Abstract
Limited expression of the amphotropic envelope receptor is a recognized barrier to efficient oncoretroviral vector–mediated gene transfer. Human hematopoietic cell lines and cord blood–derived CD34+ and CD34+, CD38− cell populations and the progenitors contained therein were transduced far more efficiently with oncoretroviral particles pseudotyped with the envelope protein of feline endogenous virus (RD114) than with conventional amphotropic vector particles. Similarly, human repopulating cells from umbilical cord blood capable of establishing hematopoiesis in immunodeficient mice were efficiently transduced with RD114-pseudotyped particles, whereas amphotropic particles were ineffective at introducing the proviral genome. After only a single exposure of CD34+ cord blood cells to RD114-pseudotyped particles, all engrafted nonobese diabetic/severe combined immunodeficiency mice (15 of 15) contained genetically modified human bone marrow cells. Human cells that were positive for enhanced green fluorescent protein represented as much as 90% of the graft. The use of RD114-pseudotyped vectors may be advantageous for therapeutic gene transfer into hematopoietic stem cells.
Introduction
The ability to transfer genes into hematopoietic stem cells would provide the opportunity for somatic gene therapy for malignant and nonmalignant disorders that affect bone marrow and peripheral blood function.1,2 Extensive efforts have been invested in adapting oncoretroviral vectors for gene transfer into stem cells. Despite considerable success in murine models, retrovirus-mediated gene transfer into human stem cells has been difficult to achieve.3-8 Recognized barriers to human stem cell gene transfer include low expression of viral receptors and the relative quiescence of this target cell population.9-11
A useful surrogate for human stem cells are primitive hematopoietic cells that are able to establish human hematopoiesis in immunodeficient mice, such as the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) strain. Human NOD/SCID repopulating cells (SRCs) can be recovered from murine bone marrow months after transplantation, as reflected by their ability to establish human hematopoiesis in secondary recipients.12-15 This quality plus the multilineage engraftment observed in the NOD/SCID model suggest that gene-transfer strategies that result in retroviral marking of a significant proportion of the progeny of SRCs would be a better predictor of stem cell gene transfer than surrogates evaluated by in vitro assays.15 Accordingly, we used umbilical cord blood SRCs as targets in our experiments designed to improve the efficiency of oncoretrovirus-mediated gene transfer into primitive human hematopoietic cells.
Oncoretroviral vectors require cell division to achieve genome integration and long-term gene expression.11 Many combinations of cytokines have been tested in experiments designed to initiate cell-cycle activation of primitive hematopoietic cells without loss of repopulating potential.16-18 Recent evidence suggests that short-term cultures with high concentrations of cytokines19 20 allow limited division and expansion of SCID repopulating cells, raising the possibility that receptor deficiency may be the major remaining barrier to stem cell–targeted gene transfer.
Most vector preparations used for gene transfer into human cells contain particles that have amphotropic specificity based on the structure of their envelope protein. Amphotropic viral particles have a broad host range that includes human cells,9 and they rely on a phosphate transporter for cell entry21-23 that is now known to be expressed at very low levels on primitive human hematopoietic cells.10 To overcome this barrier to oncoretrovirus-mediated gene transfer, researchers have generated and studied vector preparations pseudotyped with envelope proteins from other viruses.
Oncoretroviral vectors pseudotyped with the envelope protein of the gibbon ape leukemia virus (GALV) can transduce human clonogenic hematopoietic progenitor cells24 and SRCs from cord blood.25,26 The GALV receptor, also a phosphate transporter, is expressed at a somewhat higher level than the amphotropic receptor on the CD34+ population of cells from human and baboon bone marrow.21-23,27,28 The frequency of retrovirus-marked human cells achieved with the GALV-pseudotyped particles exceeds that achieved with amphotropic vector particles in the NOD/SCID model under similar transduction conditions.25,26,29-33 GALV-pseudotyped murine oncoretroviral vector particles are somewhat more efficient than amphotropic particles at transducing canine and baboon repopulating cells, but the gene-transfer efficiency, as reflected by the proportion of genetically modified peripheral blood or bone marrow cells in these myeloablated animal models, remains less than or equal to 10%.8,28,34-36 This suggests that repeated exposure to conditioned medium affects the long-term repopulating potential of transduced cells, which may be reflected in the larger animal models but not in the NOD/SCID assay.33
The G-envelope protein of vesicular stomatitis virus (VSV-G), a rhabdovirus, has been used to pseudotype both lentiviral and murine retroviral vector particles.37-41 VSV-G–pseudotyped particles can be concentrated 100- to 1000-fold by centrifugation, thereby removing the conditioned medium.42 Successful gene transfer into SRCs with concentrated VSV-G–pseudotyped oncoretroviral or lentiviral vector particles has been reported.43,44 However, only with VSV-G–pseudotyped lentiviral vector particles was the vector genome documented to be present in human cells of multiple lineages in the NOD/SCID recipients of transduced cells.44
Murine retroviral vector particles pseudotyped with the envelope protein of the feline endogenous virus, RD114, have been shown to have tropism for human hematopoietic cells.45,46 The RD114 retrovirus is a member of the large interference group 1 of retroviruses, all of which use the same receptor on human cells,47 recently identified as a neutral amino acid transporter.46 We have evaluated the use of RD114-pseudotyped oncoretroviral vector particles for transducing primitive human hematopoietic cells, including those that repopulate NOD/SCID mice.
Materials and methods
Preparation of cord blood cells
Human umbilical cord blood specimens were obtained from delivered placentas following uncomplicated births at a local delivery center. Mononuclear cell preparations were recovered by centrifugation on Histopaque-1077 (Sigma, St Louis, MO). The CD34+cells in the cord blood mononuclear cell specimens were purified using a CD34-specific magnetic cell selection system according to instructions provided by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). Two to 3 CD34+ preparations (greater than 90% purity) were pooled for each in vitro and in vivo experiment. CD34+, CD38− cells were recovered from the pooled CD34+-enriched populations by labeling with anti-CD34 (clone My10) and anti-CD38 (clone HB-7) monoclonal antibodies (Becton Dickinson, San Jose, CA) conjugated to fluorescein isothiocyanate or phycoerythrin (PE), respectively, and then sorted for the CD34+, CD38− population (less than 5% of the total CD34+ population) using a vantage flow cytometer (Becton Dickinson).
Retroviral vector preparation
Vector particles pseudotyped with the feline endogenous virus (RD114) envelope protein were derived by generating producer cells from a packaging cell line designated FLYRD18,48 which was obtained from Mary Collins (Chester Beatty Laboratories, London, UK). This packaging cell line had been derived from human sarcoma cells (HT1080) by introducing the GAG/POL genes from murine leukemia virus and the gene encoding the envelope protein of the RD114 virus. We derived producer cells from the FLYRD18 packaging cell line by introducing a vector genome that encodes the enhanced green fluorescent protein (EGFP) and a drug-resistant variant of human dihydrofolate reductase (L22Y).3,5 The reading frames for these proteins are separated by an internal ribosomal entry site and are transcribed into a bicistronic transcript under the control of the mouse stem cell virus long terminal repeat.49 This vector genome designated MGirL22Y was introduced into FLYRD18 cells in the form of vector particles pseudotyped with the VSV-G envelope protein. These particles had been generated by transfection of 293T cells, as described previously.50 Individual clones were recovered by limiting dilution, and a high-titer clone designated RD114/MGirL22Y was identified. This methodology was also used to generate an amphotropic producer cell population (AM13/MGirL22Y) beginning with FLYA13 packaging cells, a derivative of HT1080.48 Control amphotropic vector particles containing the MGirL22Y genome were derived from an amphotropic retroviral producer clone (AM/MGirL22Y) derived from PA317 packaging cells, kindly provided by Derek Persons (St Jude Children's Research Hospital, Memphis, TN). GALV-pseudotyped particles were generated by a producer clone derived from PG13 cells.51 VSV-G–pseudotyped particles were generated by a producer clone derived from 293T cells.52The capacity for vector production for all producers was determined by assaying serial dilutions of conditioned medium on human HeLa cells (HEL-ATCC TIB 180). The titers of infectious vector particles in medium conditioned by the producer clones were as follows: amphotropic, 1 to 2 × 105/mL; GALV, 1 to 2 × 105/mL; RD114, 2 to 10 × 105/mL; and VSV-G, 1 to 5 × 105/mL or 1 to 2 × 107/mL before or after ultracentrifugation, respectively.
Vector particles pseudotyped with the RD114 envelope protein were also generated in 293T cells that had been transfected with vector, helper, and envelope plasmids. For RD114-pseudotyped particles, the 293T cells were transfected with a plasmid encoding the vector genome (pMGirL22Y), a second encoding the GAG and POL proteins of murine leukemia virus (pEQPAM3-E), and a third plasmid encoding the gene for the RD114 envelope (pRDF48). Between 48 and 72 hours after transfection, conditioned medium was harvested and titered on HeLa cells. The titers ranged from 0.5 to 2 × 105 infectious vector particles per milliliter.
In vitro analysis of gene transfer
CD34+ or CD34+, CD38−purified cell populations were cultured in Iscove's modified Dulbecco's medium plus 1% bovine serum albumin, human insulin (5 μg/mL), human transferrin (100 μg/mL), low-density lipoproteins (10 μg/mL), 0.1 mmol/L β-mercaptoethanol, stem cell factor (SCF, 300 ng/mL), Flt-3 ligand (300 ng/mL), interleukin-3 (IL-3, 10 ng/mL), and IL-6 (10 ng/mL). The cytokines were obtained from R&D Systems (Minneapolis, MN). The cells were incubated for 24 hours (or as otherwise indicated) at 37°C in 5% CO2 before transduction.
Transduction of the CD34+ and CD34+, CD38− cells was performed in 48-well plates (1-2 × 104 cells per well at a concentration of 1-2 × 105 cells/mL) coated with retronectin (CH-296; Takara Shuzo, Otsu, Japan) at a concentration of 20 μg/cm2. When indicated, the retronectin-coated wells were preloaded with retroviral vector particles by placing 0.25 mL/cm2 of medium conditioned by producer cells in each well and incubating for 20 to 30 minutes at room temperature. This medium was then aspirated, and serum-free culture medium as specified above, containing CD34+ or CD34+, CD38−purified cells, was added. When transductions were performed without preloading, serum-containing medium (10% fetal calf serum [FCS]) conditioned by producer cells was added to achieve the specified multiplicity of infection (MOI) in amounts up to 40% of the initial culture volume.
After a total of 96 hours in culture, the CD34+ or CD34+, CD38− cells were washed in phosphate-buffered saline (PBS) containing 2% heat-inactivated FCS and then stained with mouse anti-CD38 (clone HB-7) and anti-CD34 (clone My10) monoclonal antibodies conjugated to PE or allophycocyanin (APC; Becton Dickinson), respectively. The cells were then washed and resuspended in PBS plus 2% FCS. Four-color flow cytometry was performed, and the data were analyzed using the Cell Quest software package (Becton Dickinson).
To assay gene transfer into clonogenic progenitors, we replated transduced and control hematopoietic cells after 96 hours of culture into Methocult GF (H4434; Stem Cell Technologies, Vancouver, BC, Canada), which had been pretreated with 1.2 U/mL thymidine phosphorylase at 37°C for 2 hours. Cultures were established with or without 100 nmol/L trimetrexate. At this concentration of trimetrexate, no colonies formed in the cultures of control, untransduced cells. Hematopoietic cells were cultured in 35-mm plates (1 mL of medium per plate) at 37°C in a 5% CO2 humidified atmosphere for 10 to 15 days, after which the colonies were enumerated.
Analysis of gene transfer into cells that establish human hematopoiesis in immunodeficient (NOD/SCID) mice
In a series of 3 experiments, purified CD34+ cells were prestimulated in medium containing SCF, Flt-3 ligand, IL-3, and IL-6, as described earlier, for 24 hours or 48 hours at a concentration of 1 to 2 × 105 cells/mL. The cells in this medium were then transferred to retronectin-coated culture plates to which amphotropic or RD114-pseudotyped vector particles had been absorbed (preloaded) or to retronectin-coated plates without virus. All cultures were diluted 2-fold with serum-free medium containing cytokines at 48 hours and harvested for injection at 96 hours. Where indicated, amphotropic vector particles were also added in the form of conditioned medium at up to 50% of the culture volume daily for 2 days to maximize transduction of CD34+ cells with this pseudotyped virus.
The NOD/SCID mice (original stocks kindly provided by M. Pallavicini, University of California, San Francisco, CA) were housed in sterile microisolator cages and supplied with sterile food, acidified water, and bedding. Each mouse was injected with 1 to 1.5 × 105freshly isolated CD34+ cells (greater than 95% purity) or after expansion of this input cell number for 96 hours in culture. Six- to 8-week-old mice were used after sublethal irradiation (325 cGy-127Cs source). The mice were killed 8 to 10 weeks after injection, and bone marrow cells were harvested for flow cytometric analysis and in vitro culture.
Bone marrow cells from animals injected with human cells were subjected to flow cytometric analysis with the use of conjugated antibodies against human surface antigens, as follows: (1) human hematopoietic cells, CD45-APC; (2) B lymphocytes, CD19-PE; and (3) myeloid cells, CD33-PE. These antibodies were obtained from Pharmingen (San Diego, CA). Bone marrow cells at 5 to 10 × 105 were mixed with either rat anti-mouse CD16/CD32 Fc block (clone 2.4G2; Pharmingen) or 10% heat-inactivated, pooled mouse serum to block nonspecific antibody binding and then incubated with saturating amounts of one of the conjugated antibodies. Cells from each animal were also stained with appropriate conjugated, isotype-matched, control antibodies obtained from Becton Dickinson or Pharmingen. After incubation, cells were resuspended in red cell lysis buffer (0.83% NH4Cl, 0.1% KHCO3, 0.004% EDTA) and washed twice in PBS containing 2% FCS. In all experiments, cells stained with the isotype control antibody were used to set the quadrant markers such that the negative quadrant contained at least 97% of the control cells. The percentage of engrafted human cells was determined by CD45 positivity, and lineage marker and EGFP expression were determined on the CD45+-gated population.
Aliquots of bone marrow cells were assayed for total and trimetrexate-resistant human clonogenic progenitors, as described earlier. After 14 to 21 days, individual colonies were plucked from the methylcellulose and processed to recover DNA. The DNA samples were assayed for EGFP coding sequences with the polymerase chain reaction (PCR) methodology. After scoring the plates, we picked 20 colonies (or fewer, if fewer were present) at random and incubated them in lysis buffer (50 nmol/L Tris, pH 8.5, 1 mmol/L EDTA, 0.5% Tween 20, and 100 μg/mL proteinase K) at 56°C for 2 hours. To inactivate the proteinase K, we heated the samples at 95°C for 10 minutes. PCR was performed with the PCR Core Kit (Boehringer Mannheim) according to the manufacturer's instructions. The amplification conditions were as follows: 92°C for 2 minutes, then 35 cycles of 92°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute, followed by a final elongation step of 7 minutes at 72°C. Primers that amplify an 829-base pair (bp) fragment of human α-satellite DNA, 5′-AATTTCAGCTGACTAAACA-3′ and 5′-TTTAGTTAGGTGCAGTTAT-3′, were used to confirm the presence of human DNA in each sample. The EGFP gene was amplified using the primers 5′-ACCCCGACCACATGAAGCAGC-3′ and 5′-CGTTGGGGTCTTTGCTCAGGG-3′, giving a 417-bp product. Primers specific to the β-actin gene were used as an internal control: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′, giving a 604-bp product. The PCR products were subjected to electrophoresis on a 1% agarose gel with ethidium bromide. Samples that failed to show PCR products for either internal control β-actin or α-satellite DNA were not included in the calculation of gene-transfer efficiency.
Evaluation of RD114-pseudotyped vector preparations, the RD114/MGirL22Y producer clone, and genetically modified bone marrow cells for replication-competent retroviruses
Murine retroviral particles pseudotyped with the RD114 envelope protein do not infect murine cell lines. Vector preparations were therefore screened for replication-competent virus by a marker rescue assay with HeLa or K562 cells (K562-ATCC CCL 243), which contained an integrated vector genome encoding neomycin resistance (G1Na).53 These cells were repeatedly exposed to RD114-pseudotyped, MGirL22Y vector particles in conditioned medium, resulting in 100% transduction on the basis of fluorescence-activated cell sorter (FACS) analysis for EGFP expression. The transduced cells were expanded for 2 weeks while conditioned medium was collected, filtered, and stored. Neomycin-sensitive HeLa and K562 cells were expanded and exposed twice daily to the collected medium over 7 to 10 days before being placed under G418 selection for 14 days in G418-containing medium, which was changed every 48 hours. Fresh medium without G418 was then added twice weekly for an additional 2 weeks. No viable colonies were detected in the target cells and negative control cells, whereas the positive control cells (G1Na-transduced HeLa cells) expanded exponentially. Additionally, EGFP-positive HeLa cells were mixed with unexposed (EGFP-negative) HeLa cells at a 1:10 dilution and expanded over 4 weeks. No increase in EGFP-expressing cells was detected.
DNA was recovered from the RD114/MGirL22Y producer clone used in these experiments and from the bone marrow of 3 mice that engrafted with high levels of EGFP+ human CD45+ cells. The DNA samples were assayed by PCR for a potential recombination product that would indicate the presence of replication-competent retrovirus (RCR). The RCR primers were designed to specifically recognize a recombination event 3′ to the packaging signal in the vector genome and 5′ to a portion of the GAG gene that was not included in the vector genome but was present in the pEQPAM3-E plasmid within the RD18 packaging cell line. The primers used were 5′-GTGGAACTGACGAGTTCTGAACAC-3′ and 5′-GAGGAGAACGGCCAGTATTGAAGC-3′, which amplified a 995-bp sample in a positive control of rhesus DNA derived from an animal that had previously been shown to be infected with RCR.54
Results
Enhanced transduction of human hematopoietic cells with RD114-pseudotyped vector
Human cord blood CD34+ cells were cultured for 24 hours in cytokine-containing, serum-free medium (see “Materials and methods”) and then transduced on retronectin-coated plates. Serum-containing (10% FCS), conditioned medium from RD114-, amphotropic-, GALV-, and VSV-G–pseudotyped producer cells was added in amounts necessary to achieve the specified MOIs. After 24 hours, fresh, serum-free, cytokine-containing medium in amounts equal to the culture volume (2× dilution) was added, and after an additional 48 hours, the cells were analyzed for EGFP expression. With a single exposure at an MOI of 5, the RD114-pseudotyped particles were far more efficient at transducing human CD34+ cells than were vector particles pseudotyped with the amphotropic, GALV, or VSV-G envelope proteins (Figure 1).
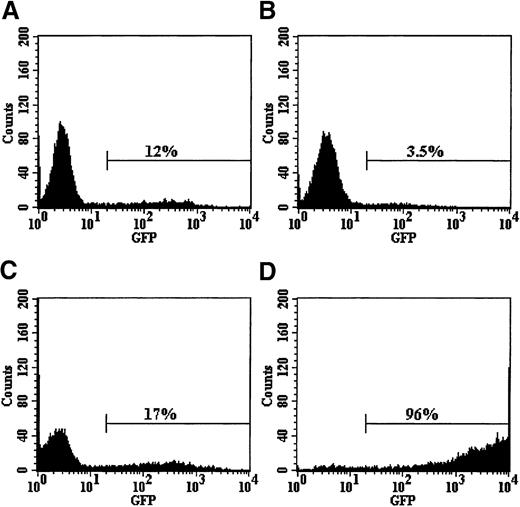
Transduction of umbilical cord blood CD34+ cells with pseudotyped retroviral vector particles.
Cells were prestimulated for 24 hours in serum-free medium containing high-dose cytokines before a single exposure to viral supernatant on retronectin-coated plates. Cells were then expanded for a total of 96 hours in vitro and analyzed for EGFP expression. Cells transduced with retroviral particles pseudotyped with the amphotropic (A), GALV (B), or VSV-G (C) envelope proteins failed to significantly transduce the bulk CD34+ population at the low MOI of 5 used. However, retroviral particles pseudotyped with the RD114 envelope protein (D) transduced the target cell population at a high efficiency. Pseudotransduction was also ruled out by pretreating CD34+cells with 50 nmol/L AZT 1 hour before transduction with each vector. EGFP expression in all cases was less than 1% (data not shown). These results demonstrate that in this cell population, the major barrier to gene transfer is at the receptor level and is not due to the quiescence of the target cell.
Transduction of umbilical cord blood CD34+ cells with pseudotyped retroviral vector particles.
Cells were prestimulated for 24 hours in serum-free medium containing high-dose cytokines before a single exposure to viral supernatant on retronectin-coated plates. Cells were then expanded for a total of 96 hours in vitro and analyzed for EGFP expression. Cells transduced with retroviral particles pseudotyped with the amphotropic (A), GALV (B), or VSV-G (C) envelope proteins failed to significantly transduce the bulk CD34+ population at the low MOI of 5 used. However, retroviral particles pseudotyped with the RD114 envelope protein (D) transduced the target cell population at a high efficiency. Pseudotransduction was also ruled out by pretreating CD34+cells with 50 nmol/L AZT 1 hour before transduction with each vector. EGFP expression in all cases was less than 1% (data not shown). These results demonstrate that in this cell population, the major barrier to gene transfer is at the receptor level and is not due to the quiescence of the target cell.
Aliquots of cells transduced with either RD114- or amphotropic-pseudotyped particles were replated in methylcellulose immediately after transduction (48 hours of culture) and incubated for an additional 10 to 15 days, and resistance to trimetrexate was determined (Figure 2). The RD114-pseudotyped vector efficiently transduced CD34+ cells and their progenitors at a very low MOI, indicating the presence of the appropriate receptor and cycling of a significant proportion of the cell population. Amphotropic-pseudotyped particles failed to efficiently transduce the same population even at increased particle concentrations, consistent with a block of transduction at the receptor level. These preliminary results led us to focus our efforts on evaluating RD114-pseudotyped particles for transducing primitive human hematopoietic cells under conditions that preserved their repopulating activity in the NOD/SCID model.
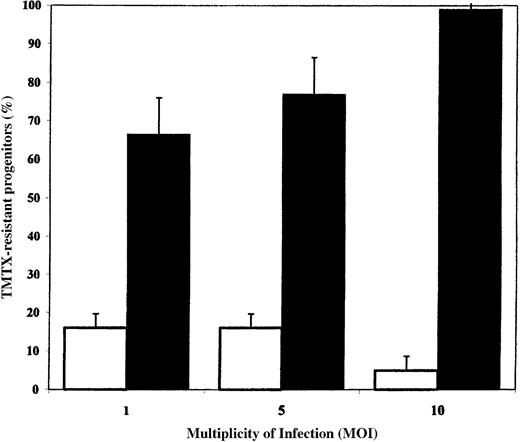
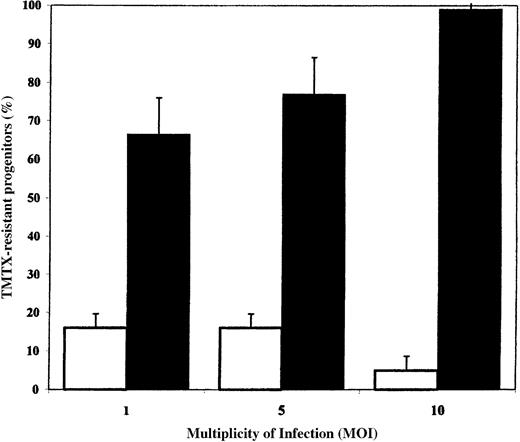
Transduction of human cord blood progenitors with RD114-pseudotyped particles.
Target CD34+ cells were cultured in serum-free medium for 24 hours and then transduced on retronectin-coated plates at various MOIs. The open bars depict results obtained with amphotropic vector particles, and the solid bars depict results obtained with RD114-pseudotyped vector particles. The percentages of colonies that grew in the presence of 100 nmol/L trimetrexate (TMTX) were compared with the total number of colonies observed in control cultures. The means and standard deviations for 4 replicate experiments are shown.
Transduction of human cord blood progenitors with RD114-pseudotyped particles.
Target CD34+ cells were cultured in serum-free medium for 24 hours and then transduced on retronectin-coated plates at various MOIs. The open bars depict results obtained with amphotropic vector particles, and the solid bars depict results obtained with RD114-pseudotyped vector particles. The percentages of colonies that grew in the presence of 100 nmol/L trimetrexate (TMTX) were compared with the total number of colonies observed in control cultures. The means and standard deviations for 4 replicate experiments are shown.
Medium conditioned by derivatives of the HT1080 cell line altered the immunophenotype of primitive human hematopoietic cells during in vitro culture
We routinely monitored the immunophenotype of purified CD34+ and CD34+, CD38− cells after in vitro culture and transduction. After 96 hours in serum-free culture with high-dose cytokines, purified CD34+, CD38− cells retained their phenotype during in vitro culture (Figure 3B).19Exposure of CD34+, CD38− cells to medium conditioned by the RD114/MGirL22Y producer cells during transduction caused the majority of CD34+, CD38− cells to become CD38+ (Figure 3C). Although only a small decrease in total clonogenic progenitors accompanied this phenotypic change (95% ± 9%, P > .05), we found that CD34+cells exposed to conditioned medium from the RD114/MGirL22Y producer cells failed to engraft in murine NOD/SCID recipients (n = 4). In the same experiment, control CD34+ cells cultured identically but without exposure to vector particles in conditioned medium during in vitro culture exhibited multilineage engraftment (data not shown).
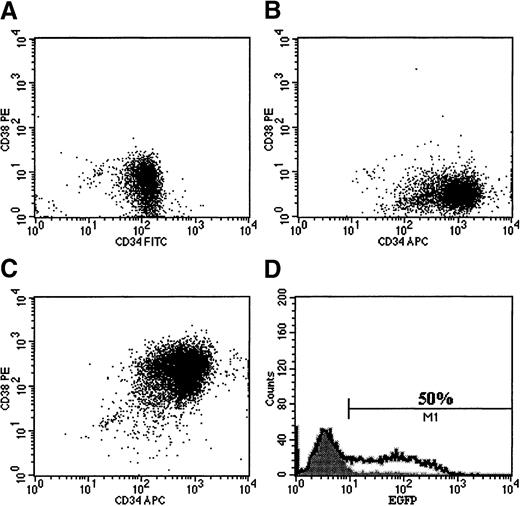
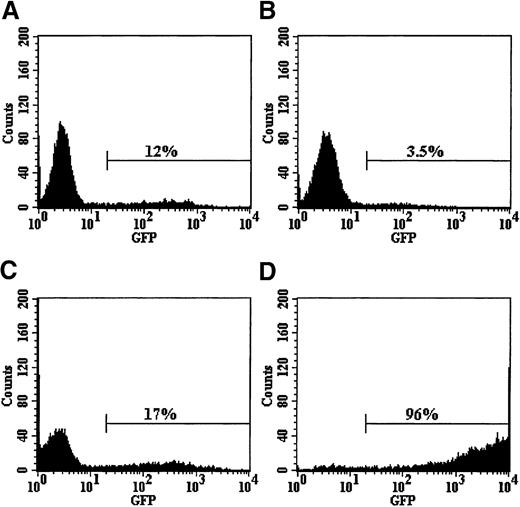
The effect of conditioned medium from the RD114/MGirL22Y producer cells on the immunophenotype of purified cord blood CD34+, CD38− cells.
FACS profiles are shown for the following: (A) purified CD34+, CD38− cells before culture; (B) CD34+, CD38− cells after 96 hours of culture in serum-free medium without addition of vector particles; (C) CD34+, CD38− cells after 96 hours in medium containing RD114-pseudotyped vector particles; and (D) population of cells shown in panel C analyzed for EGFP expression.
The effect of conditioned medium from the RD114/MGirL22Y producer cells on the immunophenotype of purified cord blood CD34+, CD38− cells.
FACS profiles are shown for the following: (A) purified CD34+, CD38− cells before culture; (B) CD34+, CD38− cells after 96 hours of culture in serum-free medium without addition of vector particles; (C) CD34+, CD38− cells after 96 hours in medium containing RD114-pseudotyped vector particles; and (D) population of cells shown in panel C analyzed for EGFP expression.
The observed phenotypic changes during culture and the loss of primitive repopulating cells could have been due to a direct effect of the RD114 envelope protein or to the action of some other component within the medium conditioned by the RD114/MGirL22Y producer cells. The latter seemed more likely when we found that medium from an amphotropic producer cell population derived from FLYA13, also a derivative of the HT1080 cell line used to generate FLYRD18,48 induced a similar immunophenotypic change in CD34+, CD38− cells (Figure 4C). Medium from an amphotropic producer line derived from PA317 cells did not affect the immunophenotype of the CD34+, CD38− cells (Figure 4D). We also generated RD114-pseudotyped vector particles following transient transfection of 293T cells, as described in “Materials and methods.” The immunophenotype was preserved after exposure to these vector preparations (Figure 4E). Significant gene transfer was observed only in CD34+, CD38− cells transduced with the RD114-pseudotyped vector (data not shown).
Immunophenotypic changes in cultured, primitive hematopoietic cells reflect the action of an unknown component in medium conditioned by derivatives of HT1080 cells rather than RD114-pseudotyped vector particles.
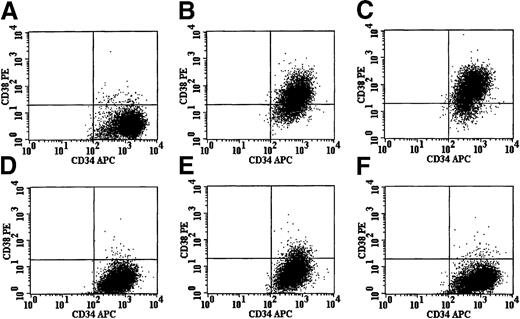
CD34+, CD38− cells were isolated and placed in serum-free culture containing high-dose cytokines. After 24 hours of prestimulation, cells were transduced once at an MOI of 5 by various pseudotyped retroviral particles. Cells were then expanded for a total of 96 hours in culture and analyzed for phenotype. Under these conditions, untransduced cells maintained their phenotype (A). However, cells exposed to conditioned media derived from HT1080 cells and containing particles pseudotyped with either the RD114 envelope protein (B) or the amphotropic envelope protein (C) showed a significant shift to a CD38+ phenotype. Interestingly, no significant change in phenotype was noted in cells exposed to conditioned media from 3T3 cells containing amphotropic-pseudotyped vector particles (D) or RD114-pseudotyped particles derived transiently in 293T conditioned media (E). Cells exposed to RD114-pseudotyped retroviral particles derived from the HT1080 producer clone that had been preloaded on retronectin-coated dishes with removal of conditioned medium allowed efficient transduction without a change in the CD34+, CD38− phenotype (F). Transduction based on EGFP expression was seen only in CD34+, CD38− cells transduced with RD114/MGirL22Y vector particles (data not shown). These results suggest that the conditioned medium from the HT1080 cells and not the RD114-pseudotyped retroviral particles is responsible for the differentiation of the CD34+, CD38−cells.
Immunophenotypic changes in cultured, primitive hematopoietic cells reflect the action of an unknown component in medium conditioned by derivatives of HT1080 cells rather than RD114-pseudotyped vector particles.
CD34+, CD38− cells were isolated and placed in serum-free culture containing high-dose cytokines. After 24 hours of prestimulation, cells were transduced once at an MOI of 5 by various pseudotyped retroviral particles. Cells were then expanded for a total of 96 hours in culture and analyzed for phenotype. Under these conditions, untransduced cells maintained their phenotype (A). However, cells exposed to conditioned media derived from HT1080 cells and containing particles pseudotyped with either the RD114 envelope protein (B) or the amphotropic envelope protein (C) showed a significant shift to a CD38+ phenotype. Interestingly, no significant change in phenotype was noted in cells exposed to conditioned media from 3T3 cells containing amphotropic-pseudotyped vector particles (D) or RD114-pseudotyped particles derived transiently in 293T conditioned media (E). Cells exposed to RD114-pseudotyped retroviral particles derived from the HT1080 producer clone that had been preloaded on retronectin-coated dishes with removal of conditioned medium allowed efficient transduction without a change in the CD34+, CD38− phenotype (F). Transduction based on EGFP expression was seen only in CD34+, CD38− cells transduced with RD114/MGirL22Y vector particles (data not shown). These results suggest that the conditioned medium from the HT1080 cells and not the RD114-pseudotyped retroviral particles is responsible for the differentiation of the CD34+, CD38−cells.
Transduction of primitive human hematopoietic cells without immunophenotypic change by RD114-pseudotyped vector particles that had been preloaded onto retronectin-coated plates
An alternative experiment to test whether the conditioned media from the HT1080 cells is causing the differentiation would be to absorb (preload) the particles on retronectin-coated plates. Retroviral vector particles can be preloaded onto retronectin-coated plates by a brief incubation of viral conditioned medium.55 The vector particles become concentrated on the retronectin, allowing the conditioned medium to be removed and replaced by medium containing the target cell population. Using conditioned medium from the RD114/MGirL22Y producer cells, we absorbed RD114-pseudotyped particles onto retronectin and then transduced CD34+, CD38− cells. By this approach, we maintained the immunophenotype of the CD34+, CD38− cells (Figure 4F) with preservation of transduction efficiency (data not shown).
Our objective in these studies was to efficiently transduce SRCs. We were concerned about potential toxicity to these cells because of the manipulations required to repeatedly expose the CD34+ cells to our vector particles, preloaded on retronectin-coated plates. Therefore, we performed experiments with CD34+, CD38− cells to determine the optimum period of prestimulation before a single exposure of this target population to retronectin-coated plates onto which RD118-pseudotyped particles had been preloaded (n = 4). Significant transduction of clonogenic progenitors, as reflected by trimetrexate resistance, was achieved with only 24 hours of prestimulation (38% ± 15%), but maximal effect was seen with 48 hours of prestimulation (73% ± 12%). These results suggested that we could achieve significant marking of primitive hematopoietic cells by preloading retronectin-coated plates with RD114-pseudotyped viral particles alone after only 24 to 48 hours of activation in vitro.
Transduction of human cells capable of establishing hematopoiesis in immunodeficient mice with RD114-pseudotyped vector particles
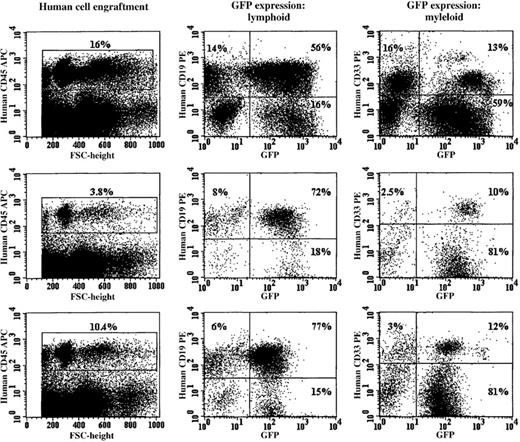
Purified CD34+ cells that had been prestimulated for 24 or 48 hours in serum-free medium were transduced by a single exposure to RD114 vector particles that had been preloaded onto retronectin-coated plates. After a maximum of 96 hours of culture, expanded cells derived from an input volume of 1.0 to 1.5 × 105 cells were injected into NOD/SCID murine recipients. Control cells were either not exposed to retroviral vector particles or transduced with amphotropic vector particles preloaded alone or additionally exposed to fresh viral supernatant at 48 and 72 hours in vitro to maximize transduction efficiency (Figure6). Each method of transduction was performed at least twice in a series of 3 experiments. Figure5 shows the results of analysis of the bone marrow cells of 3 animals that had received CD34+cells transduced with RD114 vector particles preloaded onto retronectin-coated plates after 24 or 48 hours of prestimulation. In each animal, there was a substantial population of bone marrow cells that reacted with a human CD45-specific monoclonal antibody. EGFP+ cells were found in both the lymphoid (CD19+) and myeloid (CD33+) cell populations (Figure 5).
RD114-pseudotyped vector particles efficiently transduced cells capable of establishing multilineage hematopoiesis in immunodeficient (NOD/SCID) mice.
The 3 rows show the FACS profile of bone marrow cells obtained from NOD/SCID mice killed 8 weeks after receiving in vitro–expanded human CD34+ cells (1.0 × 105 input cells). The cells were stained with a human-specific CD45 antibody and analyzed for human engraftment. The gated human CD45+ cells from these animals were analyzed for human CD19 (B-lymphoid) or CD33 (myeloid) expression. Each row shows the FACS profile of bone marrow cells obtained from a NOD/SCID recipient of RD114-transduced cord blood CD34+ cells. The bone marrow from the animals shown was negative for RCR by PCR analysis (data not shown).
RD114-pseudotyped vector particles efficiently transduced cells capable of establishing multilineage hematopoiesis in immunodeficient (NOD/SCID) mice.
The 3 rows show the FACS profile of bone marrow cells obtained from NOD/SCID mice killed 8 weeks after receiving in vitro–expanded human CD34+ cells (1.0 × 105 input cells). The cells were stained with a human-specific CD45 antibody and analyzed for human engraftment. The gated human CD45+ cells from these animals were analyzed for human CD19 (B-lymphoid) or CD33 (myeloid) expression. Each row shows the FACS profile of bone marrow cells obtained from a NOD/SCID recipient of RD114-transduced cord blood CD34+ cells. The bone marrow from the animals shown was negative for RCR by PCR analysis (data not shown).
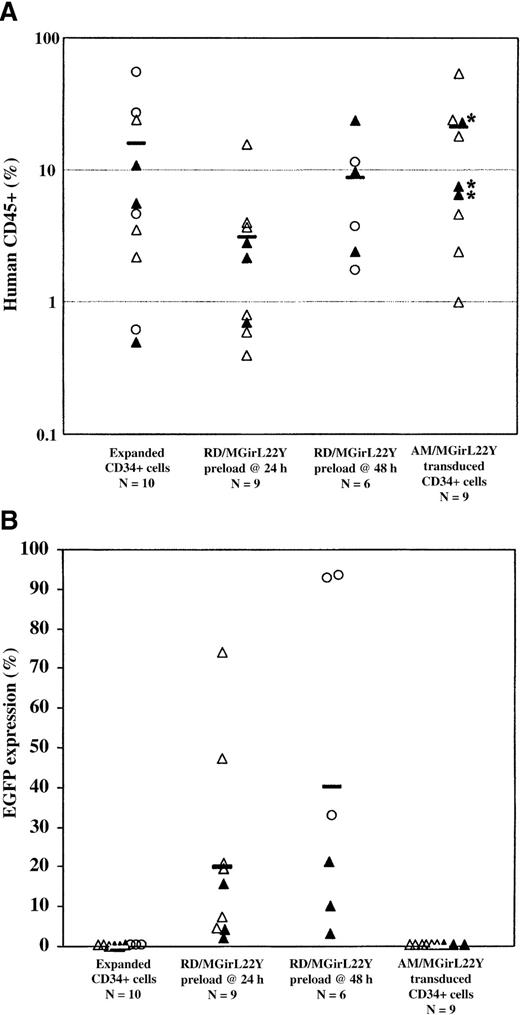
Overall rates of human engraftment in the NOD/SCID recipients and the level of EGFP expression are shown in Figure 6. EGFP+ cells were found in significant numbers only in animals that received RD114-transduced CD34+ cells. The overall level of EGFP expression varied among experiments but was consistent within individual cohorts of animals. Although we observed decreased human engraftment in NOD/SCID mice that received CD34+ cells transduced by the RD114-pseudotyped vector after only 24 hours of prestimulation as compared with untransduced CD34+ cells (P < .05 compared with controls), this effect could be ameliorated by slightly longer prestimulation (48 hours) before a single transduction. Extending the period of prestimulation also resulted in a higher proportion of gene-modified human cells in transplant recipients, which correlated with our in vitro studies and those of others,20 30 suggesting that the optimal timing for transduction of NOD/SCID repopulating cells is after 48 hours in culture.
Analysis of human CD45+engraftment and EGFP expression in human hematopoietic cells in NOD/SCID mice.
Transduction efficiency of SCID repopulating cells (SRCs) by a single exposure of cord blood CD34+ cells to RD114-pseudotyped vector particles preloaded on retronectin after only 24 hours or 48 hours of preactivation was assessed over a series of experiments (Experiment 1, open triangles; Experiment 2, closed triangles; Experiment 3, open circles). Cells were cultured for a total of 96 hours before injection into recipient animals. After 8 weeks in vivo, the animals were killed and analyzed for human engraftment (greater than 0.5%) based on CD45+ expression (A) and functional marking based on EGFP expression (B). The number (N) of animals in each group is indicated. Engraftment was notably decreased in animals that received CD34+ cells transduced at 24 hours in vitro with RD114/MGirL22Y vectors, but this effect was not as significant in animals that received cells transduced at 48 hours. Remarkably, all animals that received RD114/MGirL22Y-transduced CD34+ cells engrafted with EGFP+ cells. Indeed, the best engrafted animals also had the highest level of EGFP+ cells. Control animals that received untransduced cells or cells either transduced by preloaded am/MGirL22Y vectors alone (*) or transduced by preloaded am/MGirL22Y particles at 24 hours, followed by exposure to viral supernatant at 48 hours and 72 hours, engrafted only with unmarked human cells (despite the presence of 10% serum in the conditioned medium).
Analysis of human CD45+engraftment and EGFP expression in human hematopoietic cells in NOD/SCID mice.
Transduction efficiency of SCID repopulating cells (SRCs) by a single exposure of cord blood CD34+ cells to RD114-pseudotyped vector particles preloaded on retronectin after only 24 hours or 48 hours of preactivation was assessed over a series of experiments (Experiment 1, open triangles; Experiment 2, closed triangles; Experiment 3, open circles). Cells were cultured for a total of 96 hours before injection into recipient animals. After 8 weeks in vivo, the animals were killed and analyzed for human engraftment (greater than 0.5%) based on CD45+ expression (A) and functional marking based on EGFP expression (B). The number (N) of animals in each group is indicated. Engraftment was notably decreased in animals that received CD34+ cells transduced at 24 hours in vitro with RD114/MGirL22Y vectors, but this effect was not as significant in animals that received cells transduced at 48 hours. Remarkably, all animals that received RD114/MGirL22Y-transduced CD34+ cells engrafted with EGFP+ cells. Indeed, the best engrafted animals also had the highest level of EGFP+ cells. Control animals that received untransduced cells or cells either transduced by preloaded am/MGirL22Y vectors alone (*) or transduced by preloaded am/MGirL22Y particles at 24 hours, followed by exposure to viral supernatant at 48 hours and 72 hours, engrafted only with unmarked human cells (despite the presence of 10% serum in the conditioned medium).
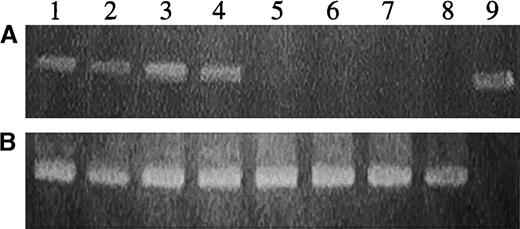
The efficiency of gene transfer was also evaluated by plating bone marrow from the experimental animals engrafted with human cells into methylcellulose in the presence of human cytokines. DNA recovered from such individual, plucked human, secondary hematopoietic colonies was analyzed by PCR analysis for the presence of EGFP (Figure7). Progenitors from every animal that received CD34+ cells transduced with RD114-pseudotyped particles and that grew human progenitors were analyzed. We limited the number analyzed per mouse to prevent skewing of the data (the best engrafted mice had the best marking). In the 24-hour RD114 cohort, a mean of 7 progenitors per engrafted animal were analyzed (maximum of 15). For the 48-hour RD114 cohort, a mean of 16 progenitors were analyzed per animal (maximum of 20). The results for all animals in each experimental cohort were combined. A high percentage of the colonies from animals that had received cells transduced with RD114-pseudotyped vector particles was positive by PCR analysis (Table1).
Detection of the MGirL22Y genome in DNA from human hematopoietic cells derived from secondary progenitors cultured from a NOD/SCID recipient of CD34+ cells transduced with RD114-pseudotyped vector particles.
(A) PCR analysis with primers specific for EGFP sequences. (B) PCR analysis with the internal control β-actin primers. Lanes 1 to 8 represent analysis of DNA from individual hematopoietic colonies. Lane 9 represents analysis of plasmid DNA encoding EGFP.
Detection of the MGirL22Y genome in DNA from human hematopoietic cells derived from secondary progenitors cultured from a NOD/SCID recipient of CD34+ cells transduced with RD114-pseudotyped vector particles.
(A) PCR analysis with primers specific for EGFP sequences. (B) PCR analysis with the internal control β-actin primers. Lanes 1 to 8 represent analysis of DNA from individual hematopoietic colonies. Lane 9 represents analysis of plasmid DNA encoding EGFP.
We regard the scoring of individual colonies for the proviral genome by PCR as being the most valid measure of gene transfer into SRCs because silencing or expression variegation may affect the percentage of cells scored as EGFP+ or trimetrexate resistant. This is best illustrated by the analysis of human progenitors derived from the bone marrow of NOD/SCID mice that received human CD34+ cells transduced by RD114/MGirL22Y at 48 hours. In 2 separate experiments, 3 mice received CD34+ cells transduced by RD114/MGirL22Y at 48 hours (Figure 6). One cohort of animals was 71% positive by EGFP expression and 66% positive (39 of 59) by progenitor analysis. Analysis of the other cohort of animals showed that only 10% of the engrafted human cells expressed EGFP, but 49% (19 of 39) of the progenitors were positive for the proviral genome. Thus, the marking in the human progenitors (58 of 98 combined) derived from the NOD/SCID mouse bone marrow was equivalent to the gene-transfer efficiency into the progenitors from the transduced CD34+ population used for transplantation (45% to 66%; Table 1).
The significant marking of the SRCs achieved by a single exposure to RD114-pseudotyped retroviruses raised the possibility of RCR contamination. We therefore performed PCR analysis on DNA from the producer cell clone and the bone marrow of 3 NOD/SCID recipients that had received human CD34+ cells transduced with RD114-pseudotyped retroviral particles and had the highest levels of EGFP expression. Primers specific for a recombination product that would reflect RCR were used.54 The RCR recombination product was not present in either the producer clone DNA or bone marrow DNA. Sensitivity of this assay, based on the positive control rhesus DNA, containing 36 copies of RCR per cell was 0.1% (data not shown).
Discussion
Our results demonstrate that murine oncoretroviral vector particles pseudotyped with the envelope protein of feline endogenous virus (RD114) transduced human hematopoietic cell lines and the CD34+ and CD34+, CD38− cell populations from cord blood and the progenitors found in these populations far more efficiently than conventional amphotropic particles at equivalent MOIs. The FLYRD18 cells (HT1080) from which the RD114-pseudotyped vector particles were produced apparently generate a substance that induces differentiation, as reflected by immunophenotypic changes and depletion of repopulating cells. These undesirable effects were avoided by preloading the RD114-pseudotyped retroviral particles onto retronectin-coated plates for transduction of hematopoietic cell populations. The cells in the cord blood CD34+ population that are capable of establishing human hematopoiesis in immunodeficient (NOD/SCID) mice were also efficiently transduced by preloaded RD114-pseudotyped vector particles, as reflected by the presence of the proviral genome in as many as 90% of the myeloid and lymphoid cells in transplant recipients.
The nature and identity of the substance(s) produced by derivatives of the FLYRD18 cell line that induced phenotypic changes and depletion of NOD/SCID repopulating cells are unknown. The evidence indicates that the RD114-pseudotyped particles themselves are not responsible because the effect was observed with amphotropic particles produced by a derivative of this HT1080-derived cell line and was not observed with RD114 particles produced by human 293T cells. CD34+ cells stained with the cell membrane dye PKH26 on day 0 and analyzed after 96 hours in culture showed that virtually all cells divided 1, 2, or 3 times in culture. There was no difference in the mean fluorescence pattern of CD34+ cells exposed to either preloaded RD114-pseudotyped virus or HT1080-derived conditioned medium compared with untransduced controls (P. Kelly, unpublished observations). Thus, the substance in the conditioned medium seems to alter the immunophenotype without altering proliferation. The CD38 antigen is a bifunctional ectoenzyme that participates in signal transduction pathways involved in the regulation of cell growth and differentiation.56 The retinoic acid receptor-α–mediated signaling pathway results in induction of the CD38 cell surface antigen.57 58 It is possible that the HT1080 cell line produces a factor that mimics the effects of all-trans–retinoic acid, resulting in differentiation and loss of long-term repopulating cell potential.
The proportions of genetically modified cells within the human myeloid and lymphoid lineages achieved by transduction with RD114-pseudotyped particles have not been achieved with oncoretroviral vector particles pseudotyped with other envelope proteins. In addition, we achieved high-frequency transduction of human NOD/SCID repopulating cells with a single exposure to vector particles at a low MOI, whereas the lower levels of gene transfer into these cells achieved with amphotropic-pseudotyped particles25,31 or GALV-pseudotyped particles25,26,29-33 required multiple exposures to vector particles at generally higher MOIs. VSV-G–pseudotyped oncoretroviral vector particles have been reported to transduce NOD/SCID repopulating cells with a single exposure at an MOI of greater than 100, resulting in marking of 25% of the progenitors in animals undergoing transplantation under conditions that were toxic to the repopulating cells.43 Moreover, we have found that CD34+, CD38− cells are poorly transduced with VSV-G–pseudotyped particles59 compared with the high levels of transduction achieved with a single exposure with preloaded RD114-pseudotyped particles. In the present studies, we did not directly compare marking of SRCs with VSV-G–pseudotyped particles to marking with RD114-pseudotyped particles. However, in the serum-free culture conditions used in these experiments, multiple exposures of CD34+ cells to concentrated VSV-G–pseudotyped retroviral particles over 96 hours in vitro resulted in only a low proportion (less than 10%) of genetically modified human cells in NOD/SCID recipients (P. Kelly, unpublished observations).
The frequency of genetically modified human progenitors present in bone marrow 8 to 10 weeks after transplantation was approximately equivalent to the frequency of genetically modified primary progenitors in the transduced population used for transplantation of the immunodeficient mice. These results imply that the frequency of transduction of NOD/SCID repopulating cells was equal to the transduction frequency of progenitors detected in vitro. In this respect, the results obtained in transducing human cord blood cells with RD114-pseudotyped vector particles are comparable to those generally obtained in transducing murine hematopoietic cells with ecotropic vector particles.3,5 These results contrast with the general experience with amphotropic- and GALV-pseudotyped oncoretroviral vector particles, in which the transduction frequency of more mature human CD34+ cells and progenitors exceeds that of repopulating cells.25,26,29-31 It is interesting that RD114- and ecotropic-pseudotyped particles use amino acid transporters as receptors.46 60 We infer that the neutral amino acid transporter that serves as the receptor for RD114-pseudotyped particles is expressed at functionally higher levels on primitive human hematopoietic cells than are the phosphate transporters that serve as receptors for amphotropic- and GALV-pseudotyped vector particles.
In a recent study, a significant proportion of engrafted human hematopoietic cells were genetically modified (mean, 18%) after multiple exposures of CD34+ cells to a GALV-pseudotyped vector over 96 hours of culture, but with a decline in SRC frequency believed to be secondary to the presence of serum in the conditioned medium containing vector particles.32 Of greater concern are the findings by Demaison et al33 of preferential marking of myeloid lineage–committed progenitors after 72 hours in culture and marking of lymphoid-committed progenitors after an additional 24 hours of exposure to vector in vitro. These findings suggest significant heterogeneity in the population scored as SRCs after repeated exposure to vector containing conditioned medium over 96 to 120 hours in culture. The ability to achieve a high frequency of marking after a single exposure to RD114-pseudotyped vector particles preloaded on retronectin-coated plates in the NOD/SCID model may be advantageous with respect to genetic modification of true multilineage repopulating cells.
In this report, experiments were performed using umbilical cord blood–derived CD34+ cells, which are not the most clinically relevant stem cell source. Indeed, studies are now ongoing using a similar transduction strategy on peripheral blood CD34+ cells. In initial studies, we have achieved greater than 80% transduction efficiency of peripheral blood CD34+cells (n = 3) on the basis of EGFP expression with only a single exposure to retronectin-coated plates preloaded with RD114-pseudotyped particles after 48 hours of prestimulation (unpublished observations, P. Kelly and K. Pollok). We anticipate that given the more quiescent nature of peripheral blood CD34+ cells, additional exposures to vector may be necessary to achieve therapeutic levels of gene transfer but that this may be determined only by larger animal models, such as the rhesus monkey autologous transplant model.
In conclusion, we report that RD114-pseudotyped murine retroviral vectors can efficiently transduce umbilical cord blood SRCs after only 24 to 48 hours of prestimulation before a single exposure to the viral particles. Preloading the viral particles onto retronectin-coated plates with removal of the conditioned medium allowed significant gene transfer to occur. Such an approach may bypass deleterious effects to the true long-term repopulating cells not appreciated when using other pseudotyped retroviruses to mark SRCs. We predict that RD114-pseudotyped retroviral vectors may result in gene-transfer efficiencies at levels that may be curative for hematopoietic genetic diseases.
Acknowledgment
We acknowledge the expert assistance of Jean Johnson in preparation of the manuscript.
Supported by NHLBI Program Project Grant P01 HL 53749, The ASSISI Foundation of Memphis 94-00, Cancer Center Support CORE Grant P30 CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Patrick F. Kelly, Division of Experimental Hematology, St Jude Children's Research Hospital, 332 N Lauderdale, Room D-4026, Memphis, TN 38105.