Abstract
Vaccination of patients with cancer using dendritic cells (DCs) was shown to be effective for B-cell lymphoma and malignant melanoma. Here we provide evidence that patients with advanced breast and ovarian cancer can be efficiently vaccinated with autologous DCs pulsed with HER-2/neu– or MUC1-derived peptides. Ten patients were included in this pilot study. The DC vaccinations were well tolerated with no side effects. In 5 of 10 patients, peptide-specific cytotoxic T lymphocytes (CTLs) could be detected in the peripheral blood using both intracellular IFN-γ staining and 51Cr-release assays. The major CTL response in vivo was induced with the HER-2/neu–derived E75 and the MUC1-derived M1.2 peptide, which lasted for more than 6 months, suggesting that these peptides might be immunodominant. In addition, in one patient vaccinated with the MUC1-derived peptides, CEA- and MAGE-3 peptide-specific T-cell responses were detected after several vaccinations. In a second patient immunized with the HER-2/neu peptides, MUC1-specific T lymphocytes were induced after 7 immunizations, suggesting that antigen spreading in vivo might occur after successful immunization with a single tumor antigen. Our results show that vaccination of DCs pulsed with a single tumor antigen may induce immunologic responses in patients with breast and ovarian cancer. This study may be relevant to the design of future clinical trials of other peptide-based vaccines.
Introduction
The identification of tumor-associated antigens (TAAs) opened new opportunities for the treatment of patients with malignant diseases.1,2 For the treatment of breast and ovarian cancer, however, only few T-cell epitopes have been identified, including those derived from the HER-2/neu protooncogene and the epithelial mucin MUC1. HER-2/neu is overexpressed in 20% to 30% of patients with breast and ovarian cancer and correlates with a poor prognosis.3-5 Two HLA-A2 binding peptides (E75 and GP2) derived from the HER-2/neu protein were identified6,7 and in vitro studies in our laboratory using these peptides for cytotoxic T-lymphocyte (CTL) induction demonstrated that these epitopes efficiently elicit antigen-specific T-cell responses against a variety of solid tumors, including renal cell and colon carcinoma when loaded on DCs.8 In contrast to the restricted expression of HER-2/neu, the MUC1 protein is overexpressed on more than 90% of breast and ovarian cancers and is therefore a suitable candidate for broadly applicable vaccine therapies.9-13 Mucins are transmembrane type I glycoproteins with a unique extracellular domain consisting mostly of 20 to 60 tandem repeats (VNTRs).9 We recently identified 2 novel 9-mer peptides, M1.1 and M1.2, with a high-binding probability to HLA-A2; the M1.1 peptide is derived from the VNTR domain of the MUC1 protein, whereas the M1.2 peptide is located in the leader sequence.14 CTL induced with these peptides efficiently lysed target cells pulsed with the cognate peptide or tumor cells naturally expressing MUC1 in major histocompatibility complex (MHC)–restricted and antigen-specific manner.14
Dendritic cells (DCs) are the most potent antigen-presenting cells with the unique ability to initiate and maintain primary immune responses when pulsed with antigens.15-20 They originate from the bone marrow and their precursors migrate via the blood stream to almost all the organs, where they can be found in an immature state characterized by a high rate of antigen uptake.21 On stimulation with bacterial products, cytokines, or CD40 ligation, DCs undergo characteristic modulations of their phenotype, antigen-presenting function and the ability to migrate to the secondary lymphoid organs. These mature DCs express high levels of costimulatory and MHC molecules and are regarded as the initiators of primary immune responses. In vitro, DCs can develop from peripheral blood CD14+ monocytes when grown in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4). These cells have the characteristics of immature DCs and can be further induced to mature by inflammatory stimuli such as tumor necrosis factor α (TNF-α), IL-1, lipopolysaccharides (LPS), or CD 40 ligation.22-24
Vaccinations using DCs pulsed with TAAs were shown to be effective for patients with B-cell lymphoma and malignant melanoma,25-29for which spontaneous remissions30 31 due to immunologic reactions as well as responses to immunotherapy based treatments were reported. In this phase I/II study, we analyzed the feasibility and efficacy of HER-2/neu or MUC1 peptide-pulsed “mature” DC vaccinations in heavily pretreated patients with metastatic breast and ovarian cancer. We show that this approach is feasible and induces specific cytotoxic T-cell responses without any side effects. In addition, we demonstrate that vaccinations with a single tumor antigen can induce responses against different epitopes derived from other tumor antigens not used for vaccination, suggesting that epitope spreading might occur in vivo after the vaccination with a single tumor antigen.
Patients, materials and methods
Patient characteristics and clinical protocol
The clinical protocol was approved by the Institutional Ethical Review Board at the University of Tübingen. All patients had histologically confirmed metastatic breast or ovarian cancer that expressed HLA-A2 and HER-2/neu or MUC1 and underwent a complete clinical evaluation, including measurements and radiologic examination of all available tumor sites. Inclusion criteria were bidimensionally measurable metastatic lesions, an ECOG score less than 3, and a positive delayed hypersensitivity test for common recall antigens (Multitest Merieux, Leimen, Germany). All patients were heavily pretreated, including surgery, radiation, multiple cycles of chemotherapy, as well as antihormonal treatment. No patient had received any systemic treatment in the prior 4 to 6 weeks or any immunosuppressive drugs, including steroids. Patients with brain metastasis or second malignancy as well as participants in other clinical studies were excluded. Ten patients were recruited and basic data are presented in Table 1.
Peptide-pulsed DCs generated from peripheral blood monocytes were injected subcutaneously into the upper limb close to the inguinal lymph nodes on days 1, 14, and 28, respectively. On day 35, an evaluation of clinical responses was performed. The vaccine treatment was continued in case of stable disease or tumor regression every 28 days until tumor progression. Patients with progressive disease after 3 vaccinations went off study. The World Health Organization (WHO) definitions of clinical responses and adverse effects were applied.
Cell isolation and cultures
Peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll/Paque (Gibco-BRL, Grand Island, NY) density gradient centrifugation of 100 mL of heparinized blood. Isolated PBMNCs were plated (1 × 107 cells/3 mL per well) into 6-well plates (Costar, Cambridge, MA) in serum-free X-VIVO 20 medium. After 2 hours of incubation at 37°C, nonadherent cells were removed and the adherent cells (12%-19% of the incubated cells) were cultured in X-VIVO 20 medium supplemented with IL-4, GM-CSF, and TNF-α. The population of adherent cells remaining in the wells comprised of more than or equal to 90% CD14 positive cells, 2% to 6% CD3 positive cells, and 0% to 2% CD19 positive cells. The percentage of CD1a+ or CD83+ cells was less than 1%.24
IL-4 (1000 IU/mL) and TNF-α (10 ng/mL) were purchased from Genzyme (Cambridge, MA) and Boehringer Mannheim (Mannheim, Germany), respectively. Human recombinant GM-CSF (Leucomax, 100 ng/mL) was from Novartis (Basle, Switzerland). The cultures were fed with fresh medium and cytokines every 2 to 3 days, and cell differentiation was monitored by light microscopy. The phenotype of DCs was analyzed after 7 days of culture.
The peptides derived from HER-2/neu (E75: KIFGSLAFL, GP2: IISAVVGIL) and MUC1 (M1.1 STPPVHNV, M1.2: LLLLTVLTV) were synthesized using standard Fmoc chemistry on a peptide synthesizer (432A, Applied Biosystems, Weiterstadt, Germany) and analyzed by reverse phase high-performance liquid chromatography (HPLC) and mass spectrometry. The patients received either HER-2/neu or MUC1 peptides, corresponding to the histologic analysis. If the tumor samples expressed both antigens, patients received HER-2/neu peptides only. DCs were separately pulsed for 2 hours with 50 μg/mL of each peptide and washed 3 times with phosphate-buffered saline (PBS) before application.
Immunostaining
Cell staining was performed using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse mAbs against CD86, CD40 (all purchased from Pharmingen, Hamburg, Germany); CD3, CD19, CD20, CD80, HLA DR, CD54, CD14 (Becton Dickinson, Heidelberg, Germany), CD83 (Coulter-Immunotech Diagnostics, Hamburg, Germany), CD1a (OKT6, Ortho Diagnostic Systems; and T6-RD1, Coulter Immunology, Hialeah, FL), and mouse IgG isotype controls. Samples were analyzed on a FACScan Calibur (Becton Dickinson, Seattle, WA).
Intracellular IFN-γ staining
The intracellular staining for IFN-γ production was performed as recently described.30 The 2.5 × 105PBMNCs obtained before and after the vaccinations were stimulated with the MUC1- or HER-2/neu–derived peptides (50 μg/mL) for 6 hours. The 10 mmol Brefeldin A (Sigma Chemical Co, St Louis, MO) were added during the last 3 hours. An HLA-A2 binding HIV peptide (pol HIV-1 reverse transcriptase peptide, amino acids 476-484, ILKEPVHGV) was used as a negative control. In addition, HLA-A2 binding peptides derived from CEA (CAP-1, amino acids 571-579, YLSGANLNL) and MAGE-3 (M3-271, amino acids 271-279, FLWGPRALV) were used in the assay. Positive controls were performed by stimulating the cells with PMA (50 ng/mL, Sigma) and ionomycin (500 ng/mL, Sigma). Cells were stained with a FITC-conjugated anti-CD8 (Becton Dickinson, Heidelberg, Germany). After washing, cells were fixed with 2% formaldehyde/PBS for 15 minutes at room temperature, permeabilized in 0.5% saponin, 2% bovine serum albumin in PBS, and stained with a PE-labeled antibody reactive with human IFN-γ (Pharmingen, Hamburg, Germany).
To confirm that the results obtained with this method are not a reflection of random fluctuations, blood samples from patients were split into several portions and analyzed 2 to 3 times at different time points. These repeated analyses of samples drawn from the same patient at the same time gave identical results. Furthermore, blood samples of 5 healthy donors and 9 patients with metastatic malignant diseases, including pancreatic cancer (n = 3), cancer of unknown primary (n = 1), and renal cell carcinoma (n = 5), were analyzed. The level of IFN-γ secreting CD8+ T cells (measured with an irrelevant HIV-peptide, as well as with the MUC1- or HER-neu–derived peptides) never exceeded more than 0.3%.
Analysis of antigen-specific cytotoxic T-lymphocyte responses after in vitro restimulation
The 5 × 105 DCs were pulsed with 50 μg/mL of the synthetic peptides for 2 hours, washed, and incubated with 2.5 × 106 autologous PBMNCs in RP10 medium. Cells were restimulated after 7 days of culture and 1 ng/mL human recombinant IL-2 (Genzyme) was added every other day.8 The cytolytic activity of the CTLs was analyzed on day 5 after the restimulation in a standard 51Cr-release assay.
Cytotoxic T-lymphocyte assay
The standard 51Cr-release assay was performed with some modifications as described.8 MCF-7 (breast cancer cell line, HLA-A2+, MUC1+), A498 (renal cell carcinoma, HLA-A2+, HER-2/neu+), SK-OV-3 cells (ovarian cancer, HLA-A2−, MUC1+, HER-2/neu+), Croft cell line (immortalized B-cell line, HLA-A2+, HER-2/neu−, MUC1−, kindly provided by O. J. Finn, University of Pittsburgh, PA), K562 (no MHC expression, MUC1+, sensitive to natural killer [NK]-cell–mediated lysis), and T2 cells (174 × CEM.T2 hybridoma, HLA-A2+, and TAP1 and TAP2 deficient) were used as target cells in the assay. T2 cells were pulsed with 25 μg/mL peptide for 2 hours and labeled with [51Cr]-sodium chromate in RP10 for 1 hour at 37°C. The 104 cells were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTLs were added to give a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, supernatants (50 μL per well) were harvested and counted in a microbeta counter (Wallac). The percentage specific lysis was calculated as: 100 × (experimental release − spontaneous release/maximal release − spontaneous release). Spontaneous and maximal release were determined in the presence of either medium or 1% Triton X-100, respectively.
Results
Clinical results of vaccinations using HER-2/neu or MUC1 peptide-pulsed dendritic cells
DCs were generated under serum-free conditions from peripheral blood monocytes using GM-CSF and IL-4 and activated by the addition of TNF-α to the culture medium, as previous in vitro studies demonstrated that TNF-α may increase the antigen-specific stimulatory capacity of T cells by DCs.8
DCs obtained after 7 days of culture expressed high levels of CD83, HLA-DR, and costimulatory molecules, corresponding to the mature phenotype, as a result of TNF-α stimulation (data not shown, Brossart et al24). These DCs were pulsed with the antigenic peptides derived from either the HER-2/neu (E75 and GP2) or the MUC1 (M1.1 and M1.2) protein, depending on the expression of the corresponding TAA by the tumor, as indicated in Table 1.
DCs were injected subcutaneously close to the inguinal lymph nodes of the patients. The patients received a median of 6.5 × 106 DCs (range 2-17 × 106 DCs) per vaccine. The administration of DCs was performed every 14 days 3 times and repeated afterward every 28 days until tumor progression was observed. A total of 53 vaccinations were performed. The DC injections were well tolerated with no side effects.
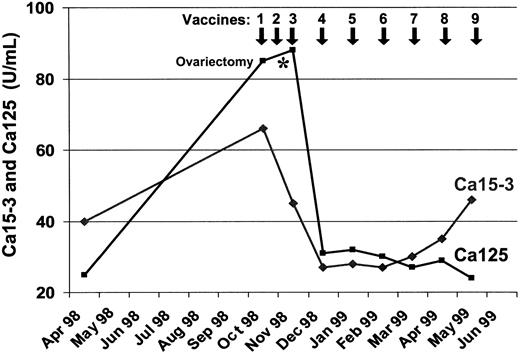
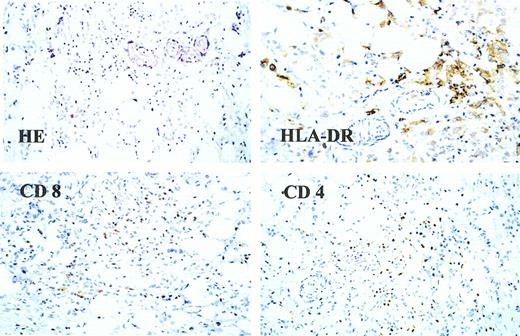
One patient (no. 8) with metastatic breast cancer who was treated with MUC1 peptide-pulsed DCs developed a regression of her multiple subcutaneous lesions. In parallel with the disappearance of the soft-tissue metastases, the serum levels of CA125 and CA15.3 continuously decreased after the third vaccination but started to increase again 5 months later (Figure 1). Extensive staging procedures 6 months after starting DC therapy revealed multiple central nervous system (CNS) metastases but no signs of visceral disease, with the exception of a small soft-tissue nodule in her left axilla that was detected by the patient and that was not clinically evident before. This residual nodule was removed and analyzed histologically for the presence of infiltrating T lymphocytes. As shown in Figure 2, both tumor-infiltrating CD8 and CD4 positive T cells were detected. HLA-DR staining of the tumor sample revealed the presence of large MHC class II–positive cells in the lesion that might represent antigen-presenting cells such as DCs or macrophages.
Levels of Ca125 and Ca15-3 tumor markers in serum of patient no. 8 with metastatic breast cancer during the vaccination with MUC1 peptide-pulsed DCs.
Levels of Ca125 and Ca15-3 tumor markers in serum of patient no. 8 with metastatic breast cancer during the vaccination with MUC1 peptide-pulsed DCs.
Histologic analysis of a soft-tissue nodule from patient no. 8.
This lesion was analyzed for infiltrating lymphocytes using monoclonal antibodies against CD4, CD8, and HLA-DR. In addition, conventional hematoxylin-eosin staining was performed.
Histologic analysis of a soft-tissue nodule from patient no. 8.
This lesion was analyzed for infiltrating lymphocytes using monoclonal antibodies against CD4, CD8, and HLA-DR. In addition, conventional hematoxylin-eosin staining was performed.
Before entering the DC vaccination trial, this patient was on palliative gosereline and tamoxifen treatment since July 1997, when she developed multiple progressive soft-tissue nodules, ovarian enlargement, and a continuous increase in her tumor marker levels until October 1998 when we started the DC vaccinations. Between the second and third DC vaccination, her tumor-infiltrated ovaries were removed by a gynecologist working outside the clinical trial by laparoscopy. Although we cannot exclude that ovariectomy contributed to the decline in the CA125 levels, it seems unlikely that the soft-tissue metastases disappeared as a result of ovariectomy, because the patient was already functionally ovariectomized since 1997 on the gosereline and tamoxifen treatment, and the soft-tissue nodules developed during this antihormonal therapy. However, the clinical response observed in this individual cannot be considered as proof that the vaccination works, particularly because the vaccination and ovariectomy were performed concurrently.
Another patient with ovarian cancer (no. 6) had stable disease (8+ months) after having had progressive disease before the vaccination and a debulking surgery. She is currently continuing the DC vaccinations. One additional patient (no. 7) had a short period of disease stabilization (approximately 8 weeks) after the third vaccination with MUC-1 peptide-pulsed DCs.
Peptide-specific T-cell responses in patients after dendritic cell immunizations
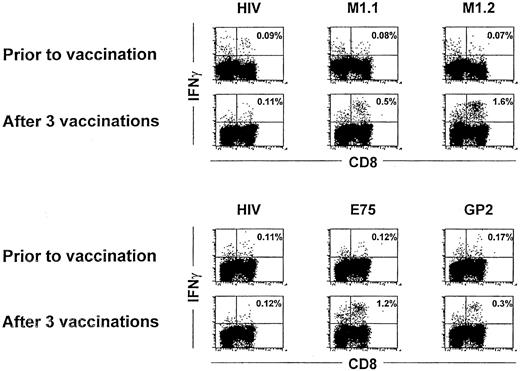
The antigen-specific response in the 10 patients receiving peptide-pulsed DC immunizations was determined by analysis of IFN-γ production by T lymphocytes on stimulation with the cognate peptide in vitro. IFN-γ production in CD8+ lymphocytes was assessed by flow cytometry after intracellular staining with IFN-γ–specific antibody. No reactivity against the used peptide antigens was observed in any tested patient before vaccination or after the first 2 DC vaccinations (Figure 3 and data not shown). After 3 vaccinations, however, in 5 of 10 patients (3 immunized with the MUC1 and 2 with HER-2/neu peptides), antigen-specific T-cell responses could be detected in peripheral blood using intracellular IFN-γ staining (Table 2 and Figure 3). In HER-2/neu–immunized patients, the major CTL response in vivo was induced with the E75 peptide. Patients receiving MUC1 peptides had a stronger response to the M1.2 peptide derived from the leader sequence, suggesting that the E75 and M1.2 peptides might be immunodominant. After 3 vaccinations, approximately 0.5% to 2% of all CD8+ lymphocytes (Table 2 and Figure 3) produced IFN-γ on stimulation with the cognate peptide. The HIV peptide was used as a negative control and considered as a background reactivity.
IFN-γ staining before and after 3 vaccinations.
Intracellular IFN-γ staining in patient no. 10 (upper panel) and patient no. 6 (lower panel) before and after 3 vaccinations. PBMNCs from each patient were incubated with autologous PBMNCs pulsed with the indicated peptides for 6 hours, and IFN-γ production was assessed by flow cytometry after intracellular staining for IFN-γ in CD8 positive lymphocytes. Numbers represent the percentage of IFN-γ–expressing cells in the lymphocyte gate.
IFN-γ staining before and after 3 vaccinations.
Intracellular IFN-γ staining in patient no. 10 (upper panel) and patient no. 6 (lower panel) before and after 3 vaccinations. PBMNCs from each patient were incubated with autologous PBMNCs pulsed with the indicated peptides for 6 hours, and IFN-γ production was assessed by flow cytometry after intracellular staining for IFN-γ in CD8 positive lymphocytes. Numbers represent the percentage of IFN-γ–expressing cells in the lymphocyte gate.
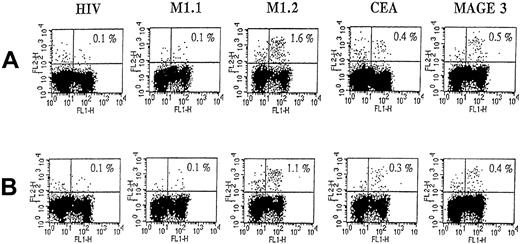
Interestingly, as analyzed in 2 patients (no. 6 and no. 8), E75 and M1.2 peptide-specific T-cell responses could be detected even at 6 and 9 months after initiation of DC vaccinations, as analyzed by intracellular IFN-γ staining (Figure 4, Table 3, and Table4).
Intracellular IFN-γ staining of peptide-specific CD8+ T cells in patient no. 8 after 6 and 9 vaccinations.
For details, see legend to Figure 3. In addition, CEA and MAGE-3 peptides were used in this assay. (A) T-cell response after 6 vaccinations. (B) T-cell response after 9 vaccinations. FL-1 represents staining with anti-CD8, FL-2 represents IFN-γ staining.
Intracellular IFN-γ staining of peptide-specific CD8+ T cells in patient no. 8 after 6 and 9 vaccinations.
For details, see legend to Figure 3. In addition, CEA and MAGE-3 peptides were used in this assay. (A) T-cell response after 6 vaccinations. (B) T-cell response after 9 vaccinations. FL-1 represents staining with anti-CD8, FL-2 represents IFN-γ staining.
Immunization with a single tumor antigen can induce T-cell responses against epitopes not used for vaccination
To analyze whether immunizations with a single TAA can lead to induction of CTL specific for antigens not used for vaccination (antigen spreading) as a result of cross priming, we used a set of several HLA-A2 binding peptides, including the CAP-1 (CEA) and M3-271 (MAGE-3) peptides derived from antigens known to be expressed in breast and ovarian cancers. This analysis has been performed by intracellular IFN-γ staining in 4 patients who showed peptide-specific reactivity against antigens used for vaccination. In patient no. 8 who was vaccinated with the MUC1 peptides and responded to the treatment, CEA- and MAGE-3 peptide-specific T cells were observed after 6 and 9 vaccinations, suggesting that epitope spreading might occur in vivo after successful vaccination (Figure 4, Table 3). No such reactivity could be detected in this patient before the therapy or after the first 5 vaccinations (Table 3). Interestingly, whereas the T-cell response to the M1.2 peptide was still present after 9 vaccinations, no reactivity against the second M1.1 peptide could be detected in the peripheral blood of this patient.
In a second patient (no. 6) who was immunized with HER-2/neu peptides, antigen-specific reactivity against the M1.2 and M1.1 peptides was detected in peripheral blood after 7 vaccinations. This MUC1-specific T-cell response was not present after the first 5 immunizations (Table4). These findings were supported by the histologic analysis of tumor samples that confirmed MUC1 expression by tumor cells (data not shown).
In vivo–induced T cells can lyse tumor cells expressing the antigen
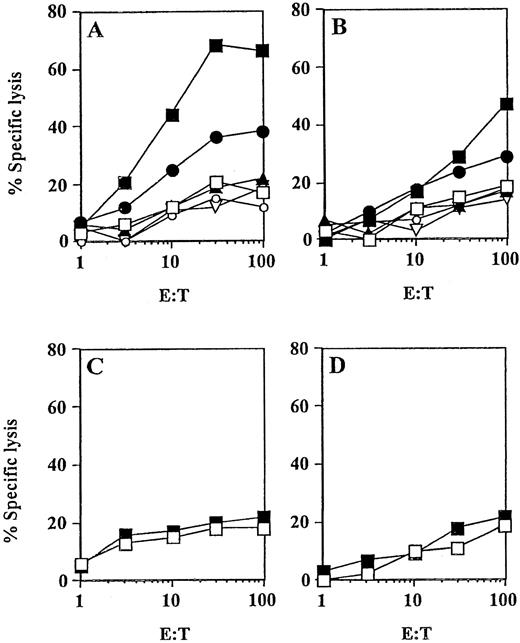
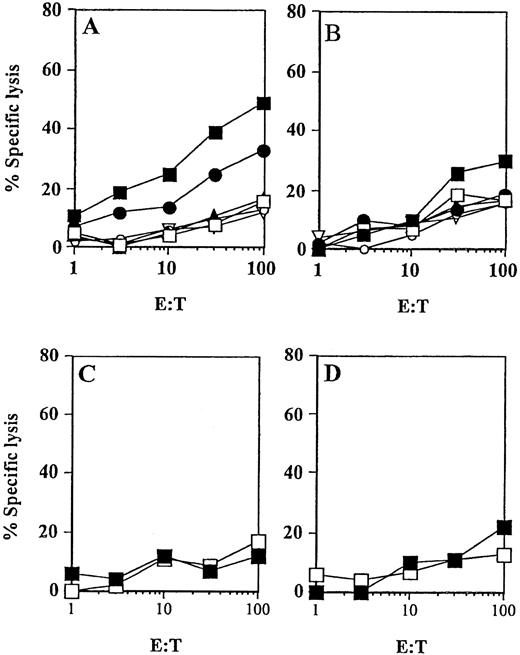
In addition to the analysis of IFN-γ production by peripheral blood T lymphocytes, analyses of peptide-specific CTL responses before and after 3 vaccinations were performed. From all 5 patients who had evidence of peptide-specific T cells by intracellular IFN-γ staining, peptide-specific CTLs were generated in vitro using peptide-pulsed DCs as antigen-presenting cells (APCs) (Table2). Results from experiments obtained with CTL derived from patient no. 8 and patient no. 2 are presented in Figures5 and 6, respectively. The CTL obtained after one in vitro restimulation efficiently lysed T2 target cells pulsed with the antigenic peptide, as well as the allogeneic HLA-A2+ tumor cell lines naturally expressing the TAA (MCF7 cells expressing MUC1 and A498 cells expressing HER2/neu). No lysis was detected when K562 or tumors missing either HLA-A2 (SK-OV-3 cells, MUC+, HER-2/neu+, HLA-A2−) or TAA (Croft cells, HER-2/neu−, MUC1−, HLA-A2+) expression were used as target cells in the assay. Consistent with the results from previous IFN-γ staining experiments, a stronger response was observed against the M1.2 (Figure 5) and E75 (Figure 6) peptides. In contrast, no in vitro CTL activity was detected in the peripheral blood obtained before the therapy in any of these patients (Figure 5C,D; Figure 6C,D).
Peptide-specific CTL responses induced in vivo (patient no. 8) by MUC1 peptide-pulsed DCs.
DCs were pulsed with the synthetic peptides derived from MUC1 and incubated with PBMNCs obtained before (C, D) or after 3 vaccinations with M1.2 and M1.1 peptides (A and B, respectively, ▪) to assess a MHC class I-restricted CTL response in vitro. Cytotoxic activity of induced CTLs was determined after one restimulation in a standard 51Cr-release assay using MCF-7 (HLA-A2+/MUC1+, ●) and T-2 (HLA-A2+) cells as targets pulsed for 2 hours with 25 μg of the cognate peptide (▪) or irrelevant HIV peptide (■). Control targets included K562 cells (▴), Croft cells (▿), and SK-OV-3 cells (○).
Peptide-specific CTL responses induced in vivo (patient no. 8) by MUC1 peptide-pulsed DCs.
DCs were pulsed with the synthetic peptides derived from MUC1 and incubated with PBMNCs obtained before (C, D) or after 3 vaccinations with M1.2 and M1.1 peptides (A and B, respectively, ▪) to assess a MHC class I-restricted CTL response in vitro. Cytotoxic activity of induced CTLs was determined after one restimulation in a standard 51Cr-release assay using MCF-7 (HLA-A2+/MUC1+, ●) and T-2 (HLA-A2+) cells as targets pulsed for 2 hours with 25 μg of the cognate peptide (▪) or irrelevant HIV peptide (■). Control targets included K562 cells (▴), Croft cells (▿), and SK-OV-3 cells (○).
Peptide-specific CTL responses induced in vivo (patient no. 2) by immunizations with HER-2/neu peptide-pulsed DCs.
DCs were pulsed with the synthetic peptides derived from HER-2/neu and incubated with PBMNCs obtained before (C,D) or after 3 vaccinations with E75 (▪, A,C) or GP2 (▪, B,D) peptides to assess a MHC class I–restricted CTL response in vitro. Cytotoxic activity of induced CTL was determined after one restimulation in a standard51Cr-release assay using A498 (HLA-A2+/HER-2/neu+, ●) and T-2 (HLA-A2+) cells as targets pulsed for 2 hours with 25 μg of the cognate peptide (▪) or irrelevant HIV peptide (■). K562, ▴; Croft cells (▿); SK-OV-3 cells (○).
Peptide-specific CTL responses induced in vivo (patient no. 2) by immunizations with HER-2/neu peptide-pulsed DCs.
DCs were pulsed with the synthetic peptides derived from HER-2/neu and incubated with PBMNCs obtained before (C,D) or after 3 vaccinations with E75 (▪, A,C) or GP2 (▪, B,D) peptides to assess a MHC class I–restricted CTL response in vitro. Cytotoxic activity of induced CTL was determined after one restimulation in a standard51Cr-release assay using A498 (HLA-A2+/HER-2/neu+, ●) and T-2 (HLA-A2+) cells as targets pulsed for 2 hours with 25 μg of the cognate peptide (▪) or irrelevant HIV peptide (■). K562, ▴; Croft cells (▿); SK-OV-3 cells (○).
Discussion
DCs are critical in the function of the immune system because they are the primary antigen-presenting cells for the initiation of T-lymphocyte responses. Several murine studies reported a successful elimination of established tumors using DCs pulsed with TAAs.17-20 In humans, vaccination therapies using DCs were shown to be effective for B-cell lymphoma and malignant melanoma.25-29 Melanoma, however, is a highly immunogenic tumor and spontaneous remissions due to immune reactions as well as remissions after IL-2– and IFN-α–based immunotherapies were reported.30,31 This led to the identification of the first human TAA from a patient with melanoma and opened a new field in the specific immunotherapy of malignant diseases.1 2
In this study, we show for the first time that a vaccination approach using HLA-A2–restricted HER-2/neu or MUC1 peptide-pulsed DCs can safely be applied in patients with advanced metastatic breast and ovarian cancers and induce immunologic responses directed against these less immunogenic tumors. No side effects were observed in these patients, particularly no clinically relevant anemia, though we have shown recently that normal erythroid bone marrow progenitor and precursor cells coexpress MUC1 molecules.33 However, most of the more mature erythroid progenitor cells are MHC class I negative and therefore are not targets for MUC-1 peptide-specific CTL. In addition, no autoimmune phenomena were observed in our study patients on the vaccination with these self-peptides.
Interestingly, in 5 of 10 vaccinated patients, peptide-specific CD8+ cytolytic T cells were detected in the peripheral blood after 3 vaccinations, suggesting that peptide-pulsed DCs can induce antigen-specific T cells in vivo in heavily pretreated patients with cancer, even after high-dose chemotherapy. Moreover, we provide evidence that epitope spreading might occur in vivo on vaccination with a single tumor antigen (Figure 4, Table 3, and Table 4). We observed MAGE-3– and CEA–peptide-specific CD8+ T cells in one patient (no. 8) treated with MUC-1 peptide-pulsed DCs, and moreover, MUC1 peptide-specific T cells were observed in another patient (no. 6) after vaccinations with HER-2/neu–derived peptides. One possible explanation for this phenomenon might be that the destruction of the tumor cells by the in vivo–induced peptide-specific T cells leads to the induction of other tumor antigen-specific CTLs as a result of tumor cell uptake and processing by APCs, such as DCs or macrophages that were demonstrated to be involved in the cross-priming phenomenon.35-38
The main immunologic responses in our study were directed against the M1.2 and E75 peptides, suggesting that these 2 peptides might be immunodominant and could be preferentially used in future vaccination trials, particularly in the setting of minimal residual disease as demontrated recently for low-grade lymphomas.29
In summary, in this report we show for the first time that vaccination therapy using DCs pulsed with HER-2/neu– or MUC1-derived peptides can be effective in patients with advanced metastatic breast and ovarian cancers. Immunologic responses were induced in patients with advanced diseases that were pretreated by multiple cycles of chemotherapy, including high-dose chemotherapy and autologous stem cell transplantation, indicating that peptide-pulsed DC vaccinations could also be successfully applied after intensive or even high-dose chemotherapy to eliminate residual disease. Furthermore, this report might be helpful to design future studies for the treatment of a variety of other tumors expressing HER-2/neu or MUC1, including renal cell carcinoma, non-small cell lung cancer, and colon and pancreatic carcinoma.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Stefanie Kurtz and Yvonne Hoffmann. We thank Prof Bültmann and Prof Kaiserling, Institute of Pathology, University of Tübingen, for performing immunohistologic analyses of the tumor specimens. Finally, we thank Prof Rammensee and Dr Stefan Stevanovic, Institute for Cell Biology, Department of Immunology, University of Tübingen, for providing the peptides.
Supported in part by grants from Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (SFB 510).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wolfram Brugger, University of Tübingen, Department of Hematology, Oncology and Immunology, Otfried-Müller-Strasse-10, D-72076 Tübingen, Germany; e-mail: wolfram.brugger@med.uni-tuebingen.de.