Abstract
A significant number of patients who relapse after allogeneic stem cell transplantation (SCT) for chronic myeloid leukemia (CML) will achieve sustained remissions after treatment with interferon-, second transplants, or donor lymphocyte infusions (DLI) from the original stem cell donor. Because leukemia-free survival (LFS) is at present defined as survival without evidence of relapse at any time posttransplant, patients who relapse but are then restored to complete remission are treated as failures when estimating LFS. We have established a new category of LFS, termed current LFS (CLFS), which we define as survival without evidence of leukemia at the time of most recent assessment. To gauge the contribution of treatment for relapse to the efficacy of allogeneic SCT in the management of CML in chronic phase, we compared conventional LFS and CLFS in 189 consecutive patients who underwent SCT over a 7-year period with a minimum follow-up of 3 years. Patients with sibling donors (n = 111) received cyclosporine and methotrexate as prophylaxis for graft versus host disease; patients with unrelated donors (n = 78) also received Campath-1G or 1H as intravenous T-cell depletion. The 5-year LFS defined conventionally was 36% (CI: 29% to 43%) versus a 5-year CLFS of 49% (CI: 36% to 62%). This new method of defining LFS confirms the view that appropriate “salvage” therapy, principally DLI, makes a major contribution to the capacity of allogeneic SCT to produce long-term LFS in patients who receive SCT for CML and emphasizes the importance of redefining LFS to take account of successful treatment of relapse.
Allogeneic hematopoietic stem cell transplantation (SCT) can cure a substantial number of eligible patients with chronic myeloid leukemia (CML), and disease-free survival rates between 45% and 80% have been achieved in patients with CML allografted in chronic phase (CP) with hematopoietic stem cells from HLA-matched siblings or unrelated volunteers.1-5 One of the major causes of treatment failure is relapse, which occurs in 10% to 30% of patients who receive unmanipulated donor marrow cells but is considerably more frequent in those who receive marrow stem cells that have been depleted of T cells in an effort to minimize or eliminate graft versus host disease (GVHD).6-8 Patients who relapse may be treated with interferon-α (IFN-α),9 by second stem cell transplants,10,11 or by infusion of lymphocytes harvested from the original stem cell donor. The use of such donor lymphocyte infusions (DLI) was reported first in 1990,12 but many subsequent studies have confirmed their efficacy.13-17 The remissions achieved seem to be durable,18 and the approach has become standard therapy for CML in relapse in many but not all transplant centers.
However, conventional measures of outcome after transplantation do not reflect the contribution of salvage therapy, including DLI, to the overall effectiveness of allogeneic transplantation.19,20At present, clinical results are reported on the basis of overall survival (OS) and leukemia-free survival (LFS). LFS is defined as survival in the absence of leukemic relapse after transplantation and, consequently, patients who relapse but achieve lasting remission after DLI are classified as treatment failures. We have established a new category of LFS after allogeneic SCT, which we call current LFS (CLFS). CLFS estimates the probability that a patient is alive in the original remission, or in a subsequent remission after treatment for relapse, at a given time after transplantation.21 By reclassifying patients allografted for CML on the basis of whether they satisfy criteria for conventional LFS or CLFS, salvage therapy can be shown to increase the ability of allogeneic transplantation to produce long-term disease-free survival in patients with CML by approximately 10% to 15%, an effect that would not have been detected using conventional measures of outcome.
Patients and methods
We reviewed clinical data on 200 consecutive patients with Philadelphia (Ph) chromosome–positive or Ph-negative BCR-ABL positive CML in CP who underwent allogeneic SCT at the Hammersmith Hospital in London between January 1, 1989, and December 31, 1995. Patients fulfilled the criteria for CP as previously described.22 Eleven patients who failed to engraft and received autologous stem cells were omitted from further analysis.
Transplant procedure
The details of the 189 patients included in this study are shown in Table 1. One hundred eleven patients underwent transplantation from HLA identical siblings and were conditioned with cyclophosphamide (120 mg/kg) and total body irradiation (TBI) (1320 cGy in 6 fractions). Seventy-eight patients received bone marrow cells from volunteer unrelated donors (VUD) and were conditioned with cyclophosphamide (120 mg/kg) and TBI (1200 to 1440 cGy in 6 fractions). Some patients also received busulfan, daunorubicin, thiotepa, or splenic irradiation. Of the 78 unrelated donors, 75 were serologically matched at the HLA A, B, and DR loci. Three of the unrelated donors were mismatched at a serologic level, two at HLA DR and one at HLA A and B. Cyclosporine and methotrexate were used as GVHD prophylaxis in all patients. In addition, HLA-identical sibling transplant recipients older than 45 and all but three VUD transplant recipients received additional T-cell depletion by intravenous administration of Campath 1G or 1H, as previously described.23
Posttransplant monitoring and definition of relapse
After transplantation, patients were monitored with monthly full blood counts. In patients who received transplants early in this series, samples of bone marrow were aspirated regularly for cytogenetic examination. In recent years, peripheral blood samples were studied 3 to 6 months after transplant for the presence of BCR-ABL transcripts using a semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) assay.24 Annual monitoring was commenced 5 years after transplantation. The frequency of cytogenetic and PCR monitoring after transplantation was similar in the sibling and VUD transplants. Relapse was defined as molecular, cytogenetic, or hematologic. Molecular relapse was diagnosed when, over a period of at least 4 weeks, a patient had (1) three consecutive samples with a BCR-ABL/ABL ratio greater than 0.02%, or (2) three samples with a rising ratio, the last two greater than 0.02%, or (3) two samples greater than 0.05%25; such patients had no detectable Ph-positive marrow metaphases. In patients in whom serial molecular data were not available, relapse was defined by cytogenetic or hematologic criteria. Cytogenetic relapse was considered to be present if one or more Ph-positive metaphases were detected without evidence of hematologic relapse. Hematologic relapse was defined as peripheral blood leukocytosis, usually with the presence of myelocytes, basophilia, and an excess of neutrophils in the differential count. This was accompanied by a hypercellular bone marrow and Ph-positive metaphases on cytogenetic analysis.
We did not routinely test patients for persistent PCR positivity immediately after SCT, and so we cannot discount the possibility that a minority of patients did not achieve a molecular remission after transplant. However, in light of our previous clinical experience and in common with other groups, we have assumed that molecular remission was achieved by all patients in the first few months after transplantation, and so all our statistical analyses have been based on this assumption.
Management of relapse
At diagnosis of relapse, cyclosporine was discontinued if the patient was still receiving it. Provided the patient's disease had not progressed beyond CP, plans were then made to administer DLI. DLI were administered in accordance with a “single bulk dose” or in accordance with a predetermined “escalating dose regimen.”26 The total number of CD3+ cells ranged from 1 × 107 to 1 × 108/kg administered on one or more occasions. Two patients received a second SCT. No patient in this series was treated for relapse primarily with IFN-α alone.
Definition of response
Patients were defined as responders to DLI or second SCT if subsequent RT-PCR analysis for the presence of BCR-ABL transcripts was negative on 2 occasions. RT-PCR analysis was performed at a minimum of 3 months after lymphocyte infusion. Patients who demonstrated persistence of BCR-ABL transcripts at 12 months or later were classified as nonresponders.
Definition of leukemia-free survival and statistical methods
Survival and leukemia-free survival probabilities may be calculated using standard actuarial methods such as Kaplan and Meier.19 Using this approach, OS is defined as survival regardless of leukemic status after SCT, and conventional LFS as survival without evidence of molecular relapse at any time after SCT. (If molecular data were not available, relapse was defined by cytogenetic or hematologic criteria.) Patients are considered as “events” for LFS at the time of relapse or death in continuing complete remission. However, both measures of outcome may provide an inaccurate assessment of transplantation effectiveness. Firstly, although OS is a surrogate measure of treatment failure in acute leukemias, where patients are likely to die soon after relapse, it does not accurately reflect events in patients allografted for chronic leukemias, where patients who relapse may still experience prolonged survival despite the presence of active disease. Secondly, conventional measures of LFS fail to take account of the capacity of DLI to produce durable molecular remissions in patients who relapse, thus significantly underestimating the ability of allografting to produce sustained disease-free survival even in patients who have relapsed. We have therefore developed a statistical method that provides an estimate of the probability that a patient is alive and not in relapse at the most recent time of assessment. We have called this probability Current Leukemia-Free Survival (CLFS).
In this paper we present a multistate Markov model that allows accurate estimation of CLFS after transplantation. This probability cannot be estimated by modifying the Kaplan-Meier statistic by only taking into account the patient's status at the last visit, as previously proposed,27 for 3 reasons. Firstly, such an approach requires anticipation of future events in order to determine the patient's eventual outcome, violating the mathematical principle on which the Kaplan-Meier curve is derived. Secondly, calculated survival curves are nonincreasing, but the probability of being alive in remission should decrease when a patient relapses and increase when a second remission is achieved. Thirdly, the Kaplan-Meier estimator is built by multiplying at each event time 1 minus the probability of experiencing the event among those who could experience the event. This probability is expressed as 1 minus the number of events at this time divided by the number at risk. The denominator constructed in this way includes patients in relapse who are not at risk of the event and is therefore larger than it should be, with the result that CLFS is overestimated.
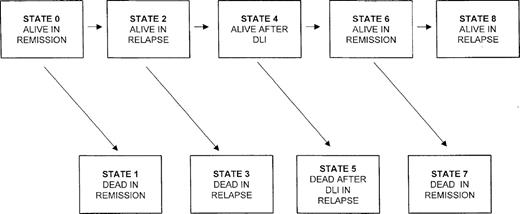
The model we present is based on a continuous time Markov process that can be used to model any physical or biologic system where the rate of transition from one state of the system to the other depends only on the current state of the system and not on the timing of when the system entered the current state. In our application there are 9 possible states that the system can be in after SCT (Figure1). The rates of transition from one state to the next are estimated directly from the data, with at least 1 patient being required to make each of the transitions. With very small numbers of patients making each transition, the standard error of the CLFS will be quite large. In our experience, reasonable standard error estimates can be obtained when there are as few as 10 patients making each type of transition. The estimated transition rate from i to j (from one state to the next; eg, from state 2 to state 3) at any time is the observed number of transitions from state i into j divided by the number who were at risk for this transition at time t. Censored data, due to different length of follow-up or due to patients being lost to follow-up, are handled as in the Kaplan-Meier estimator by modifying the risk sets for the transitions. The various transition rates are then combined to provide estimates that the patient will be in any of the 9 states after transplant. The CLFS is the sum of the probabilities that the patient is in state 0 or 6 at time t. Thus, in contrast to conventional measures of LFS, CLFS will increase if patients who have relapsed achieve a second remission after salvage therapy. It should be noted that in this model the estimated probability of being in state 0 is precisely the same as the conventional LFS and that the probability of being in state 6 is the probability that a patient who has relapsed will be in second remission. This latter probability will increase whenever a patient achieves a second remission and decrease whenever a patient in second remission dies or has a second relapse. If there is no censoring, then the CLFS estimate reduces to the proportion of patients in either a first or second remission at time t. Details of calculations of standard errors of these estimated probabilities are found in an earlier paper.21
Markov model showing possible sequence of events for patients who relapse after allografting for CML and receive DLI.
Markov model showing possible sequence of events for patients who relapse after allografting for CML and receive DLI.
Results
Relapse
Sixty patients satisfied our criteria for relapse during the period of observation. Of these, 27 had only molecular evidence of relapse, 21 were already in cytogenetic relapse, and 12 had hematologic evidence of relapse (Table 1). Of these last 12, 5 relapsed to CP disease and 7 relapsed in an advanced (accelerated or blastic) phase.
Results of treatment for relapse
Forty-eight patients were treated with DLI according to a bulk dose or escalating dose regimen.26 The median interval from diagnosis of relapse to institution of treatment with DLI was 10 months (range, 1 to 54 months). Thus, 22 of the 27 patients whose relapse was diagnosed at the molecular level had progressed to cytogenetic or hematologic relapse by the time DLI were started (Table 1). Thirty-three (69%) of the 48 patients achieved molecular remission after treatment with DLI (16 sibling and 17 VUD, Table1). Two of the nonresponders received a second SCT, and 1 survives at the time of analysis. Twelve of the 60 patients who relapsed did not receive DLI for a variety of reasons. Six patients relapsed with advanced-phase disease, of whom 3 had localized extramedullary blast cell deposits; for 3 patients the original transplant donor was no longer available, and 2 patients refused DLI. These 11 patients received a variety of different treatments, including IFN-α, hydroxyurea, and cytotoxic drug combinations. The twelfth patient received a second allo-SCT. Nine of these 12 patients died; leukemia was the primary cause of death in 8 cases and pneumonitis in 1 case.
Survival
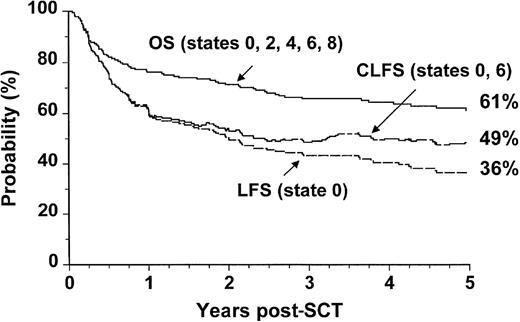
Survival for patients undergoing allogeneic SCT during the 7-year period studied is summarized in Table 2. The 5-year probability of OS was 61% (confidence interval [CI]: 54% to 68%). The conventionally defined 5-year probability of LFS was 36% (CI: 29% to 43%). When patients who relapsed but subsequently achieved durable molecular remissions were reclassified as leukemia-free survivors, the revised figure, designated CLFS, was 49% (CI: 36% to 62%) (Figure 2).
Outcome probabilities following allogeneic SCT for CML in first chronic phase showing OS, LFS, and CLFS.
The states refer to those presented in Figure 1.
Outcome probabilities following allogeneic SCT for CML in first chronic phase showing OS, LFS, and CLFS.
The states refer to those presented in Figure 1.
Difference in outcome after transplantation between sibling and VUD allografts
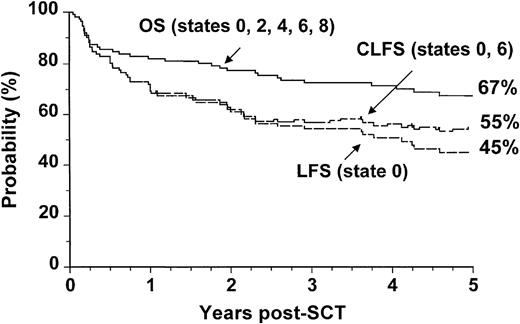
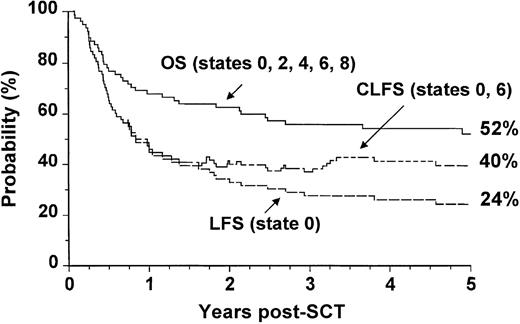
We analyzed separately the OS, LFS, and CLFS for sibling donor and VUD allografts. In patients who had undergone allogeneic SCT from HLA-matched sibling donors, the 5-year OS was 67% (CI: 57% to 75%), and the CLFS was 10% higher than the LFS (Figure3). In the 78 patients who had undergone VUD transplantation, the OS was 52% (CI: 41% to 63%), and the CLFS was 16% higher than the LFS (Figure4).
Outcome probabilities following allogeneic SCT for CML in first chronic phase using an HLA-identical sibling donor showing OS, LFS, andCLFS.
The states refer to those presented in Figure 1.
Outcome probabilities following allogeneic SCT for CML in first chronic phase using an HLA-identical sibling donor showing OS, LFS, andCLFS.
The states refer to those presented in Figure 1.
Outcome probabilities following allogeneic SCT for CML in first chronic phase using a volunteer unrelated donor showing OS, LFS, and CLFS.
The states refer to those presented in Figure 1.
Outcome probabilities following allogeneic SCT for CML in first chronic phase using a volunteer unrelated donor showing OS, LFS, and CLFS.
The states refer to those presented in Figure 1.
Discussion
There are various treatment options for patients who relapse after allografting, including IFN-α18 and second transplants,19,20 but their management has been transformed by the demonstration in 1990 that the infusion of lymphocytes from the original donor could reinduce remission.12 A series of further studies confirmed that remissions could be obtained in up to 70% of patients.13-17 Such remissions are more likely to be achieved if DLI were administered while the patient was still in molecular or cytogenetic relapse as opposed to hematologic relapse.28 The remissions usually proved to be durable. There were, however, 2 principal complications of DLI, namely the occurrence of marrow failure and the induction of GVHD, which could, on occasion, prove fatal. The first complication appears to be rare in patients who still retain some evidence of donor-derived hematopoiesis at the time for DLI – a further argument in favor of “early” use of DLI. For the second complication, there are various possible approaches. For example, the depletion of CD8 cells from the donor inoculum has proved useful in preventing GVHD after DLI.29The Sloan Kettering group has pioneered the administration of donor lymphocytes on an escalating dose schedule, starting at a low dosage and repeating the infusion at incremental doses at defined intervals until a response is achieved.30 We have recently confirmed the superiority of this approach in comparison with the administration as a single dose at relatively high cell numbers.26
These various observations have led to a need to reappraise the role of T-cell depletion in allografts for CML in CP. Sehn and colleagues31 recently compared clinical results in 46 patients who received T-cell–depleted allografts and 40 patients who received T-cell replete allografts for CML. The relapse rate for the recipients of T-cell depletion (TCD) was significantly higher than that for recipients of T-replete marrow cells, but there was no difference in overall survival at 5 years. Moreover, current leukemia-free survival calculated by the modified Kaplan-Meier method was identical for the 2 groups. More recently, Drobyski and colleagues32reported results of treating 25 CML patients by allografting with marrow cells depleted of T-cells ex vivo. They administered adjunctive DLI to the 14 patients (56%) who relapsed and obtained complete responses in 12 patients (86%). The overall 5-year survival was 80% for the entire patient population. If TCD is to be used with increasing frequency in primary transplants for patients with CML, and this seems especially probable if the patient is to receive blood-derived stem cells, an appropriate method of expressing leukemia-free survival is required.
At present, the results of allogeneic SCT are routinely based on calculation of OS and LFS after transplantation using Kaplan-Meier analyses. Neither measure of outcome is ideal in diseases in which an effective salvage strategy is available. OS does not differentiate between patients alive in remission and those in relapse. Because LFS is conventionally defined as survival without evidence of relapse at any time after transplantation, patients who relapse but subsequently respond to DLI are regarded as treatment failures. Therefore, the ability of DLI to produce sustained molecular remission after relapse is ignored and the efficacy of allogeneic SCT in producing long-term LFS in CML is underestimated. In this study, we have redefined LFS after allogeneic transplantation so that it includes all patients who are free of leukemia at a given time after transplantation regardless of whether or not they have previously relapsed. By classifying 189 patients with CML in CP who underwent allogeneic SCT according to whether they exhibit conventional LFS or newly defined CLFS, we have demonstrated that salvage therapy, principally DLI, increases the probability of being alive and free of leukemia 5 years posttransplant by approximately 13%. This figure may be higher in patient populations treated by more effective methods for GVHD prevention, such as TCD, and lower in patients who receive standard prophylaxis for GVHD.
A truly accurate assessment of the potential impact of DLI on CLFS would require that all patients who relapse receive DLI as salvage therapy. In this series, it is likely that the actual role of DLI is underestimated, because the study was conducted when use of DLI was in its infancy and some patients who relapsed were either undertreated or not treated at all. Within any single population, the more stringent or sensitive the criteria for relapse, the lower the LFS will be and thus the greater the disparity with CLFS. The evolution of relapse after allogeneic SCT for CML usually follows a sequential pattern, being recognizable first at the molecular level, then cytogenetically, and, finally, hematologically. Nonetheless, it is sometimes difficult to decide when treatment for relapse should be instituted, and this, in part, accounts for the long interval (median, 10 months) between diagnosis of relapse and initiation of treatment reported here.
This multistate model for the definition of CLFS will also be applicable to the assessment of outcome in patients with other hematologic malignancies that respond to DLI. The magnitude of the difference between CLFS and conventional LFS will reflect the degree of responsiveness to DLI and will be particularly marked in patients allografted for diseases such as CML. It is likely that conventionally defined LFS and CLFS will be broadly similar in diseases such as acute leukemia, where the majority of patients fail to respond to DLI. Novel statistical methods such as CLFS will be required to assess outcomes after procedures such as mini-allografting, in which DLI forms a central part of therapy.33
In summary, we have devised a new statistical method for assessing leukemia-free survival after allografting that reflects the efficacy of salvage therapy in restoring remission in diseases such as CML. In diseases such as acute leukemia, in which salvage therapies are less effective and the time from relapse to death is usually short, OS and conventionally measured LFS are both accurate measures of outcome after transplantation. However, in diseases in which either the time from relapse to death is prolonged or in which an effective salvage strategy is available, both conventional LFS and OS are poor measures of the ability of allografting, with adjunctive DLI where necessary, to result in survival in the absence of leukemia. The development of a meaningful measure of CLFS provides the tools necessary for accurate measurement of leukemia-free survival in these settings.
Acknowledgment
We thank John Davis, who supervises the Stem Cell Laboratory, for many hours of assiduous work.
Supported by the Leukaemia Research Fund, London, England; the Kay Kendall Leukaemia Fund (F.vR.), London, England; and grant RO1-CA54706-07 (J.P.K.) from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Reprints:John M. Goldman, Department of Haematology, Hammersmith Hospital, Imperial College School of Medicine, Du Cane Road, London W12 0NN, England; e-mail: jgoldman@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.