lansky Play Performance Scores19 of 100%. None of the engrafted patients had recurrence of vaso-occlusive crises after transplantation.
Discussion
This report updates the results of our multicenter investigation of marrow transplantation for sickle cell disease and shows that the probabilities of survival and event-free survival are currently 94% and 84%, respectively. Thus, we confirm and extend the results from European transplant centers where survival and event-free survival were 93% and 82%, respectively, from Belgian transplant sites, and 92% and 75%, respectively, from French sites.9 11 In the aggregate these published data, which account for 123 patients, demonstrate that stem cell transplantation successfully replaces sickle erythrocytes with normal donor red blood cells in most patients who receive sibling allografts.
Questions that remain have to do with the quality of survival and with the effect of donor erythropoiesis on preexisting vasculopathy caused by sickle cell disease; in other words, it has not yet been well documented that cures have been achieved in those who survive free from sickle cell disease. Accordingly, the focus of this study was to describe the late effects of stem cell transplantation in surviving patients and to follow serial studies of pulmonary function and CNS imaging as representative organs for damage from sickle vasculopathy. We observed stabilization of pulmonary function in 21 of 23 evaluable patients and stable CNS vasculopathy in all 22 patients with stable engraftment of donor cells. We also found that modest growth was sustained after transplantation, although there was considerable gonadal toxicity from BU, particularly among prepubertal females. Thus, on the basis of results during this period of follow-up, it appears that, among patients with stable engraftment of donor hematopoietic cells, clinical and subclinical manifestations of sickle cell disease are arrested or eradicated after transplantation. However, a longer period of follow-up in a larger cohort of patients will be required to confirm these preliminary observations.
We observed little change in the patients' mean SDS height after transplantation, although median SDS scores increased. There are several factors that might confound our ability to judge the impact of pretransplant conditioning on subsequent growth. These factors include delays in growth that are characteristic of sickle cell disease and transfusional iron overload.23,24 In a regression analysis of cross-sectional data from the Cooperative Study of Sickle Cell Disease and in longitudinal data from a sickle cell cohort in Jamaica, delays in growth and the adolescent growth spurt were documented, although normal adult heights were ultimately achieved.25,26 Thus, it is possible that a longer period of follow-up will be required to assess the full impact of transplantation on growth. However, on the basis of long-term data from pediatric patients with leukemia who received myeloablative doses of BU and CY, significant growth impairment stemming from this preparative regimen is not anticipated.27-30
Gonadal toxicity following high-dose therapy with alkylators has been well documented.31 In one series, this was correlated with impaired ovarian function in which only 1 of 73 postpubertal women had return of normal gonadotropin and estradiol levels and menstruation.32 In prepubertal girls, the outcome remains less certain. In another smaller series, three of six evaluable girls had normal development and gonatotropin and estradiol levels.30 In comparison, of 26 females with β-thalassemia major who were ages 3.6 to 14.5 years before transplantation, 24 had elevated gonadotropin levels 1-9 years after transplantation and 12 with no or arrested pubertal development received hormonal replacement therapy.33 Thus, the adverse effects of iron overload and alkylators on gonadal function might be amplified by exposures to both in combination.34 Although it is difficult still to predict the final outcome in our sickle cell cohort, it is likely that many of the females will require replacement therapy. In males, BU is toxic to the germinal epithelium of testes, causing Sertoli cell damage and azoospermia.35,36 In a series of leukemia patients from Seattle, 8 of 46 males experienced return of testicular function after transplantation with BU and CY, and among 11 evaluable prepubertal boys, 8 had normal pubertal development after transplantation with normal gonadotropin levels.32 There was preservation of testicular function in some ex-thalassemic males after transplantation in which 4 of 12 Tanner stage 1 and 9 of 12 Tanner stage II-V boys had elevated gonadotropin levels, suggesting that some patients will pass through puberty despite gonadal dysfunction.33 Still, efforts to identify nonmyeloablative transplant regimens as a strategy to reduce gonadal and other regimen-related toxicity appear well founded.37
A longer period of follow-up will be necessary to fully describe the natural history of stroke after transplantation. Even with regular transfusions, the prevalence of recurrent stroke in patients with sickle cell disease without transplant may reach 13%, as reported in one multicenter retrospective analysis.38 Moreover, two patients in this cohort who died of intracranial hemorrhage had HbS fractions <30%, raising the possibility that chronic transfusions did not protect against this frequent late complication of CNS vasculopathy. We have speculated that preexisting cerebral vasculopathy may predispose stroke patients to posttransplant neurologic complications.15 Although it is encouraging to note that no episodes of intracranial hemorrhage have been observed since instituting preventive measures, a longer period of follow-up will be required to determine if transplantation is superior to regular transfusions in preventing intracranial hemorrhage. Similarly, additional follow-up will be required to assess the effect of transplantation on pulmonary function. Although in most patients little change in pulmonary function was observed, it is likely that minimal change would be expected over this short interval.12 What can be concluded is that there does not appear to be a significant effect of BU toxicity on lung function after transplantation for sickle cell disease.
Results of transplantation from European and US centers show that sickle cell disease is eliminated in 75% to 84% of patients.39 After transplantation, the quality of life among patients with stable engraftment of donor cells, as measured by the Lansky Play Performance Scale or the Karnofsky Performance Scale, was good, with all but one patient reporting 100% scores, and the sickle vasculopathy was stabilized in most patients. These encouraging results challenge us to determine the appropriate role and eligibility for transplantation amid other therapies such as chronic red blood cell transfusions and pharmacological interventions. On the basis of current findings, it appears appropriate now to consider comparative clinical trials that document short- and long-term effects of each of these treatments of sickle cell disease.
Acknowledgments
The authors thank the data managers, nurses, and physicians from the sickle cell and transplantation centers for their participation in this study. We also thank Dr. Helena Mishoe and members of the Data and Safety Monitoring Board from the National Heart, Lung and Blood Institute for their suggestions and assistance.
The following investigators and centers participated in this collaborative study: Atlanta, GA (JR Eckman, A Yeager, R Vega, A Ogden, L Hsu, T Adamkiewicz, Emory University); Birmingham, UK (PJ Darbyshire); Boston, MA (J Antin, E Guinan, O Platt, Dana Farber Cancer Institute and The Children's Hospital, Harvard University; L McMahon, Boston Comprehensive Sickle Cell Center); Bronx, NY (L Benjamin, R Nagel, Montefiore Medical Center, Albert Einstein College of Medicine); Brooklyn, NY (L Guarini, Interfaith Medical Center; K Viswanathan, Brookdale Hospital; S Sadananda, Brooklyn Hospital Center); Chapel Hill, NC (R Redding-Lallinger, J Wiley, E Orringer); Cleveland, OH (M Nieder, Rainbow Babies Hospital, Case Western University); Creteil, France (F Bernaudin, G Souillet, Hospital Henri Mondor); Dallas, TX (GR Buchanan, ZR Rogers, V Aquino, University of Texas Southwestern Medical Center at Dallas); Denver, CO (R Giller, PA Lane, University of Colorado); Detroit, MI (I Sarnaik, P Swerdlow, J Levine, Wayne State University); Durham, NC (M Telen, T Kinney, J Kurtzberg, R Ware, KM Sullivan, Duke University Medical Center); Fort Worth, TX (G Eames, D Friedman); Gainesville, FL (A Kedar, J Wingard, University of Florida); Houston, TX (KW Chan, University of Texas; R Krance, H Heslop, Baylor College of Medicine); London, UK (SC Davies, I Dokal, I Roberts, Royal Postgraduate Medical School); Los Angeles, CA (R Parkman, D Powars, N Kapoor, W-Y Wong, T Coates, University of Southern California; A Thompson, S Feig, University of California at Los Angeles); Miami, FL (AS Wayne, T Harrington, C Pegelow, University of Miami); Milwaukee, WI (B Camitta, D Margolis, JP Scott, Medical College of Wisconsin); Minneapolis, MN (P Orchard, S Nelson, J Wagner, University of Minnesota); New Haven, CT (B Sleight, J Rappeport, Yale University); Montreal, Quebec (H Hume, Hospital Ste-Justine); New Orleans, LA (L Yu, R Veith, Louisiana State University; C Scher, Tulane Medical Center); New York, NY (R Bellevue, NY Methodist Hospital; F Blei NYU Medical Center; J Lipton, Mt Sinai Medical Center; D Wethers, St Luke's Roosevelt Hospital; S Piomelli, Columbia University); Oakland, CA (MC Walters, L Styles, E Vichinsky, Children's Hospital of Oakland); Philadelphia, PA (K Ohene-Frempong, K Smith-Whitley, N Bunin, University of Pennsylvania); Pittsburgh, PA (J Hord); St Louis, MO (D Wall, A Chu, St Louis University, S Shenoy, R Hayashi, Washington University); Rochester, NY (R Duerst, University of Rochester); St Petersburg, FL (M Klemperer, University of S Florida); Sao Paolo, Brazil (C Bonfim, Federal University of Parana; S Brandalise, R Pasquini, University of Campinas); San Francisco, CA (WC Mentzer, M Cowan, University of California, San Francisco); St Augustin, Germany (R Dickerhoff, T Klingebiel, University of Bonn); Seattle, WA (R Storb, A Woolfrey, JE Sanders, Fred Hutchinson Cancer Research Center and the University of Washington); Stanford, CA (M Amylon, B Glader, Stanford University); Tucson, AZ (ML Graham, University of Arizona); and Washington, DC (P Dinndorf, E Bayever, O Castro, Children's Hospital National Medical Center, George Washington University, and Howard University).
Supported in part by National Institutes of Health grants HL36444 and CA15704.
Reprints:Keith M. Sullivan, Division of Medical Oncology and Transplantation, DUMC 3476, Duke University Medical Center, Durham, NC 27710; e-mail: sulli025@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.