Anaplastic large-cell lymphoma (ALCL) accounts for approximately 10% of pediatric non-Hodgkin lymphoma (NHL). Previous experience from NHL–Berlin-Frankfurt-Münster (BFM) trials indicated that the short-pulse B-NHL–type treatment strategy may also be efficacious for ALCL. The purpose of this study was to test the efficacy of this protocol for treatment of childhood ALCL in a large prospective multicenter trial and to define risk factors. From April 1990 to March 1995, 89 patients younger than 18 years of age with newly diagnosed ALCL were enrolled in trial NHL-BFM 90. Immunophenotype was T-cell in 40 patients, B-cell in 5, null in 31, and not determined in 13. Stages were as follows: I, n = 8; II, n = 20; III, n = 55; IV, n = 6. Extranodal manifestations were as follows: mediastinum, n = 28; lung, n = 13; skin, n = 16; soft tissue, n = 13; bone, n = 14; central nervous system, n = 1; bone marrow, n = 5. After a cytoreductive prephase, treatment was stratified into 3 branches: patients in K1 (stage I and II resected) received three 5-day courses (methotrexate [MTX] 0.5 g/m2, dexamethasone, oxazaphorins, etoposide, cytarabine, doxorubicin, and intrathecal therapy); patients in K2 (stage II nonresected and stage III) received 6 courses; patients in K3 (stage IV or multifocal bone disease) received 6 intensified courses including MTX 5 g/m2, high-dose cytarabine/etoposide. The Kaplan-Meier estimate for a 5-year event-free survival was 76% ± 5% (median follow-up, 5.6 years) for all patients and 100%, 73% ± 6%, and 79% ± 11% for K1, K2, and K3, respectively. Events were as follows: progression during therapy, n = 2; progression or relapse after therapy, n = 20; second malignancy, n = 1. It was concluded that short-pulse chemotherapy, stratified according to stage, is effective treatment for pediatric ALCL. B symptoms were associated with increased risk of failure.
Introduction
Anaplastic large-cell lymphoma (ALCL) is a distinct entity of non-Hodgkin lymphoma originally described by Stein et al1 in 1985. It is characterized by large anaplastic cells of T-cell or null-cell phenotype expressing CD30 (Ki-1 antigen).2-8 The morphological aspect of these tumors is variable, ranging from small-cell to large-cell variants with some resembling cells of malignant histiocytosis.3-6,9 A putative Hodgkin-like ALCL as well as ALCL of B-cell immunophenotype is still a matter of debate.3,5,6 The variability of morphology as well as immunophenotype and clinical presentations suggest that ALCL is probably a heterogeneous group of malignant non-Hodgkin lymphomas (NHLs).6,8 However, certain common characteristic features in terms of morphology (large cells with eccentrically located, horseshoe-shaped nucleus), immunophenotype (expression of the surface antigen CD30), and genetics (translocation t[2;5], leading to the expression of the fusion-protein nucleolar protein nucleophosmin–anaplastic lymphoma kinase [NPM/ALK] and reactivity to the ALK-1 antibody) provided further evidence for the hypothesis that most of the variant types of ALCL represented a group of NHLs derived from a common precursor cell of still undetermined origin.1,3,4,6 10-16
ALCL accounts for approximately 10% to 15% of all childhood NHL and is clinically characterized by the frequent presence of B-symptoms and involvement of extranodal sites, such as skin, lung, bone, and soft tissue.17-19 Therapy for pediatric ALCL varies considerably in different study groups.18-22 Although some groups apply a short-pulse chemotherapy strategy of different durations, others treat these patients according to more prolonged LSA2-L2–derived protocols. In previous trials on NHL of childhood and adolescence of the Berlin-Frankfurt-Münster (BFM) group, patients with the newly described entity ALCL were treated according to the short-pulse chemotherapy strategy proven to be effective for mature B-cell neoplasms (B-NHL). This strategy proved to also be efficacious for ALCL patients.17
A striking observation in the first 62 ALCL patients enrolled in 3 consecutive BFM group trials was, however, the favorable chance of survival after relapse of ALCL patients in contrast to children suffering from mature B-cell neoplasms. Owing to their distinct clinicopathological features, in study NHL-BFM 90, ALCL patients constituted a distinct therapy group. Treatment consisted of short chemotherapy pulses derived from the B-NHL strategy without radiotherapy. The number and intensity of therapy courses were stratified for stage of disease.
In the previous studies NHL-BFM 83 and 86, patient accrual for pediatric patients with ALCL was incomplete owing to the fact that patients with this disease were treated partly according to protocol HX 83 (Austrian-German cooperative trial for the treatment of histiocytic disease) or protocols for the treatment of acute myeloid leukemias. However, with the introduction of therapy protocol NHL-BFM 90, all pediatric patients with newly diagnosed ALCL from Germany, Austria, and large parts of Switzerland were enrolled in trial NHL-BFM. Trial NHL-BFM therefore represents a population-based prospective trial comprising a nonselected group of uniformly treated pediatric patients with ALCL. Here we present therapy and results of 89 pediatric ALCL patients enrolled in the NHL-BFM 90 study.
Patients and methods
Patients
Children and adolescents up to 18 years of age with NHL were eligible for trial NHL-BFM 90. Exclusion criteria were HIV infection, severe immunodeficiency, posttransplantation lymphoma, NHL as a second malignancy, previous cytostatic treatment, and pre-existing disease prohibiting chemotherapy. From April 1990 through March 1995, 682 eligible patients were enrolled from 90 centers in Austria, Germany, and Switzerland. Informed consent was obtained from the parents or guardians of all patients according to the Helsinki protocol. The study population was subdivided according to NHL subtype into 3 groups with different therapy strategies: non-B therapy group (patients with B-ALL lymphoblastic lymphoma or peripheral T-cell lymphoma) (n = 162); B-NHL/B acute lymphoblastic leukemia (B-ALL) therapy group (n = 431); and ALCL therapy group (patients with ALCL) (n = 89). We report here on treatment and results of the ALCL therapy group with 89 eligible patients. Of these patients, 37 were included in a previous report.17 A further 11 patients with ALCL were registered but were considered noneligible for the following reasons: the fact that ALCL was a second malignancy (1 patient, 1 relapse); pre-existing severe combined immunodeficiency (1 patient, death in complete remission); previous chemotherapy (7 patients, 3 relapses); and previous radiotherapy (1 patient, 1 relapse). One patient had a diagnosis of lymphomatoid papulosis and was considered noneligible because he did not receive any form of cytostatic treatment. He underwent surgical excision of localized skin and was lost to follow-up after a progression-free survival of 31 months.
Diagnosis
Diagnosis of ALCL was based on histopathology and immunohistochemistry according to the updated Kiel classification and the revised European-American classification of lymphoid neoplasms for NHL.3,5 Of 89 patients, 78 had tumor slides reviewed by central reference pathology in the Lymph Node Registry Kiel. The following diagnostic criteria were applied: typical pattern of tumor cell distribution in paracortical areas and nodal sinuses and coherent growth pattern of tumor cells together with anaplastic cytology, ie, one or more irregularly shaped vesicular nuclei containing one or multiple prominent nucleoli. Histologic subclassification of ALCL types was restricted to the diagnosis of the lymphohistiocytic variant. This variant was distinguished from typical classical ALCL by less densely packed or evenly dispersed uniform tumor cells of medium size containing indented nuclei and small inconspicuous nucleoli and the abundance of broad cytoplasmic macrophages or histiocytes, which made up more than 80% of the infiltrates in some areas.23
Immunohistochemistry on conventional paraffin sections was performed with avidin-biotin-peroxidase complex24 or alkaline phosphatase antialkaline phosphatase25 techniques; paraffin-resistant monoclonal antibodies to CD20 (L26) (Dianova, Hamburg, Germany), Ki-B3 and Ki-B5 (Laboratory of R.P.), and CD3 and CD45RO (Dako, Hamburg, Germany) were used for the detection of B and T cells. CD68 (Ki-M1P) (laboratory of R.P.) and antilysozyme (Dako) were used to detect monocytes and macrophages.26-28 CD30 was visualized by means of the monoclonal antibodyBerH2.29 Cytokeratin and melanoma-associated antigens were detected by KL1, HMB45, and anti–S 100 (Dianova). During the accrual period of study NHL-BFM 90, methods to detect characteristic chromosomal translocations of ALCL in formalin-fixed material were not routinely available. However, after the availability of the ALK-1 monoclonal antibody (Dako) directed to the NPM/ALK fusion protein,13 43 of 89 cases were re-examined for ALK-1 expression.
Staging
Clinical investigations included physical examination, peripheral blood smear, bone marrow (BM) aspirate smears, cytology of cerebrospinal fluid (CSF), ultrasonography, computed tomography (CT) or magnetic resonance tomography (MRT), and skeletal scintigraphy. Slides from BM aspirates and CSF were reviewed centrally. Patients were staged according to the St Jude staging system.30 B-symptoms were recorded as in Hodgkin disease according to the previously described criteria.31 Patients were considered to be experiencing BM involvement in the presence of any tumor cells on BM smears. Central nervous system (CNS) involvement was defined as the presence of malignant cells in the CSF on cytospin preparations or/and demonstration of a cerebral mass on cranial CT (CCT) or MRT scan and/or presence of cranial nerve palsy that was not caused by extracranial or epidural masses. Epidural manifestation was not considered to be CNS disease. All patients with mediastinal enlargement or pleural effusions on chest x-ray received thoracic CT scans.
Therapy
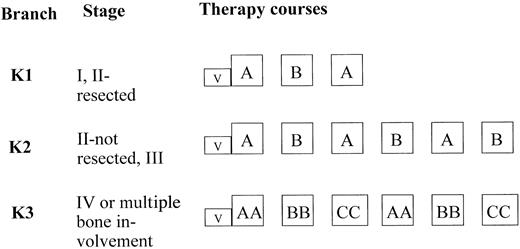
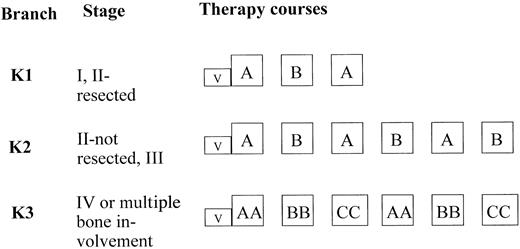
Therapy courses and dosages are given in Table1. All patients received a 5-day cytoreductive prephase. By July 1, 1993, the cytoreductive prephase was amended. Cyclophosphamide doses were reduced to 2, and prednisone was replaced by 5 mg/m2/d dexamethasone on days 1 and 2, and by 10 mg/m2/d on days 3, 4, and 5. After the 5-day prephase, patients were stratified according to clinical stage into 3 therapy branches of different intensities (Figure1). Patients with stage I or II completely resected were treated in branch K1, patients with stage II nonresected and stage III were treated in branch K2. Patients with stage IV and/or multifocal bone disease were treated in K3. Conditions for starting the second and third course of therapy were as follows: more than 50 000/μL platelets, more than 200/μL neutrophils after the nadir of postchemotherapeutic cytopenia was passed; for subsequent courses 4, 5, and 6, neutrophils should exceed 500/μL. The minimal interval between the first day of two successive courses had to be at least 2 weeks. In therapy courses AA and BB, in which 5 g/m2 methotrexate (MTX) was used, MTX serum concentration was measured at hours 24, 36, 42, and 48 from the start of the MTX intravenous (iv) infusion. The serum levels of MTX had to be 150 μM or less at hour 24 from the start of MTX infusion, less than 3 μM at hour 36, 1 μM or less at hour 42, and 0.4 μM or less at hour 48. Leucovorin (racemic folinic acid) rescue was given iv; dosages were 30 mg/m2 at hour 42 and 15 mg/m2at hours 48 and 54 after the start of MTX infusion. If the MTX serum concentration was higher than expected at hours 42 or 48, measurements of the MTX serum levels and administration of leucovorin rescue were continued every 6 hours until the serum MTX concentration decreased below 0.25 μM. The dose of leucovorin was adjusted as follows: at MTX serum level greater than 1 to 2 μM, 30 mg/m2 leucovorin; at MTX level greater than 2 to 3 μM, 45 mg/m2 leucovorin; at MTX level greater than 3 to 4 μM, 60 mg/m2 leucovorin; and at MTX level greater than 4 to 5 μM, 75 mg/m2leucovorin. If the MTX serum level exceeded 5 μM, the leucovorin dose was calculated according to the formula [mg] leucovorin = MTX serum concentrations μM × body weight [kg]. The leucovorin was administered as iv infusion in order to avoid hypercalcemia.
Therapy strategy and stratification criteria.
For therapy-group ALCL in trial NHL-BFM 90.
Therapy strategy and stratification criteria.
For therapy-group ALCL in trial NHL-BFM 90.
In patients with overt CNS disease, a device for intraventricular application of chemotherapy was implanted before the second course of therapy. In courses AA and BB, 3 mg MTX and 2.5 mg prednisolone were administered on days 1, 2, 3, and 4, and 30 mg cytarabine was given on day 5. In course CC, 3 mg MTX and 2.5 mg prednisolone were administered on days 3, 4, 5, and 6, and 30 mg cytarabine was given on day 7.
Response criteria
Treatment success was determined by event-free survival (EFS). Events were defined as death from any cause, tumor progression, and second malignancy. Tumor response was evaluated after each course of therapy. Subsequent follow-up studies were performed at 4- to 6-week intervals during the first 1.5 years after diagnosis. In patients with BM or CNS involvement, control punctures of BM and/or CSF were performed at each course until the BM or the CSF, respectively, was cleared of blasts. Progression was defined as growth of an incompletely resolved tumor or as recurrence proven by biopsy.
Statistical analysis
Analysis of EFS was performed by means of the Kaplan-Meier method with differences compared by the log-rank test.32,33 The 95% confidence intervals for the Kaplan-Meier estimate of EFS were calculated by means of the Greenwood standard error estimate.34 EFS was calculated from the date of diagnosis to the first event (death from any cause, tumor progress, or second malignancy) or to the last follow-up. Patients lost to follow-up were censored at the time of their withdrawal. Rank-order comparisons of the prognostic relevance of different parameters were examined by stepwise Cox regression analysis.35Differences in the distribution of different parameters were examined by means of the χ2 or Fisher exact test. Statistical analyses were performed by means of the SAS program (SAS-PC, version 6.12) (SAS Institute, Cary, NC). Follow-up data were actualized as of July 1, 2000.
Results
Pathology and immunohistochemistry
Among the 89 ALCL cases, 2 were diagnosed as lymphohistiocytic variant. In 31 cases (34.8%), the tumor cells did not express any lineage-specific antigens (CD3, CD45RO, CD20, Ki-B3, or Ki-B5); these cases were referred to as null phenotype (Table2). We classified 40 of 89 cases (44.9%) as being of T-cell lineage (expression of CD3 and CD45RO). In 5 cases (5.6%), tumor cells expressed B-cell antigens (CD20, Ki-B3, and Ki-B5). In 13 of 89 cases of ALCL, the immunophenotype could not be determined owing to technical shortcomings. All 89 cases were positive for CD30; CD30+ tumor cells formed dense sheaths of immunoreactive infiltrates on immunohistochemistry. In none of the 5 cases of ALCL of B-cell type were single immunoreactive cells or limited areas of reactive infiltrates seen, as was observed in cases of diffuse large B-cell lymphomas of centroblastic or immunoblastic type. Of 89 cases, 43 were re-examined for ALK-1 expression. Clinical characteristics as well as treatment outcome of these 43 patients did not differ from the remaining 46 patients who could not be re-examined for ALK-1 (Table 2). Of the 43 examined cases, 35 were ALK-1+, 8 cases were ALK-1−.
Clinical characteristics
The median age of patients was 10.5 years (range, 0.8-17.3 years). Clinical characteristics are given in Table 2. Eight patients were staged as stage I; 20 as stage II; 55 as stage III; and 6 as stage IV. In 5 of 6 patients with stage IV disease, BM involvement was present; in all 5 cases, marrow involvement was scattered and scarce with 1% to 5% blasts on BM smears. In one patient with stage IV disease, CNS involvement, diagnosed by blasts in the CSF, was the only manifestation of ALCL. Lymph nodes were involved in 90% of patients; 21.3% of patients (n = 19) had nodal disease only. Extranodal disease was present in 78.7% of patients (n = 79); B-symptoms were present in 51.7% of patients. Nine patients had multifocal bone disease and 5 patients had unilocal bone lesions. No differences in clinical presentation were found among patients with ALCL of different immunological lineages (Table 2). Even though mediastinal and skin involvement was more frequently observed in patients with ALCL of T or null immunophenotype, this difference was not statistically significant (P = .247 and P = .547, respectively). Furthermore, clinical characteristics were comparable between patients with ALK-1+ and ALK-1− tumors (Table 2).
EFS
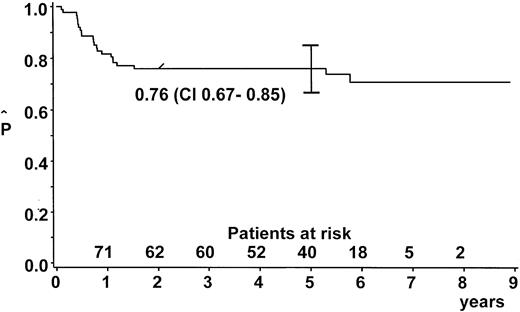
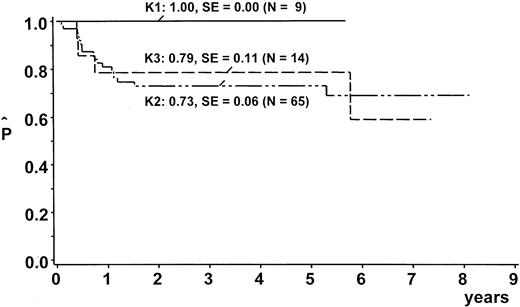
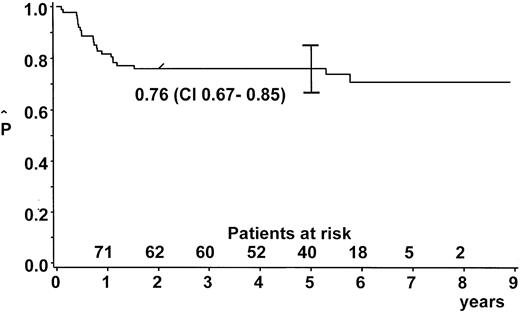
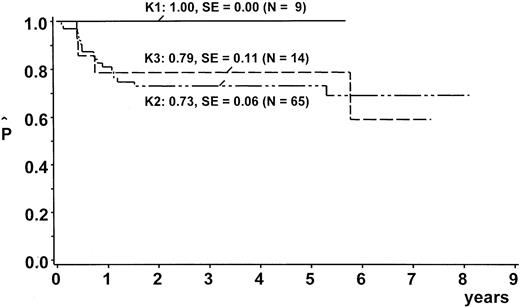
After a median observation time of 5.6 years (range, 2.0-8.9 years), the probability of EFS (pEFS) at 5 years was 76% (95% confidence interval [CI], 67%-85%) for the whole group (Figure2). We stratified 9 patients (10%) into branch K1; 65 (73%) into branch K2; and 14 (16%) into branch K3. One patient with lymphohistiocytic variant of ALCL received nonprotocol therapy of the ALL type. This patient was excluded from analysis of the outcome by therapy branch. In the intent-to-treat analysis, pEFS at 5 years was 100%, 73% ± 6%, and 79% ± 11% for patients stratified in branch K1, K2, and K3, respectively (Figure3). Owing to individual decisions of the physicians in charge, deviations from the allocated treatment branch were made for 11 patients: 2 patients were treated in K2 instead of K1; 5 patients in K3 instead of K2; 2 patients in K2 instead of K3; and 1 patient in K1 instead of K2. The pEFS at 5 years according to the treatment applied was 100%, 74% ± 6%, and 75% ± 11% for patients treated according to branches K1 (n = 8), K2 (n = 63), and K3 (n = 17), respectively. The child with CNS disease received 24 Gy cranial radiotherapy although this was not foreseen in the protocol. This patient suffered CNS relapse and died. The pEFS at 5 years according to stage was 100%, 79% ± 9%, 74% ± 6%, and 50% ± 20% for patients of stage I (n = 8), II (n = 20), III (n = 55), and IV (n = 6), respectively.
EFS estimate.
Kaplan-Meier estimate of EFS at 5 years for the whole group of patients with ALCL (n = 89). CI = 95% confidence interval.
EFS estimate.
Kaplan-Meier estimate of EFS at 5 years for the whole group of patients with ALCL (n = 89). CI = 95% confidence interval.
EFS estimates by therapy branch.
Kaplan Meier estimates of EFS at 5 years according to therapy branches. SE = standard error. One patient was excluded from this analysis because he was treated according to ALL-type chemotherapy.
EFS estimates by therapy branch.
Kaplan Meier estimates of EFS at 5 years according to therapy branches. SE = standard error. One patient was excluded from this analysis because he was treated according to ALL-type chemotherapy.
Events
Two patients died after early tumor progression while on therapy (Table 3). In one of these cases, response to therapy was nearly complete after the first course of therapy, but rapid tumor growth at new, distant sites occurred prior to the second therapy course. Twenty patients suffered from progression or relapse after completion of therapy. Of these 20 relapsed patients, 9 are in long-standing second complete remission after intensive re-induction chemotherapy (1 patient) or after re-induction chemotherapy followed by blood stem cell transplantation (autologous in 6 patients and allogeneic in 2). One patient suffered a second malignancy (ALL diagnosed 18 months after diagnosis of ALCL). Toxic deaths secondary to therapy did not occur.
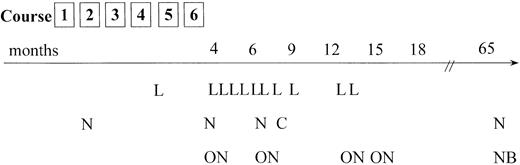
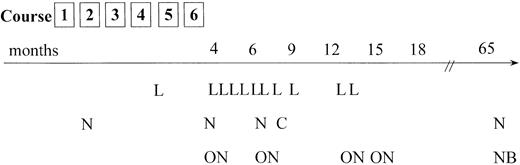
Relapses occurred most frequently at the site of the primary tumor; however, involvement of new tumor sites distant from the initial manifestations was also observed frequently (Figure4). Of 20 relapses, 18 occurred during the first 15 months after the end of chemotherapy. In 2 patients, however, very late recurrence of ALCL was observed (more than 5 years after primary diagnosis). It is not clear whether these tumors are second ALCL (ie, ALCL of different cellular origin) or true relapses. In both cases, differences in immunology or clonal identity between the first and second tumor could not be determined owing to the lack of appropriate material.
Tumor failure data.
Time and site of tumor failure. L = local relapse only; N = new tumor sites alone; ON = old and new tumor sites involved; C = CNS relapse; NB = new site and BM involvement in relapse. The patient who was treated according to an ALL-type protocol and who experienced tumor progression (local and new sites) 10 months from diagnosis is not included.
Tumor failure data.
Time and site of tumor failure. L = local relapse only; N = new tumor sites alone; ON = old and new tumor sites involved; C = CNS relapse; NB = new site and BM involvement in relapse. The patient who was treated according to an ALL-type protocol and who experienced tumor progression (local and new sites) 10 months from diagnosis is not included.
Of 28 patients with a mediastinal mass at diagnosis, 14 had a residual mediastinal mass at the end of the treatment. Three of these patients had local tumor progression; 10 remained free of tumor progression; 1 was lost to follow-up.
Prognostic factors
In univariate analysis, lung involvement (P = .03), splenomegaly (P = .04), and B-symptoms (P = .04) were associated with an increased risk of failure. Neither immunophenotype nor ALK-positivity was shown to be of prognostic value (Table 4). Patients with ALCL of B-cell immunophenotype were not included in this analysis owing to their small number. In multivariate Cox regression analysis, only B-symptoms (P = .05) proved to be significantly associated with increased risk of failure.
Discussion
Although ALCL is now a well-recognized clinicopathological entity of non-Hodgkin lymphoma, optimal treatment has not yet been defined for pediatric ALCL. Whereas some centers treat patients with prolonged T-cell–type therapy regimens,20,37 others favor a B-cell–type therapy consisting of high-dose polychemotherapy regimens administered over a relatively short period of several months.17-19,38,39,41 Comparison of treatment outcome of these different studies is difficult, owing not only to different therapy regimens applied, but also to differences in classification and, thus, in the composition of patient subpopulations. The inclusion of the B-cell phenotype in ALCL is still controversial.3-5
The present study describes a nonselected group of ALCL patients in a population-based prospective trial, because the NHL-BFM multicenter trial covers all of Germany and Austria as well as large parts of Switzerland with all newly diagnosed pediatric cases of ALCL being registered in the BFM study center. Clinical data on symptoms and signs of disease, age distribution, involved tumor sites, and therapy results have a high probability of being epidemiologically representative for ALCL in childhood.39 Factors influencing outcome, such as positive selection of patients or treating centers, can be excluded owing to the nearly complete patient accrual of pediatric ALCL in trial NHL-BFM 90.
Compared with most previously published series of pediatric ALCL,18-20,36,37,41,42 all patients in the present study were treated according to the same therapy protocol and were diagnosed and stratified according to identical criteria. Therapy results of study NHL-BFM 90 for pediatric patients with ALCL were similar to previously published preliminary data from 62 patients treated according to studies NHL-BFM 83 and 86 and to the first 20 months of the accrual period of study NHL-BFM 9017: the pEFS at 5 years was 76% ± 5% for the 89 ALCL patients treated in study NHL-BFM 90, compared with 76% ± 8% for 25 ALCL patients treated in the preceeding studies NHL-BFM 83 and 86. Thus, the results of study NHL-BFM 90 confirm the efficacy of a short-dose, intensive polychemotherapy for pediatric ALCL in a larger group of nonselected pediatric ALCL patients.
The results of study NHL-BFM 90 for ALCL patients compare favorable with recently presented results from other study groups.19When different therapy regimens for pediatric ALCL are compared in detail, total duration of therapy is shorter in trial NHL-BFM 90 than in other published trials on pediatric ALCL.18-20,36-41Whereas minimum duration of therapy was only 2 months for patients with stage I or resected stage II disease and maximum therapy duration in therapy branch K3 was 5 months, total duration of therapy in other trials was 10 months to 2 years. In addition, cumulative dosages of critical drugs were comparably low in trial NHL-BFM 90, with a maximum of 3.4 g/m2 for cyclophosphamide, 12 g/m2 for ifosfamide, 150 mg/m2 for anthracyclines, and 1300 mg/m2 for etoposide (for 16% of patients; 84% of patients received 600 mg/m2 etoposide or less). The major apparent differences between the protocol of study NHL-BFM 90 and other published treatment regimens for pediatric ALCL19,38,40 41are the use of dexamethasone instead of prednisone, the use of ifosfamide, the infusion of methotrexate over a long period of 24 hours with late leucovorin rescue, and intrathecal chemotherapy. It is not clear whether these differences influence response to therapy and, if so, in which ways. However, in view of favorable therapy results despite lower cumulative dosages and a shorter duration of therapy compared with other treatment programs, these factors may be of relevance.
In our study, only the presence of B-symptoms was a significant negative prognostic factor in multivariate analysis whereas other biological parameters, such as the immunophenotype of the lymphoma cells, had no additional impact on treatment outcome. Other study groups, applying a nonstratified treatment for pediatric ALCL were able to define several negative prognostic risk factors in similarly large series of patients: visceral involvement, mediastinal involvement, and elevated levels of lactate dehydrogenase exceeding 800 U/L were associated with a worse prognosis in multivariate analysis.19 None of these factors reached statistical significance in multivariate analysis in trial NHL-BFM 90. Likewise, uncertainty exists as to whether multifocal bone involvement is prognostically equivalent to BM involvement. In our trial, 8 patients with multifocal bone disease were assigned to the most intensive therapy branch K3; 1 of them failed therapy. However, it remains an open question whether multifocal bone disease without concomitant BM involvement is really associated with an increased risk of failure. The differences in risk factors for failure in different studies are probably a result of small patient numbers in each study. Interestingly, in a previous report by our group on the first 62 ALCL patients enrolled in 3 consecutive studies, skin involvement proved to be a negative prognostic parameter.17 In the present series of 89 patients enrolled and treated in one study, skin involvement was not associated with an increased risk of failure in either univariate or multivariate analysis. These observations suggest that much larger series of patients are required to allow valid evaluation of prognostic risk factors. This may also be true for the impact of ALK-1 expression on treatment outcome. The number of ALK-1− cases in our series was too small for meaningful statistical analysis on the prognostic strength of ALK-1 negativity.
Although this short B-cell–type chemotherapy with moderate cumulative dosages of alkylating agents, anthracyclines, and epipodophyllotoxins and stratified treatment turned out to be efficient for pediatric ALCL patients, further improvement is necessary. Local recurrences of initial manifestations in our series raise the question of additional local therapy modalities, eg, irradiation. This may be of equivocal value, however, considering the current treatment results with chemotherapy alone and the potential late risks of radiotherapy.43-45 In addition, one third of recurrences were at new sites and would most probably not have been prevented by irradiation of the primary. On the other hand, residual mediastinal masses at the end of chemotherapy (observed in 50% of patients with mediastinal tumors) were not associated with an increased risk of subsequent tumor progression, thus causing difficulties in the decision of whom to irradiate.
To answer these questions and further improve outcome, proper histological subclassification, definition of reliable prognostic factors, and evaluation of the role of defined treatment components in large randomized trials at an international level are required.
The authors thank Edelgard Odenwald for expert work in cytomorphology; Ulrike Meyer and U. Regelsberger for expert work in data management; and especially all the doctors and nurses in participating hospitals for their continuous care for sick children and their excellent cooperation with the NHL-BFM study center. Please see the for a listing of the participating individuals.
Study committee for NHL-BFM 90: W. Dörffel (Berlin), W. Ebell (Berlin), H. Gadner (Vienna), N. Graf (Homburg), G. Henze (Berlin), G. Janka-Schaub (Hamburg), T. Klingebiel (Tübingen), S. Müller-Weihrich (Munich), I. Mutz (Leoben), R. Parwaresch (Kiel), H. J. Plüss (Zurich), A. Reiter (Hannover), H. Riehm (Hannover), G. Schellong (Münster), M. Schrappe (Hannover), F. Zintl (Jena).
Principal investigators contributing ALCL patients to study NHL-BFM 90: R. Mertens (Aachen), I. Imbach (Basel), G. Henze (Berlin-Charité), W. Dörffel (Berlin-Buch), U. Bode (Bonn), H. J. Spaar (Bremen), H. Breu (Dortmund), G. Weissbach (Dresden), U. Göbel (Düsseldorf), G. Weinmann (Erfurt), D. Beck (Erlangen), W. Havers (Essen), B. Kornhuber (Frankfurt), C. Niemeyer (Freiburg), M. Lakomek (Göttingen), K. Welte (Hannover), B. Selle (Heidelberg), N. Graf (Homburg-Saar), K. Dengg (Innsbruck), F. Zintl (Jena), G. Nessler (Karlsruhe), W. Kaulfelsch (Klagenfurt), M. Rister, (Koblenz), M. Domula (Leipzig), K. Schmitt (Linz), P. Bucsky (Lübeck), U. Mittler (Magdeburg), P. Gutjahr (Mainz), W. Tillmann (Minden), C. Eschenbach (Marburg), K. D. Tympner (Munich-Harlaching), R. J. Haas (Munich), C. Bender-Götze (Munich), S. Müller-Weihrich (Munich), H. Jürgens (Münster), A. Jobke (Nuremberg), R. Geib (Saarbrücken), F. R. Dickerhoff (St Augustin), A. Feldges (St Gall), Haschke (Salzburg), J. Treuner (Stuttgart), D. Niethammer (Tübingen), K. M. Debatin (Ulm), H. Gadner (Vienna), J. Kühl (Würzburg), J. Otte (Wolfsburg), H. J. Plüss (Zurich).
Contributing pathologists: H. Mittermeyer (Aachen); R. Backmann (Augsburg); H. Ohnacker (Basel); H. Stein (Berlin), M. Dietel (Berlin); M. Ruhnke, W. Schneider (Berlin-Buch); H. J. Födisch (Bonn); F. K. Kössling (Bremen); E.W. Schwarze (Dortmund); M. Müller (Dresden); W. Hort (Düsseldorf); D. Schreiber (Erfurt); V. Becker (Erlangen); L. D. Leder (Essen); S. Falk (Frankfurt); H. E. Schäfer (Freiburg); E. Kunze (Göttingen); A. Georgii (Hannover); F. Otto (Heidelberg); K. Remberger (Homburg/Saar); G. Mikuz (Innsbruck); D. Katenkamp (Jena); W. Gusek (Karlsruhe); W. Wagner (Klagenfurt); F. deLeon (Koblenz); C. Wittekind (Leipzig); M. Weber (Linz); A. Feller (Lübeck); A. Roessner (Magdeburg); W. Thoenes (Mainz); C. Thomas (Marburg); E. Jehn (Minden); K. Wurster (Munich), U. Löhrs, P. Meister (Munich); G. Grundmann (Münster); P. H. Wünsch (Nuremberg); H. Mitschke (Saarbrücken); O. Dietze (Salzburg); A. Diener (St Gall); B. Kraus-Hounder (Stuttgart); P. Kaiserling (Tübingen); O. Haferkamp (Ulm); T. Radaszkiewicz (Vienna); H. K. Müller-Hermelink (Würzburg); F. Rühl (Wolfsburg); T. Stallmach (Zurich).
Reference laboratories for histopathology and immunophenotyping: Lymphnode Registry Kiel founded by the Society of German Pathologists, Institute of Hematopathology, University of Kiel (R. Parwaresch, M. Tiemann); Institute of Pathology, University of Würzburg (H. K. Müller-Hermelink); Institute of Pathology, University of Berlin (H. Stein); Institute of Pathology, University of Lübeck (A. Feller); Institute of Pathology, University of Vienna, Austria (I. Simonitsch).
Supported by the Deutsche Krebshilfe, Bonn, grant M 109/91/Re1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alfred Reiter, NHL-BFM Study Center, Department of Pediatric Hematology and Oncology, Justus-Liebig-University, D-35385 Giessen, Germany; e-mail:alfred.reiter@paediat.med.uni-giessen.de.