Abstract
P-selectin is a leukocyte adhesion receptor stored in platelets and endothelial cells and is translocated to the surface upon cell activation. Purified P-selectin is oligomeric and has increased avidity for its ligand relative to the monomeric form, but whether P-selectin self-associates in the membrane of intact cells is not known. A chemical cross-linking approach was used to show that P-selectin is present as noncovalent dimers in resting platelets, human umbilical vein endothelial cells, and heterologous RIN5F cells expressing P-selectin. The results of 2-dimensional isoelectric focusing are consistent in showing P-selectin dimers as homodimers, but they are composed of a more basic subset of P-selectin than the monomers. This suggests that the dimers are a biochemically distinct subset of P-selectin. P-selectin dimers form in the endoplasmic reticulum and Golgi compartments of human umbilical vein endothelial cells only after synthesis of the mature P-selectin subunit, and are not preferentially stored in Weibel-Palade bodies as compared with the monomeric form. Platelet activation with thrombin receptor–activating peptide leads to the presence of P-selectin monomers and homodimers on the cell surface as well as P-selectin heterodimers, which are composed of P-selectin and an unidentified protein of approximately 81 kd molecular weight. In summary, these studies demonstrate that P-selectin is homodimeric in situ and that platelet activation leads to the formation of an additional activation-specific heterodimeric species. In addition, the homodimer has unique biochemical characteristics compared with the monomeric form, and dimerization occurs in the endoplasmic reticulum and Golgi compartments of endothelial cells.
Introduction
P-selectin belongs to the selectin family of leukocyte adhesion receptors and is a transmembrane protein stored in the α-granules of platelets and in the Weibel-Palade bodies of endothelial cells. Upon stimulation of these 2 cell types with secretagogues such as thrombin and histamine, the granules fuse with the plasma membrane and P-selectin is expressed on the cell surface, where it is a receptor for a number of leukocyte subsets. P-selectin promotes low-affinity interactions of leukocytes with endothelial cells and platelets deposited in the vessel wall. These interactions allow leukocytes to roll on the inflamed vessel wall, and then either detach or firmly adhere and transmigrate.1,2 Studies by our laboratory and others have suggested that P-selectin also affects leukocyte functions beyond cell–cell tethering. For example, engagement of P-selectin is important for the subsequent firm adhesion of leukocytes to endothelium,3 the functional upregulation of the β2-integrin Mac-1,4,5 and the expression of chemokines by leukocytes.6
A mechanism frequently used by low-affinity receptors to strengthen the avidity of their interactions with their ligands, and thus slow the koff (dissociation rate constant) of a biochemical reaction, is the dimerization or oligomerization of the receptor in the absence of ligand.7 Dimerization has been shown to be important in the functioning of L-selectin, present on leukocytes. The forced dimerization of this selectin using a molecular dimerization strategy led to the reduced rolling velocity of cells on inflamed endothelial cells.8 This could potentially influence the firm adhesion and migration of leukocytes. Previous studies have suggested that purified platelet P-selectin is present as dimers and high-molecular-weight oligomers in detergent solutions.9-11 Recombinant P-selectin lacking the transmembrane domain did not oligomerize, suggesting that this is the most likely site for P-selectin self-association. It is important to note that the oligomeric full-length P-selectin supported HL-60 cell adhesion at lower site densities than the mutant recombinant form lacking the transmembrane domain, which was monomeric.11Thus, dimeric or oligomeric P-selectin exists in solution and has greater binding activity for leukocytes than monomeric P-selectin, but it remains to be shown whether P-selectin self-associates in intact cells.
In the present study, we show that P-selectin forms noncovalent dimers in intact platelets, endothelial cells, and in selected heterologous cells expressing P-selectin. Using 2-dimensional isoelectric focusing analysis, we show that the dimers consist of homodimers but are composed of a more basically charged subset of P-selectin than monomeric P-selectin. Cell-fractionation studies show that P-selectin dimers form in the endoplasmic reticulum (ER)/Golgi compartment of endothelial cells and that both dimers and monomers are stored in the Weibel-Palade bodies of endothelial cells and α-granules of platelets. P-selectin homodimers and monomers are also present on the surface of platelets activated with thrombin receptor–activating peptide (TRAP) and the calcium ionophore A23187. Thus, both P-selectin species most likely play a role in P-selectin–mediated leukocyte–endothelial cell and leukocyte–platelet interactions. Furthermore, in TRAP-activated platelets, an additional heterodimeric species containing P-selectin and an unidentified biotinylated protein of approximately 81 kd molecular weight was observed. The association of P-selectin with another platelet surface protein may additionally modulate P-selectin's function on the surface of activated platelets.
Materials and methods
Cells
Human platelets from venous blood were isolated by gel filtration on a Sepharose 2B column using a previously published protocol.12 Human umbilical vein endothelial cells (HUVECs) were prepared as described previously13 and used at passage 2 or 3 for all studies. Human erythroleukemia (HEL) cells were maintained in suspension in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, 100 mmol/L l-glutamine, and 100 U/mL penicillin/streptomycin. RIN5F cells transfected with full-length wild-type P-selectin or the P-selectin C766→A mutant (single-letter amino acid codes)14 were kindly provided by Dr Denisa Wagner (Center for Blood Research, Harvard Medical School, Boston, MA) and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 mmol/L l-glutamine, and 100 U/mL penicillin/streptomycin.
Chemical cross-linking, biotinylation, and cell lysis
The cleavable sulfhydryl-reactive homobifunctional cross-linker reagent, DPDPB (1,4-Di-[3′-(2′-pyridyldithio)-proprionamido]butane) (Pierce, Rockford, IL), which has a 2.0-nm spacer arm, was prepared fresh each time as a 25-fold concentrated stock solution (12 mg/mL) in dimethylsulfoxide. DPDPB was added at a final concentration of 0.5 mg/mL to purified platelet suspensions, or to monolayers of cells, for 30 minutes at room temperature. For surface biotinylation of proteins, cells were treated concurrently with a final concentration of 0.5 mg/mL sulfo-NHS-LC-biotin (Pierce) in Hank's buffered saline supplemented with 25 mmol/L HEPES buffer for 1 hour at room temperature. Excess biotin was quenched by incubating the cells for 10 minutes in 0.1 mol/L Tris, pH 7.6. Cells were washed in phosphate-buffered saline (PBS) and then lysed on ice for 1 hour in lysis buffer containing the following: 1% Triton X-100, 50 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 2 mmol/L EDTA, 2 mmol/LN-ethylmaleimide (NEM) and 2 mmol/L iodoacetamide (IAA) to block free sulfhydryls, and protease inhibitors (2 mmol/L phenylmethylsulfonyl fluoride, 2 μmol/L leupeptin, 10 μmol/L aprotinin, 1 mg/mL benzamidine, 2 μmol/L pepstatin, 0.5 μg/mL antipain, and 0.5 μg/mL chymostatin). Cell lysates were centrifuged at 16 000g to remove detergent-insoluble components and stored at −20°C.
Treatment of platelets with DNase I
Cross-linker–treated or untreated platelets were lysed in 1% Triton X-100 buffer containing inhibitors but lacking EDTA and incubated at 4°C for 30 minutes with a highly purified form of DNAse I at 2 mg/mL (Boehringer-Mannheim, Indianapolis, IN). To avoid any nonspecific proteolytic degradation, we pretreated the DNase I with protease inhibitors before use.
Two-dimensional isoelectric focusing
Lysates from Triton X-100–solubilized cells (with or without cross-linker treatment) were separated in the first dimension on isoelectric focusing slab gels (Bio-Rad Labs, Hercules, CA). Isoelectric focusing standards (Bio-Rad) were also run and visualized by staining in Coomassie blue R-250 and crocein scarlet (Sigma, St Louis, MO). Running buffers and conditions were as suggested by the manufacturer. Gel strips were loaded at the top of 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) mini-gels with 4% stacking gels for separation in the second dimension. Additional lysate was subjected to electrophoresis in the reference well. Gels were then transferred overnight to polyvinylidene difluoride (PVDF) membrane (Bio-Rad), followed by probing with the P-selectin monoclonal antibody AC1.2 (Pharmingen, San Diego, CA).
Antibodies and immunoprecipitation
Lysates were supplemented with 0.5% deoxycholate and precleared with Protein-G beads (Amersham Pharmacia Biotech, Piscataway, NJ) in the case of polyclonal antibodies and AK-4 or goat antimouse beads (Sigma) in the case of AC1.2 for 1 hour at 4°C. Immunoprecipitations were performed at either 37°C (polyclonal) or room temperature (monoclonal) with approximately 2.5 μg of antibody per sample for 2 hours. Immunoprecipitates were collected by incubation overnight at 4°C with protein G or goat antimouse IgG beads. Beads were washed 3 times in 50 mmol/L Tris, pH 8.0, 2 mmol/L EDTA containing 1% Nonidet P40 (Sigma), and 0.5% deoxycholate (Sigma), and then eluted in electrophoresis sample buffer by boiling.
SDS-PAGE and Western blotting
Cell lysates or immunoprecipitate were subjected to electrophoresis on SDS-PAGE gels in the absence of dithiothreitol (DTT). For 2-dimensional electrophoresis, lanes were excised from the gel, reduced in DTT or β-mercaptoethanol, placed on top of a 10% polyacrylamide gel, and subjected to electrophoresis. After SDS-PAGE, proteins were transferred electrophoretically to PVDF membrane using the Bio-Rad Mini-Protean II electrophoresis/Transblot system. All buffers and conditions were as recommended by the manufacturer. Western blots were performed by blocking the PVDF with 5% nonfat dry milk in PBS and 0.05% Tween-20 (blocking buffer) for 45 minutes, followed by incubation with primary monoclonal P-selectin antibodies, 2 μg/mL AK4 or 1 μg/mL AC1.2 (Pharmingen), 1 μg/mL polyclonal antihuman P-selectin antibody (Pharmingen), monoclonal antibiotin antibody (1:1000 dilution of ascites) (Sigma), or monoclonal anti–protein disulfide isomerase antibody MA3-019 (IgG2a) (1:10 000 dilution of ascites) (Affinity BioReagents, Golden, CA). Blots were washed 3 times for 15 minutes each in blocking buffer and then incubated in horseradish peroxidase–conjugated goat antimouse IgG or goat antirabbit (Bio-Rad) at a dilution of 1:5000 or 1:10 000, respectively, for 1 hour. Blots were washed and then developed using the Supersignal West Pico substrate kit (Pierce). All incubations were performed at room temperature. Blots were exposed to x-ray film (Fuji RX, Fisher Scientific, Pittsburgh, PA) for times ranging from several seconds to 1 hour.
Cell fractionation
To separate ER and Golgi apparatus components from Weibel-Palade bodies, we used previously published protocols.15,16Briefly, HUVECs (approximately 330 cm2/gradient) were scraped into PBS; centrifuged; resuspended in 0.8 mL of homogenization buffer (HB) containing 250 mmol/L sucrose, 20 mmol/L Tris, pH 7.2, and 1 mmol/L EDTA; and then subjected to 50 strokes with a dounce homogenizer. Homogenates were centrifuged at 600g for 10 minutes. The pellet was resuspended in 0.4 mL of HB, subjected to another 50 strokes, and recentrifuged. The supernatants were combined and layered onto an 8.4-mL gradient containing 35% Percoll (Pharmacia) in 250 mmol/L sucrose and 20 mmol/L Tris, pH 7.2. Gradients were centrifuged at 40 000g in a Sorvall RC5B using an SM24 rotor for 1 hour at 4°C. Fractions of 0.8 mL were collected starting at the top of the tube, and 0.05-mL aliquots of each fraction were tested for von Willebrand factor (vWf) by a competitive enzyme-linked immunoassay (ELISA), as described previously.3 Peak fractions identified by ELISA were pooled and then immunoprecipitated for vWf.17 Immunoprecipitates of vWf were subjected to electrophoresis on 5% reducing SDS gels, transferred to PVDF membrane, and probed with the vWf polyclonal antibody. When the HUVECs were treated with the calcium ionophore A23187 (Sigma Chemical) to release Weibel-Palade bodies before fractionation, there was only one vWf peak detected by ELISA, in the fractions containing ER and Golgi apparatus. The same peak-fraction pools were immunoprecipitated with the P-selectin polyclonal antibody, subjected to electrophoresis on nonreducing 6% SDS gels, transferred to PVDF, and probed with the AC1.2 monoclonal antibody. Fractions from gradients containingA23187-treated samples were immunoblotted directly.
Results
P-selectin is present as noncovalent dimers in platelets and endothelial cells
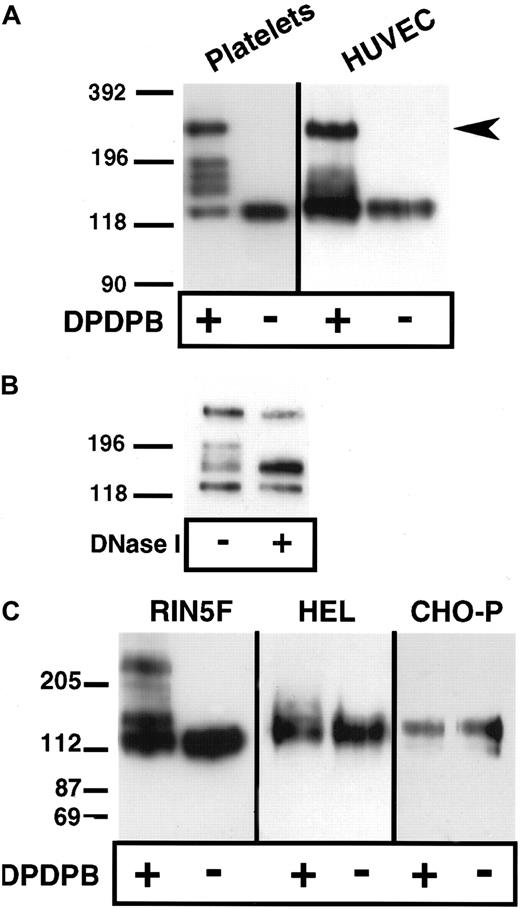
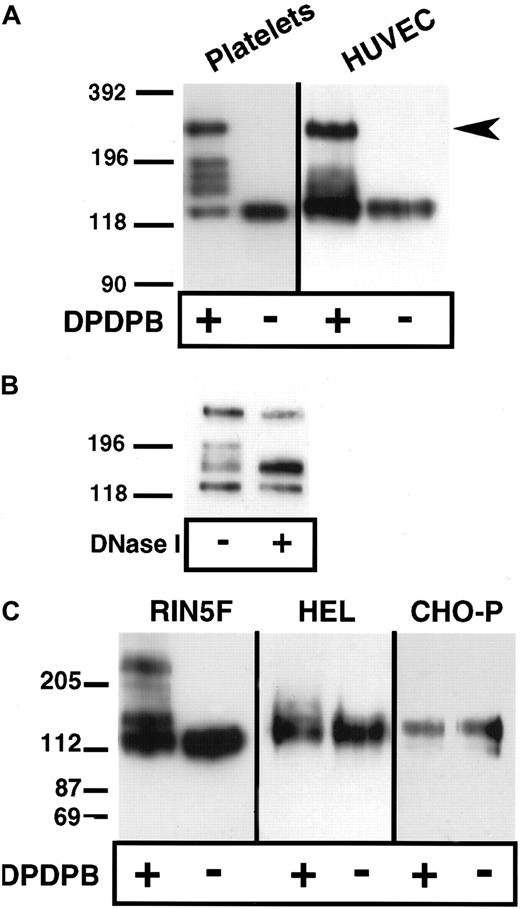
Lysis of platelets and endothelial cells in the absence of cross-linker but in the presence of the alkylating agents IAA and NEM, followed by analysis of P-selectin under nonreducing conditions, showed that P-selectin is monomeric (Figure1A). Thus, covalent, disulfide-bonded P-selectin dimers are not present in situ. To determine whether noncovalent dimers of P-selectin exist in situ, we used a chemical cross-linking approach. The membrane-permeable, thiol-specific cross-linking reagent DPDPB was added to platelets and HUVECs, and the cells were lysed in nonionic detergent in the presence of alkylating agents and then subjected to immunoblot analysis using anti–P-selectin antibody. Three species were detected in HUVECs. One migrated at a position consistent for monomeric P-selectin (approximately 140 kd), another migrated slightly above the monomeric band (approximately 180 kd), and the third migrated at approximately twice the size of the monomer (approximately 270 kd) (Figure 1A). In platelets, monomer and dimer were present as well as 3 additional species that migrated between monomer and dimer. The molecular weights of each of these 3 species were averaged over 3 experiments and found to be approximately 204, 189, and 167 kd. These species may be conformers of P-selectin captured by the cross-linker, as seen in the case of the microtubule-associated protein tau.18 Alternatively, some or all of these species may consist of heterodimers between P-selectin and intracellular free sulfhydryl–containing proteins such as actin monomers or actin-binding proteins. To help determine the contribution of the cytoskeleton to the generation of P-selectin cross-linked species, we treated soluble platelet lysates from cross-linker–treated resting cells with DNase I to depolymerize actin filaments. As shown in Figure 1B, DNase I treatment resulted in collapse of the upper 2 bands of the triplet into the lower band, suggesting that actin polymerization is associated with the generation of different cross-linked species. In activated platelets, DNase I treatment gives the same result (data not shown). The same patterns of P-selectin monomer, dimer, and intermediate species were obtained if the cross-linker was quenched with alkylating agents before cell lysis, which indicates that the cross-linking occurred in the intact cells and not during lysis (data not shown). None of the cross-linked species were detected if cells were incubated with IAA and NEM before the addition of cross-linker, which suggests that the species detected after the addition of cross-linker were cross-linked through free sulfhydryls (data not shown).
Chemical cross-linking of P-selectin in platelets, endothelial cells, HEL cells, and heterologous cells expressing P-selectin.
Purified resting platelets in suspension and nonactivated HUVECs in monolayer (A) and RIN5F cells transfected with P-selectin, HEL cells, and CHO cells expressing P-selectin (C) were treated with the sulfhydryl-specific, membrane-permeable cross-linker DPDPB. The cells were then lysed in buffer containing 1% Triton X-100 in the presence of alkylating agents. Lysates were separated by SDS-PAGE on 6% gels, followed by immunoblotting using P-selectin monoclonal antibodies AK4 for platelets and AC1.2 for all other cells. (B) Lysates from cross-linked, resting platelets were treated with DNase I, separated electrophoretically, and immunoblotted as described in (A).
Chemical cross-linking of P-selectin in platelets, endothelial cells, HEL cells, and heterologous cells expressing P-selectin.
Purified resting platelets in suspension and nonactivated HUVECs in monolayer (A) and RIN5F cells transfected with P-selectin, HEL cells, and CHO cells expressing P-selectin (C) were treated with the sulfhydryl-specific, membrane-permeable cross-linker DPDPB. The cells were then lysed in buffer containing 1% Triton X-100 in the presence of alkylating agents. Lysates were separated by SDS-PAGE on 6% gels, followed by immunoblotting using P-selectin monoclonal antibodies AK4 for platelets and AC1.2 for all other cells. (B) Lysates from cross-linked, resting platelets were treated with DNase I, separated electrophoretically, and immunoblotted as described in (A).
P-selectin dimers were also detected by cross-linking in heterologous rat insulinoma (RIN5F) cells transfected with full-length P-selectin (Figure 1C). The unpaired cysteine residue (C766 in the cytoplasmic tail of P-selectin) is probably the site of cross-linking because a P-selectin mutant (C766→A) expressed in these cells did not dimerize (data not shown). Under the same conditions of cross-linking, dimers were not detected in CHO cells transfected with full-length P-selectin (CHO-P) or in HEL cells, which share several features with megakaryocytes (Figure 1C). The presence of dimers in RIN5F cells but not in CHO-P or HEL cells is not related to the ability of RIN5F to store heterologous P-selectin in intracellular granules, a property also of platelets and endothelial cells, because dimer formation occurred at steps before storage (Figure 3). It is possible that P-selectin dimers are not detected in CHO-P and HEL cells because posttranslational modifications that are necessary for dimer formation do not occur in these cells, or the reducing environment in these cells is too strong to allow reactivity of the thiol-specific cross-linker. For example, a modification may occur in the intracellular redox potential in these cells, which has been suggested to be the case in plasma cells as compared with B-cells.19
Characterization of P-selectin cross-linked species: analysis by isoelectric focusing
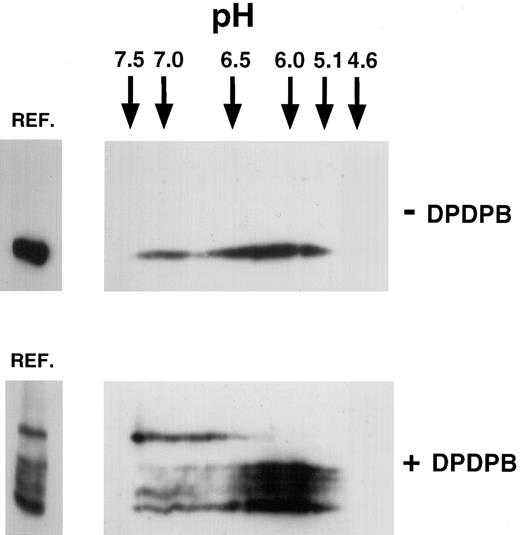
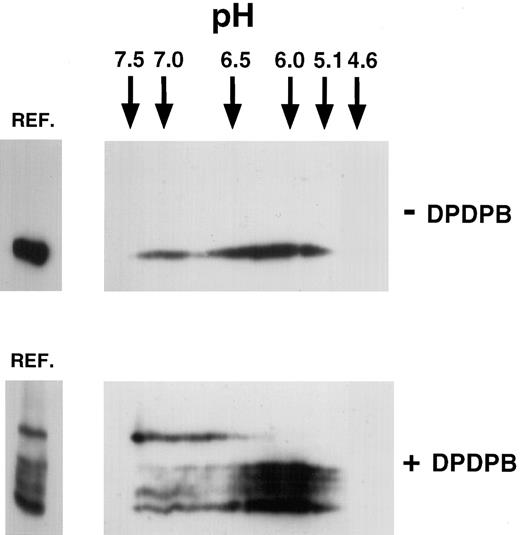
To determine whether the P-selectin dimers observed upon cross-linking represented homodimers or heterodimers of P-selectin, we analyzed the isoelectric point (pI) of the cross-linked and monomeric species by isoelectric focusing (IEF). Homodimers would be expected to have the same pI as monomeric P-selectin, whereas heterodimeric species would not. The theoretical pI of human P-selectin has been calculated as pH 6.17, as reported in the SWISS-PROT data bank. Cross-linker–treated and untreated lysates of resting platelets were separated on isoelectric focusing slab gels, followed by separation in the second dimension by SDS-PAGE (2-dimensional IEF) (Figure 2). P-selectin from cross-linker–untreated platelets gave a separation upon 2-dimensional IEF, ranging from pH 5.0 to pH 7.5. The broad separation of pI for P-selectin is typical for platelet glycoproteins.20 In lysates from platelets subjected to cross-linking, all cross-linked species of P-selectin were detected in the same range as that detected for P-selectin in cross-linker–untreated platelets. However, the dimeric species was polarized to the basic end of this range, whereas monomeric P-selectin and the triplet bands were polarized toward the acidic end. This finding suggests that a more basic subset of P-selectin forms homodimers. However, this finding is not inconsistent with P-selectin being a heterodimer composed of P-selectin and a protein with the same pI as P-selectin. Because the pI range of the triplet bands overlaps with that of the monomer, the triplet bands could represent conformers of P-selectin monomer captured by the cross-linker, as discussed earlier. On the other hand, they could also be P-selectin heterodimers with molecules that have a similar pI to P-selectin. For example, monomeric actin has a theoretical pI of 5.1,21 and heterodimers between P-selectin and monomeric actin would fall within the broad pI range seen for P-selectin in Figure 2.
Two-dimensional isoelectric focusing of P-selectin in platelets.
Triton X-100 lysates were made from resting untreated platelets (−DPDPB) or platelets treated with DPDPB (+DPDPB) and were subjected to electrophoresis on isoelectric focusing slab gels, as described in “Materials and methods.” The positions of IEF standards electrophoresed simultaneously are indicated. Gel lanes were then excised and subjected to electrophoresis in the second dimension on nonreducing 6% SDS-PAGE gels. Aliquots of appropriate lysate were run alongside in the reference well (REF.) for comparison. Samples were analyzed by immunoblotting using the P-selectin monoclonal antibody AC1.2.
Two-dimensional isoelectric focusing of P-selectin in platelets.
Triton X-100 lysates were made from resting untreated platelets (−DPDPB) or platelets treated with DPDPB (+DPDPB) and were subjected to electrophoresis on isoelectric focusing slab gels, as described in “Materials and methods.” The positions of IEF standards electrophoresed simultaneously are indicated. Gel lanes were then excised and subjected to electrophoresis in the second dimension on nonreducing 6% SDS-PAGE gels. Aliquots of appropriate lysate were run alongside in the reference well (REF.) for comparison. Samples were analyzed by immunoblotting using the P-selectin monoclonal antibody AC1.2.
Generation of P-selectin dimers in vitro
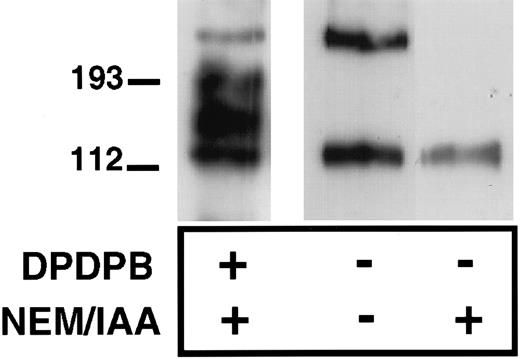
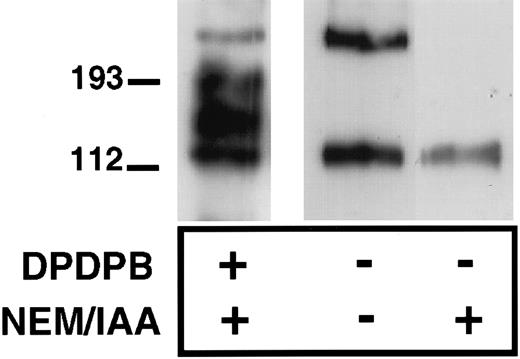
To determine further whether P-selectin can form homodimers, we assessed the ability of immunopurified P-selectin to dimerize in vitro. We reasoned that the unpaired cysteine, when removed from the reducing environment of the cell cytoplasm, would be able to form disulfide-bonded P-selectin dimers in vitro. P-selectin was immunopurified from platelet lysates in the presence or absence of IAA and NEM using a polyclonal P-selectin antibody, followed by collection of the immunoprecipitates on Protein G–Sepharose beads. The sample was then denatured and examined, nonreduced by immunoblot analysis using anti–P-selectin antibody. P-selectin dimers were detected under these conditions with the dimer having the same electrophoretic mobility as dimers detected in cross-linker–treated platelets (Figure 3). Thus, P-selectin dimers can be recreated in vitro, in the absence of other platelet proteins, suggesting that these are P-selectin homodimers. As expected, no P-selectin disulfide-bonded dimers were detected when NEM and IAA were included during immunoprecipitation of P-selectin because these agents alkylate free-sulfhydryl groups and would thus prevent the in vitro formation of disulfide bonds (Figure 3).
Formation of P-selectin dimers in vitro.
Resting platelets were lysed in the absence (−) or presence (+) of alkylating agents NEM and IAA and then immunoprecipitated with a P-selectin–specific polyclonal antibody, as described in “Materials and methods.” In the first lane, cross-linker (DPDPB)–treated platelets were lysed in the presence of alkylating agents, and P-selectin was immunoprecipitated with the polyclonal antibody. Immunoprecipitates were separated on 6% SDS gels and analyzed by immunoblotting using the P-selectin monoclonal antibody AK4.
Formation of P-selectin dimers in vitro.
Resting platelets were lysed in the absence (−) or presence (+) of alkylating agents NEM and IAA and then immunoprecipitated with a P-selectin–specific polyclonal antibody, as described in “Materials and methods.” In the first lane, cross-linker (DPDPB)–treated platelets were lysed in the presence of alkylating agents, and P-selectin was immunoprecipitated with the polyclonal antibody. Immunoprecipitates were separated on 6% SDS gels and analyzed by immunoblotting using the P-selectin monoclonal antibody AK4.
P-selectin dimers are formed in the ER/Golgi apparatus and are not preferentially stored
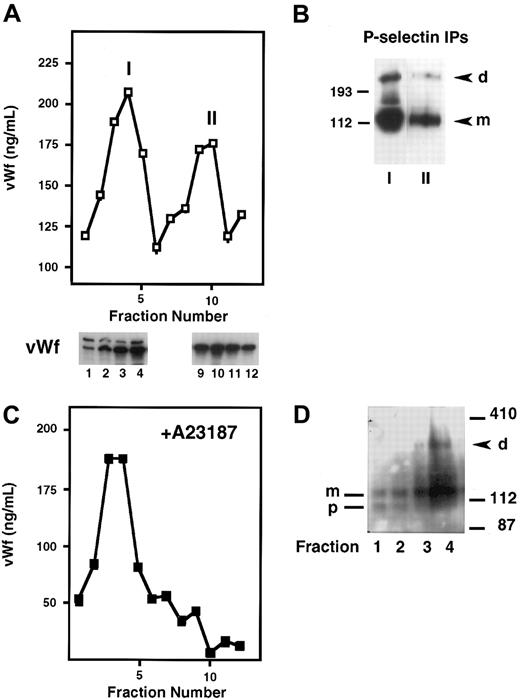
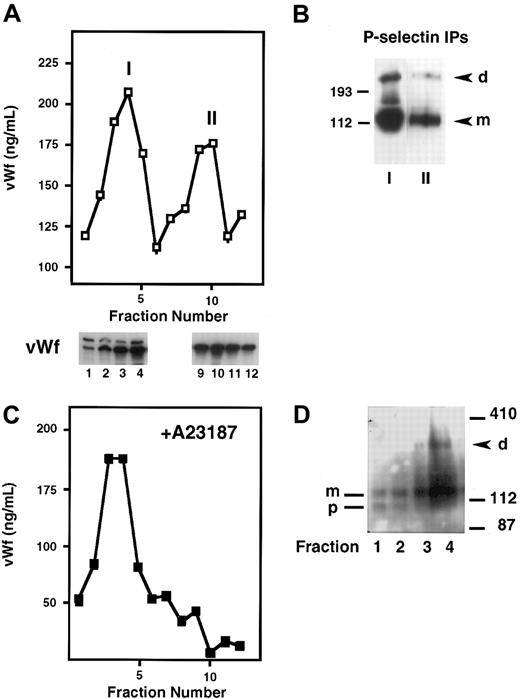
P-selectin is stored in platelets and endothelial cells as well as in transfected RIN5F cells and is dimeric in these cell types. To determine whether P-selectin dimerization occurs before or after storage in Weibel-Palade bodies, we fractionated homogenates of cross-linker–treated HUVECs on Percoll gradients. The fractions were then analyzed by a vWf ELISA to identify where the ER/Golgi apparatus versus Weibel-Palade bodies eluted. vWf is useful as a marker of Weibel-Palade bodies because it is packaged along with P-selectin into these granules in endothelial cells, and its fractionation pattern in HUVECs has been well described.15,16 Figure4A shows that there were 2 peaks of vWf, as described previously. “I” corresponds to lighter fractions containing vWf that represents the ER/Golgi compartment, and “II” shows heavier fractions representing Weibel-Palade bodies. To confirm further the intracellular identities of the components in peaks I and II, we analyzed the gradient fractions for the subunit composition of vWf by SDS-PAGE, followed by Western blotting. Previous studies have shown that vWf precursor (pro-vWf) and mature subunits are present in the ER/Golgi compartment, whereas only the mature subunit (m-vWf) is present in the post-Golgi/Weibel-Palade body compartment.22 Indeed, the earlier fractions contained pro-vWf subunit migrating at 260 kd and the mature subunit at 220 kd, whereas the later fractions contained only the mature 220-kd subunit, indicative of vWf in Weibel-Palade bodies (Figure 4A). Next, P-selectin was immunoprecipitated from peaks I and II. Dimer was found in both pools, and the ratio of dimer to monomer was similar in both compartments (Figure 4B). This indicates that P-selectin dimerization occurs before its storage in Weibel-Palade bodies. Additionally, these results indicate that dimer is not preferentially packaged into granules because the dimer/monomer ratio in the granule fraction is not enhanced compared with the ER/Golgi fraction.
Subcellular fractionation of HUVECs on Percoll gradients.
Resting HUVECs (A,B), or HUVECs stimulated with A23187 (C,D) were treated with DPDPB, homogenized, and fractionated on a 35% Percoll gradient. (A) Fractions were collected and analyzed by ELISA for the presence of vWf, which is present in 2 peaks: the first represents the ER/Golgi fraction (I) and the second represents Weibel-Palade bodies (II). Fractions 1 to 4 from peak I and 9 to 12 from peak II were analyzed by immunoblotting with a vWf polyclonal antibody. The Weibel-Palade fraction is recognized by the presence of only the mature subunit of vWf in this fraction, whereas both the precursor (260 kd) and mature subunit (220 kd) are present in the ER/Golgi fractions. (B) P-selectin was immunoprecipitated with a polyclonal antibody from pooled fractions 3 to 5 (peak I) and fractions 9 and 10 (peak II) and then immunoblotted with monoclonal P-selectin antibody AC1.2. P-selectin dimer (d) and monomer (m) are detected in both samples. (C) vWf ELISA profile obtained from A23187-treated HUVEC homogenates fractionated on a Percoll gradient. (D) P-selectin immunoblot of early fractions from a Percoll gradient of cross-linked, A23187-treated HUVECs. Fractions 1 and 2 contain P-selectin precursor (p) and mature subunit (m). Fractions 3 and 4 contain mature subunit and P-selectin dimer (d).
Subcellular fractionation of HUVECs on Percoll gradients.
Resting HUVECs (A,B), or HUVECs stimulated with A23187 (C,D) were treated with DPDPB, homogenized, and fractionated on a 35% Percoll gradient. (A) Fractions were collected and analyzed by ELISA for the presence of vWf, which is present in 2 peaks: the first represents the ER/Golgi fraction (I) and the second represents Weibel-Palade bodies (II). Fractions 1 to 4 from peak I and 9 to 12 from peak II were analyzed by immunoblotting with a vWf polyclonal antibody. The Weibel-Palade fraction is recognized by the presence of only the mature subunit of vWf in this fraction, whereas both the precursor (260 kd) and mature subunit (220 kd) are present in the ER/Golgi fractions. (B) P-selectin was immunoprecipitated with a polyclonal antibody from pooled fractions 3 to 5 (peak I) and fractions 9 and 10 (peak II) and then immunoblotted with monoclonal P-selectin antibody AC1.2. P-selectin dimer (d) and monomer (m) are detected in both samples. (C) vWf ELISA profile obtained from A23187-treated HUVEC homogenates fractionated on a Percoll gradient. (D) P-selectin immunoblot of early fractions from a Percoll gradient of cross-linked, A23187-treated HUVECs. Fractions 1 and 2 contain P-selectin precursor (p) and mature subunit (m). Fractions 3 and 4 contain mature subunit and P-selectin dimer (d).
To confirm further the identity of the Weibel-Palade body fraction and to rule out the possibility of contamination of the ER/Golgi fractions with Weibel-Palade components, we treated the cells first with the calcium ionophore A23187 to release granules, followed by cross-linking and fractionation. No vWf was detected in the later fractions, which should have contained Weibel-Palade bodies (Figure 4C). Aliquots of each fraction were immunoblotted for P-selectin. Dimer was detected in the fraction that contained the peak of the ER/Golgi vWf pool, thus confirming the results of our earlier experiment. Interestingly, the earliest fractions contained only P-selectin monomer and a slightly faster migrating species (Figure 4D), which has been identified previously as a precursor of P-selectin containing high mannose N-linked carbohydrates. Conversion of the high mannose chains to the complex N-linked oligosaccharides leads to the appearance of the mature subunit.23 This suggests that P-selectin dimer formation requires the synthesis of the mature P-selectin subunit and that P-selectin probably requires the final processing of its carbohydrate chains in the Golgi apparatus to be competent to dimerize.
Platelet activation leads to P-selectin homodimers and heterodimers on the cell surface
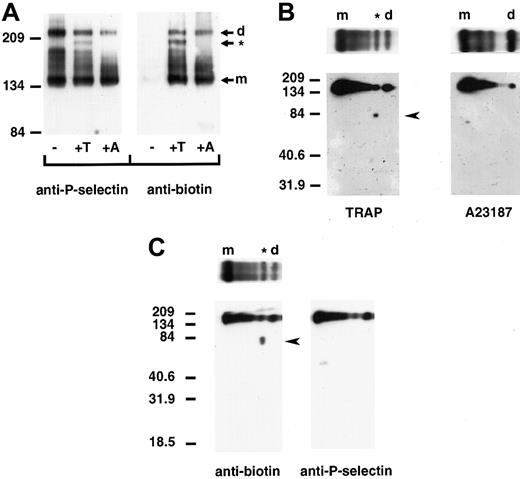
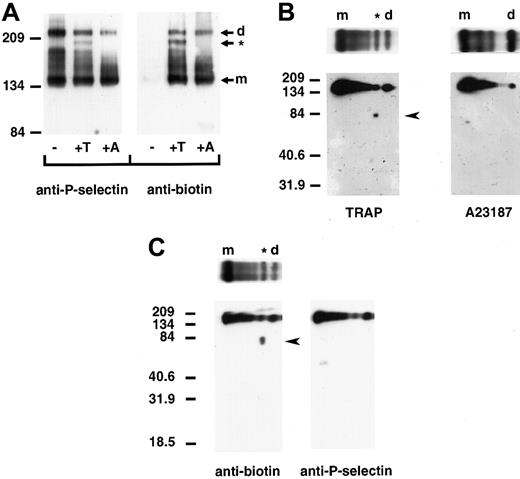
We next determined whether P-selectin dimers are present on the surface of platelets upon activation. Column-purified platelets were kept resting or activated with TRAP, which minimizes platelet aggregation, or the calcium ionophore A23187 and treated with cross-linker and a membrane-impermeable biotin to label surface proteins. Triton X-100–soluble cell lysates were immunoprecipitated with a polyclonal anti–P-selectin antibody, followed by immunoblot analysis using a monoclonal anti–P-selectin or antibiotin antibody. No P-selectin was detected on the surface of resting platelets. However, upon activation with A23187 or TRAP, biotinylated bands migrating at positions for dimeric and monomeric P-selectin were observed (Figure 5A). Thrombin activation of platelets gave the same pattern of P-selectin cross-linked species as that observed with TRAP activation (data not shown). Samples activated with TRAP plus EGTA, which prevents platelet–platelet interaction, gave a gel pattern that was indistinguishable from that of samples treated with TRAP alone (data not shown). This indicates that platelet aggregation is not responsible for the observed cross-linked P-selectin species. Thus, the data show that P-selectin dimers are present on the surface of activated platelets. In TRAP- and thrombin-activated platelets, an additional cross-linked species, migrating slightly faster than the dimer, was observed. Two-dimensional gel electrophoresis of TRAP-activated platelets revealed that this was a heterodimer of P-selectin and a biotinylated protein with an approximate molecular weight of 81 kd (Figure 5B). In agreement with the pattern obtained by one-dimensional electrophoresis, 2-dimensional gel electrophoresis did not detect the protein of approximately 81 kd in lysates of A23187-activated platelets. The 81-kd protein is not a truncated form of P-selectin because a polyclonal antibody to P-selectin did not recognize it on Western blots (Figure 5C). Assuming comparable biotin labeling of P-selectin and the 81-kd protein, the heterodimer represents 15.4% ± 0.2% of the cell surface–labeled P-selectin as determined by densitometry of the one-dimensional gels. The homodimer represents 14.4% ± 1.5%.
P-selectin dimers are present on the surface of activated platelets.
Resting (−) and TRAP (+T)- or A23187 (+A)-activated platelets were treated with DPDPB and a non–membrane permeable biotinylation reagent. The cells were then lysed in buffer containing alkylating agents, and P-selectin was immunoprecipitated with a polyclonal P-selectin antibody. (A) Samples were electrophoresed under nonreducing conditions and immunoblotted with a monoclonal antibody to P-selectin AC1.2 (left) or biotin (right). The positions of the dimer (d) and monomer (m) are indicated. The cross-linked species migrating faster than the dimer in TRAP-activated platelets is identified with an asterisk (*). (B) Gel strips of TRAP-activated (left) andA23187-activated (right) platelet samples electrophoresed in the first dimension were reduced and placed on top of a second 10% polyacrylamide gel (as shown), then subjected to electrophoresis from top to bottom and immunoblotted with a monoclonal antibody to biotin to determine the composition of the oligomers. A broad band detected between molecular-weight markers 134 and 209 migrated at the position for P-selectin. The biotinylated protein of approximately 81 kd present in TRAP- but not in A23187-activated platelet samples is indicated by an arrowhead. (C) Two gel strips of TRAP-activated platelet samples were subjected to electrophoresis in the second dimension, and gels were immunoblotted with either the monoclonal antibiotin antibody (left, as in B) or a polyclonal antibody to P-selectin (right). The biotinylated band of approximately 81 kd (arrowhead at left) was not detected in gels immunoblotted with P-selectin antibody (right).
P-selectin dimers are present on the surface of activated platelets.
Resting (−) and TRAP (+T)- or A23187 (+A)-activated platelets were treated with DPDPB and a non–membrane permeable biotinylation reagent. The cells were then lysed in buffer containing alkylating agents, and P-selectin was immunoprecipitated with a polyclonal P-selectin antibody. (A) Samples were electrophoresed under nonreducing conditions and immunoblotted with a monoclonal antibody to P-selectin AC1.2 (left) or biotin (right). The positions of the dimer (d) and monomer (m) are indicated. The cross-linked species migrating faster than the dimer in TRAP-activated platelets is identified with an asterisk (*). (B) Gel strips of TRAP-activated (left) andA23187-activated (right) platelet samples electrophoresed in the first dimension were reduced and placed on top of a second 10% polyacrylamide gel (as shown), then subjected to electrophoresis from top to bottom and immunoblotted with a monoclonal antibody to biotin to determine the composition of the oligomers. A broad band detected between molecular-weight markers 134 and 209 migrated at the position for P-selectin. The biotinylated protein of approximately 81 kd present in TRAP- but not in A23187-activated platelet samples is indicated by an arrowhead. (C) Two gel strips of TRAP-activated platelet samples were subjected to electrophoresis in the second dimension, and gels were immunoblotted with either the monoclonal antibiotin antibody (left, as in B) or a polyclonal antibody to P-selectin (right). The biotinylated band of approximately 81 kd (arrowhead at left) was not detected in gels immunoblotted with P-selectin antibody (right).
The 81-kd protein is as yet unidentified. Recent studies have suggested that protein disulfide isomerase (PDI; molecular weight approximately 61 kd), which catalyzes thio-disulfide interchange, is located close to platelet surface proteins such as platelet glycoprotein 1bα.24 However, antibody to PDI did not detect PDI in P-selectin immunoprecipitates under reducing or nonreducing conditions, but did detect PDI in platelet cell lysates (T.N.M., F.J.B., unpublished data).
Discussion
The selectins mediate leukocyte rolling through rapid association and dissociation with their ligands. The avidity of this interaction is sufficient to allow the leukocytes to resist premature dissociation by shear forces encountered in blood. Dimerization or oligomerization of cell adhesion molecules may be a physiologically relevant feature to enhance the avidity of ligand–receptor pairs. Dimerization of P-selectin glycoprotein ligand-1 (PSGL-1) is important for functional interactions with P-selectin.25 P-selectin isolated from platelets, observed to be predominantly dimeric by sedimentation velocity and equilibrium studies, has a higher affinity for PSGL-111 than monomeric P-selectin because it supports HL-60 cell adhesion at lower site densities than P-selectin monomers.11 Finally, the forced dimerization of L-selectin increased the number of rolling lymphocytes on endothelium, reduced their rolling velocity, and decreased receptor-detachment rates.8 Thus, there is compelling evidence for the physiologic importance of selectin dimerization in increasing the avidity of selectin–ligand interactions.
Here, we show that P-selectin is present as noncovalent dimers in platelets and endothelial cells. P-selectin dimers are present both within the α-granules of resting platelets and on the surface of activated cells. Thus, although functional assays using recombinant P-selectin and L-selectin forced to dimerize have suggested the importance of dimeric selectins, this is the first demonstration that a member of the selectin family is dimeric in intact cells. P-selectin dimers were not detected in cells lysed in the presence of sulfhydryl inhibitors but were detected only using a chemical cross-linker that would stabilize existing noncovalent associations between proteins. Thus, our data suggest that P-selectin dimers are noncovalent, unlike PSGL-1, which forms covalent dimers on leukocytes26,27 even when sulfhydryl inhibitors are included during cell lysis to avoid spurious disulfide-bond formation (T.N.M., unpublished data). Because the thiol-specific cross-linker used in our study can stabilize the association of only 2 molecules, our studies do not rule out the possibility that higher-molecular-weight multimers of P-selectin exist in cells, as has been observed for purified P-selectin in detergent solution.11
As in the case of L-selectin, P-selectin dimerization may dictate the rolling velocity of cells or the detachment rate once rolling is established. Furthermore, interactions between dimeric PSGL-1 and dimeric/oligomeric P-selectin could lead to the formation of large multivalent complexes that are important in signaling or other cellular events. For example, clustering of P-selectin with intact antibody has been shown to trigger transient increases in cytosolic Ca++,28 which is required for the transmigration of neutrophils across the endothelial cell barrier.29 Recent studies by Setiadi et al30suggested that interaction of P-selectin with clathrin-coated pits may enhance leukocyte adhesion under flow by providing a microdomain of clustered P-selectin. This provides a mechanism in addition to dimer formation for increasing the clustering and avidity of the P-selectin–PSGL-1 pair.
Two lines of evidence point to the theory that P-selectin dimer in cross-linker–treated platelets and endothelial cells being homodimeric. The isoelectric profile of dimeric P-selectin was contained within the range seen for P-selectin in untreated platelets, and immunopurified P-selectin formed dimers in vitro in the absence of other platelet proteins. Because the dimeric P-selectin was more basic than the monomeric protein, it is not inconsistent with it being a heterodimer. However, this is unlikely because the other putative molecule would have to be of the same molecular weight and pI of P-selectin; have a free sulfhydryl; and be present in platelets, endothelial cells, and RIN5F cells. We favor the interpretation that P-selectin is homodimeric and is composed of a subset of basic P-selectin, which may be differentially modified relative to the monomer. P-selectin is posttranslationally modified by N- but not O-linked carbohydrates. Accordingly, sialic acid, neutral sugars, and glucosamine residues, but not galactosamine, are detected on P-selectin.31 It is possible that preferential modification of the dimer versus monomer with more negatively charged carbohydrates such as sialic acid could polarize the pI of P-selectin dimers to the basic range as compared with the monomer. Phosphorylation of a molecule changes the pI to an acidic range. P-selectin has been shown to be constitutively phosphorylated,32,33 with phosphorylation increasing and then decreasing on selected amino acids after platelet activation.33 Thus, the dimer may be a dephosphorylated subset of P-selectin. P-selectin is also acylated and palmitoylated at C766 through a thioester bond,34 although these modifications are unlikely to affect the pI of the molecule. The intermediate bands between monomer and dimer lie within the pI range observed for P-selectin but are skewed toward the acidic end along with monomeric P-selectin. These bands may represent P-selectin cross-linked to components of the actin cytoskeleton, such as actin in platelets, because the actin-depolymerizing agent DNase I collapses the triplet bands to one band, giving a pattern of cross-linked P-selectin species that is similar to that seen in HUVECs. It is possible that the triplet bands are not observed in HUVECs because there are known differences between the cytoskeleton of platelets and endothelial cells. For example, the platelet cytoskeleton is very dynamic, with actin polymer content doubling from 40% at rest to 80% upon activation,35 whereas in confluent HUVECs, the cytoskeleton is more stable, functioning to provide resistance to shear stress.36,37 Furthermore, in platelets, α-actinin, an F-actin–binding protein, has been shown to be associated with the α-granules,38 whereas similar findings have not been reported for the Weibel-Palade bodies of endothelial cells.
Dimerization or multimerization may represent a signal for storage of proteins, or it may actually occur in storage granules. In the case of vWf, another protein stored in the Weibel-Palade bodies of endothelial cells, dimerization occurs in the ER and multimerization occurs in the Golgi apparatus, with only the highly multimeric, most biologically potent forms of vWf being stored.22 In the case of P-selectin, we have shown that dimerization occurs in the ER/Golgi apparatus. The mature subunit dimerizes, whereas the precursor, which does not have the proper carbohydrate processing, does not. However, the P-selectin dimers are not preferentially stored in either the Weibel-Palade bodies or the α-granules, and both monomers and dimers are present on the surface of activated platelets. This suggests that both the monomer and dimer of P-selectin may be functionally important. It is possible that laterally diffusing monomer may be important in making low-affinity tethers with its ligand to initiate leukocyte rolling, whereas the dimeric form may exhibit strong binding to its ligand by decreasing the detachment rate (ie, increasing the koff rate) and thus stabilizing leukocyte rolling. This would likely influence subsequent leukocyte migration. In addition, dimerization may be particularly important at sites where the density of P-selectin or its ligands may be low.
Platelet activation with TRAP or thrombin led to the appearance of a heterodimeric P-selectin species on the platelet surface in addition to P-selectin homodimers. This association occurred in the absence of any other detectable associations of P-selectin with cell-surface proteins and involved approximately 15% of cell-surface P-selectin. Thus, P-selectin closely associates with another protein on the platelet cell surface upon thrombin-receptor activation of platelets; this represents the first demonstration that a selectin can associate with other proteins. This finding suggests that P-selectin may influence the function of another protein, and vice versa, on activated platelets. PDI has been identified recently on the platelet surface39to be physically associated with the platelet glycoprotein 1bα.24 Furthermore, PDI has been reported as catalyzing the formation of complexes between thrombin-antithrombin and either vitronectin40 or thrombospondin-1.41 In the current study, however, PDI was not found to be present in P-selectin immunoprecipitates probed with a polyclonal antibody specific for PDI. Future identification of the approximately 81-kd protein and the functional relevance of the P-selectin–81-kd association may provide key insights into possible additional functions of P-selectin in activated platelets.
Acknowledgments
We thank Kay Case (Tissue Culture Facility, Vascular Research Division, Department of Pathology, Brigham and Women's Hospital, Boston, MA) for providing HUVECs, Dr Denisa Wagner (Center for Blood Research, Harvard Medical School, Boston, MA) for providing RIN5F cells transfected with P-selectin, and Dr Michael Berndt (Baker Medical Research Institute, Monash University, Prahran, Victoria, Australia) for providing polyclonal anti–P-selectin antibody.
Supported by National Institutes of Health grants DK51643 and NS33296 (T.N.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tanya N. Mayadas, Brigham and Women's Hospital and Harvard Medical School, 221 Longwood Ave, Room 404, Boston, MA 02115; e-mail:tmayadas@rics.bwh.harvard.edu.