Abstract
The CD34 antigen serves as an important marker for primitive hematopoietic cells in therapeutic transplantation of hematopoietic stem cells (HSC) and gene therapy, but it has remained an open question as to whether or not most HSC express CD34. Using a competitive long-term reconstitution assay, the results of this study confirm developmental changes in CD34 expression on murine HSC. In fetuses and neonates, CD34 was expressed on Lin−c-Kit+ long-term repopulating HSC of bone marrow (BM), liver, and spleen. However, CD34 expression on HSC decreased with aging, and in mice older than 10 weeks, HSC were most enriched in the Lin−c-Kit+CD34− marrow cell fraction. A second transplantation was performed from primary recipients who were transplanted with neonatal Lin−c-Kit+ CD34high HSC marrow. Although donor-type HSC resided in CD34-expressing cell fraction in BM cells of the first recipients 4 weeks after the first transplantation, the stem cell activity had shifted to Lin−c-Kit+CD34− cells after 16 weeks, indicating that adult Lin−c-Kit+CD34− HSC are the progeny of neonatal CD34-expresssing HSC. Assays for colony-forming cells showed that hematopoietic progenitor cells, unlike HSC, continue to express CD34 throughout murine development. The present findings are important because the clinical application of HSC can be extended, in particular as related to CD34-enriched HSC and umbilical cord blood HSC.
Introduction
CD34 is a glycoprotein expressed on hematopoietic cells, vascular endothelium, and embryonic fibroblasts.1-3Based on in vitro studies identifying cells with the capacity to differentiate into various hematopoietic lineages in clonal culture4 and to generate hematopoietic progenitor cells (HPC) in long-term culture,5 CD34 has served as the most important marker for primitive hematopoietic cells. However, despite the widespread clinical use of CD34 antibodies for enumeration and isolation of human primitive hematopoietic cells in therapeutic hematopoietic stem cell (HSC) transplantation and gene therapy, it has remained an open question as to whether or not all HSC express CD34. Krause and colleagues6,7 and Morel and coworkers8 reported that murine adult bone marrow (BM) CD34+ cells were capable of long-term hematopoietic reconstitution and allow survival of lethally irradiated mice. Conversely, Osawa and associates9 reported that CD34low/− rather than CD34high cells in the lineage markers-negative (Lin−) c-Kit+Sca-1+ cell fraction possess the most long-term reconstitution ability, but not short-term radioprotection ability, as determined in a competitive long-term reconstitution analysis, although it recently has been shown that a small population of c-Kit− dormant HSC are present in adult BM.10 Goodell and colleagues11 carried out a dual-wavelength flow cytometric analysis of BM cells stained with the fluorescent DNA-binding dye Hoechst 33342 and found that adult HSC capable of reconstituting lethally irradiated recipients express low or undetectable levels of CD34. In humans, engraftment models using immunodeficient mice12-14 and preimmune fetal sheep,15 and the clinical transplantation of CD34-enriched cell populations16 indicated long-term repopulating ability of CD34+ HSC. However, recent studies raised doubts as to whether the CD34+ cell fraction includes all human stem cell activity.11,17 18
A great deal of progress has been made in characterizing murine fetal HSC. It has been shown that all the stem cell activity required to reconstitute adult hematopoiesis resides in the AA4.1+c-Kit+CD34+ population at 14 days postcoitum (dpc) fetal liver cells and that the AA4.1+c-Kit+CD34− population does not repopulate.19 Transplantation experiments in adult mice also indicated that all HSC in 11 dpc aorta-gonad-mesonephros region and fetal liver reside in a c-Kit+CD34+population.20 The presence of CD34+ HSC capable of reconstituting hematopoiesis of pretreated neonate, but not adult, recipients in 9 dpc para-aortic splanchnopleura and yolk sac was demonstrated.21 Thus, in contrast to reports on adult murine HSC, CD34 expression on fetal murine HSC at early stages is in agreement, irrespective of their location, which means that CD34 expression on murine HSC may vary during development from fetus to adult. Indeed some phenotypic variations between fetal and adult HSC have been reported for CD45RB,22 major histocompatibility complex class I,23 and Mac-1 antigens.24
We investigated CD34 expression on HSC in the murine fetus, neonate, and adult at various ages, using competitive long-term reconstitution analyses. Our data showed that HSC from the fetus express CD34 at late gestational stages and continue to express it in neonates and younger adults, but that this expression decreases with aging. Second transplantation experiments showed that the decrease in CD34 expression on HSC occurs with a similar time course, even in older recipients engrafted with neonatal Lin−c-Kit+CD34+ HSC. The present findings have important implications for the clinical applications of HSC, in particular CD34-enriched HSC transplantation and umbilical cord blood transplantation.
Materials and methods
Mice
C57BL/6 Ly-5.1 mice were kindly provided by Dr Koichi Ikuta (Kyoto University, Kyoto, Japan) and C57BL/6 Ly-5.2 mice were obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan). These mice were bred and maintained in a specific pathogen-free microisolator environment. For experiments on fetuses, one or 2 female mice were caged with a male for 2 hours late in the afternoon and then examined for vaginal plugs. The appearance of the vaginal plug was designated as day 0 of gestation. Mice within 24 hours from birth were used as neonatal mice. In the transplantation experiments, recipient mice were given neomycin (1.1 g/1000 mL) in the drinking water for the first month after irradiation and transplantation.
Antibodies
The antibodies used in immunofluorescence staining included 49E8 (anti-CD34, kindly provided by Dr Hirohumi Hamada, Cancer Chemotherapy Center, Tokyo, Japan), ACK45 (anti–c-Kit), A20 (anti–Ly-5.1), and 104 (anti–Ly-5.2). Lineage marker antibodies included RB6-8C5 (anti–Gr-1), M1/70 (anti–Mac-1), RA3-6B2 (anti-CD45R/B220), 30-H12 (anti–Thy-1.2), L3T4 (anti-CD4), 53-6.72 (anti-CD8a), and TR-119 (anti–TER-119). All the antibodies except 49E8 were purchased from Pharmingen (San Diego, CA). Antibodies for Mac-1 and Thy-1, markers for macrophage/monocyte lineage cells and T lymphocytes, respectively, were omitted from the cocktail of lineage marker antibodies, as their expression on HSC has been reported.22 25 All antibody incubations were carried out for 30 minutes on ice.
Cell preparation
The BM cells were flushed from femurs and tibiae of fetal, neonatal, and adult mice. Liver and spleen cells were obtained by rubbing tissue between 2 pieces of glass and repeated pipetting. Cell suspensions were then filtered through a sterile 40-μm Cell Strainer (No. 2340; Falcon, Lincoln Park, NJ), stained with biotinylated antilineage markers, and enriched for cells not expressing the lineage markers (Lin−), using streptavidin-conjugated magnetic beads (PerSeptive Biosystems, Framingham, MA). Lin− cells were then stained with fluorescein isothiocyanate (FITC)–anti-CD34 and phycoerythrin (PE)–anti–c-Kit, and sorting was performed on a FACS Vantage (Becton Dickinson, Mountain View, CA).26,27 In second transplantation experiments, Lin− cells prepared by a method using immunomagnetic beads coated with sheep antirat IgG (Dynal AS, Oslo, Norway)28 29 were stained with biotinylated anti–Ly-5.2, followed by FITC–anti-CD34, PE–anti–c-Kit, and PE–cyanine 5–succinimidylester-streptavidin.
Transplantation and analysis of recipients
Varying numbers of sorted cells from Ly-5.2 mice were injected into sublethally irradiated Ly-5.1 mice together with 1 × 105 unfractionated BM cells from Ly-5.1 mice. In a preliminary experiment, we determined that 1 × 105 BM cells was the minimum dose of cells required for more than 95% recipient survival. Eight to 10 weeks after transplantation, peripheral blood (PB) was collected from the tail veins of the recipient mice. Red blood cells were removed, and the nucleated PB cells were stained with FITC–anti–Ly-5.2 and PE-antimyeloid cells (Mac-1 and Gr-1), anti-B lymphocytes (B220), or anti-T lymphocytes (Thy-1), and analyzed on a FACScan (Becton Dickinson). The mice in which donor-derived (Ly-5.2+) cells made up more than 1% of all B220+, Thy-1+, and Mac-1+/Ga-1+ cells in PB were scored as positive for successful reconstitution. Stable chimerism was maintained for over 6 months in all engrafted mice, although the reconstitution of T lymphocytes was slightly late compared with that of B lymphocytes or myeloid cells. The secondary transplants into Ly-5.1 mice were carried out using Ly-5.2 cells sorted from BM cells of the primary recipients (Ly-5.1 mice) 4 and 16 weeks after the first transplantation of Ly-5.2 mouse HSC.
Assay for colony-forming cells
Clonal cell culture was done in triplicate, as described.30,31 Briefly, 1 mL culture mixture containing 2.5 × 102 cells sorted from BM cells of Ly-5.2 mice at various developmental stages, α-modified Eagle medium (Flow Laboratories, Rockville, MD), 1.2% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal bovine serum (Hyclone Laboratories, Logan, UT), 1% deionized fraction V bovine serum albumin (Sigma Chemical, St. Louis, MO), 10−4 M mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), 100 ng/mL rat stem cell factor (SCF; Amgen, Thousand Oaks, CA) and human interleukin (IL)-6 (Tosoh, Kanagawa, Japan), 20 ng/mL mouse IL-3 (Kirin Brewery, Tokyo, Japan) and human thrombopoietin (Tpo) (Kirin), 2 U/mL of human erythropoietin (Epo) (Kirin), and 10 ng/mL of human granulocyte colony-stimulating factor (G-CSF) (Kirin) was plated in each 35-mm suspension culture dish (No. 171099; Nunc, Naperville, IL), which was incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Colony types were determined on days 7 to 14 of incubation by in situ observation using an inverted microscope and according to the criteria described.30 32 Abbreviations for the colony types are as follows: GM, granulocyte and/or macrophage colonies; E, erythroid bursts; MK, megakaryocyte colonies; and Mix, mixed hematopoietic colonies.
Results
CD34 expression on HSC in neonates
Most stem cell activity resides in the Lin−c-Kit+ cell fraction in the murine fetus19,20 and adult,33 although a small population of c-Kit− dormant HSC in adult BM has been reported.10 Therefore, we first examined CD34 expression on Lin−c-Kit+ HSC in neonatal BM cells of Ly-5.2 mice. Figure 1A shows a flow cytometric analysis of c-Kit and CD34 expression on Lin−BM cells of a murine neonate. Although most of the Lin−c-Kit− cells (57.2%) did not express CD34, Lin−c-Kit+ cells (42.8%) revealed various levels of CD34 expression. The Lin−c-Kit+ cells were fractionated into 3 subsets on the basis of CD34 expression; CD34− (6.6%, the average of 4 mice), CD34low (58.3%), and CD34high (35.1%) (R1, 2, and 3, respectively, in Figure1A). Cells from CD34−, CD34low, and CD34high fractions of Ly-5.2 mouse BM cells were injected into Ly-5.1 recipients together with 1 × 105unfractionated BM cells of Ly-5.1 mice. Eight to 10 weeks later, PB of the recipients was analyzed for Ly-5.2–expressing Gr-1+/Mac-1+ myeloid cells, B220+ B lymphocytes, and Thy-1+ T lymphocytes. Figure 1B shows the results of transplantation experiments. All 8 mice transplanted with 1 × 103Lin−c-Kit+CD34high cells and 2 of 4 mice transplanted with 1 × 102Lin−c-Kit+CD34high cells had Ly-5.2+ myeloid and lymphoid cells in the PB. Figure 1C shows a representative PB profile of a mouse transplanted with 1 × 103Lin−c-Kit+CD34high cells (R3), where 84.8% of Gr-1+/Mac-1+ cells, 98.4% of B220+ cells, and 41.3% of Thy-1+ cells were Ly-5.2+. Although the proportion of Ly-5.2+ cells depended on the number of cells injected into the recipient mice, all the mice had a higher proportion of Ly-5.2+ cells in B220+ B lymphocytes than in other lineages.
Flow cytometric analysis and transplantation results.
(A) Expression of c-Kit and CD34 on Lin− BM cells of a neonatal mouse (Ly-5.2). Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 4) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●] 1 × 103 [○] and 5 × 103 cells [▪]) in BM of neonatal mice (Ly-5.2). (C) A representative PB profile of a recipient mouse (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from Ly-5.2 mouse BM. Ly-5.2+ cells were present in all of B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
Flow cytometric analysis and transplantation results.
(A) Expression of c-Kit and CD34 on Lin− BM cells of a neonatal mouse (Ly-5.2). Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 4) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●] 1 × 103 [○] and 5 × 103 cells [▪]) in BM of neonatal mice (Ly-5.2). (C) A representative PB profile of a recipient mouse (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from Ly-5.2 mouse BM. Ly-5.2+ cells were present in all of B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
In the transplantation of Lin−c-Kit+CD34low cells, 1 of 5, and 1 of 2 mice injected with 1 × 103, and 5 × 103 cells, respectively, had Ly-5.2+ myeloid and lymphoid cells. By contrast, all 6 mice transplanted with 1 × 103Lin−c-Kit+CD34− cells had no detectable Ly-5.2+ PB leukocytes. Thus, long-term repopulating HSC were most enriched in the Lin−c-Kit+CD34high cell fraction in neonatal BM cells. Because CD34− cells accounted for only one fifiteenth of the neonatal Lin−c-Kit+BM cells, these results indicate that most neonatal BM HSC express CD34.
We then examined CD34 expression on HSC existing in neonatal liver and spleen. As shown in Figure 2, although the proportion of CD34high cells in Lin−c-Kit+ cells was smaller in neonatal liver or spleen than in BM, most long-term repopulating HSC were also included in the CD34-expressing cell fraction of neonatal liver and spleen cells, indicating that neonatal HSC express CD34, irrespective of the hematopoietic tissue in which they reside.
CD34 expression on HSC in fetal and neonatal liver and spleen.
Expression of c-Kit and CD34 on Lin− BM cells of neonatal liver, neonatal spleen, and fetal liver cells (16 dpc) of Ly-5.2 mice, and the percentages of Ly-5.2+ cells in B220+cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●], 1 × 103 [○], 2 × 103 [▪], 1 × 104 [■] and 2 × 104 cells [⧫]) in Ly-5.2 neonatal liver, neonatal spleen, and fetal liver cells (14-18 dpc). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of neonatal spleen, neonatal liver, and fetal liver cells (n = 2, 2, and 4) are shown in each window in the flow cytometry graphs.
CD34 expression on HSC in fetal and neonatal liver and spleen.
Expression of c-Kit and CD34 on Lin− BM cells of neonatal liver, neonatal spleen, and fetal liver cells (16 dpc) of Ly-5.2 mice, and the percentages of Ly-5.2+ cells in B220+cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●], 1 × 103 [○], 2 × 103 [▪], 1 × 104 [■] and 2 × 104 cells [⧫]) in Ly-5.2 neonatal liver, neonatal spleen, and fetal liver cells (14-18 dpc). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of neonatal spleen, neonatal liver, and fetal liver cells (n = 2, 2, and 4) are shown in each window in the flow cytometry graphs.
CD34 expression on HSC in fetus
Next, CD34 expression on HSC in fetal liver at 14, 16 and 18 dpc was analyzed (Figure 2). Although the proportion of CD34high cells in Lin−c-Kit+ cells was larger in 14 and 16 dpc fetal than in neonatal livers, Lin−c-Kit+ cells of 18 dpc fetal liver had a distribution of CD34-expressing cells similar to that in neonatal liver (CD34high cells, 31.9%, 28.6%, 17.1%; CD34low cells, 53.9%, 52.8%, 72.1%; and CD34− cells, 14.2%, 18.6%, 10.8%, in 14, 16 and 18 dpc fetal livers, respectively). In transplantation of the 3 fractions from 14, 16, and 18 dpc fetal liver cells, all 7 mice transplanted with over 1 × 102Lin−c-Kit+CD34high cells had Ly-5.2+ myeloid and lymphoid cells at levels that depended on the number of cells injected. In mice injected with Lin−c-Kit+CD34low cells, only one recipient receiving 2 × 104 cells from 18 dpc fetal liver showed engraftment of Ly-5.2+ cells, and 6 with less than 1 × 104 cells did not. No mouse had Ly-5.2+ PB leukocytes among the 8 mice transplanted with 1 × 102 to 2 × 104Lin−c-Kit+CD34−cells. We also carried out the transplantation using 18 dpc BM cells, in which CD34 expression in Lin−c-Kit+ cells revealed a similar distribution to that of neonatal BM cells (CD34highcells, 49.4%; CD34low cells, 42.6%; and CD34− cells, 8.0%). In the mouse injected with 3 × 103Lin−c-Kit+CD34high BM cells, 60% of myeloid cells, 84% of B lymphocytes, and 37% of T lymphocytes expressed Ly-5.2. The mouse injected with 3 × 103Lin−c-Kit+CD34low or Lin−c-Kit+CD34− cells had no Ly-5.2+ PB leukocytes. These results indicate that most stem cell activity in the fetus at late gestational stages, as well as in neonates, resides in the CD34-expressing cell fraction.
Change of CD34 expression on HSC with aging
Competitive long-term reconstitution assays were done using BM cells sorted from mice of various ages, the objective being to examine developmental changes in CD34 expression on HSC. CD34 and c-Kit expression in BM cells of 1-, 4-, 8-, and 16-week-old mice are shown in Figure 3. Although the proportion of CD34− cells in BM Lin−c-Kit+ cells showed no remarkable changes during aging, CD34low cells decreased and CD34high cells increased in BM of mice over 4 weeks of age. When transplanted with 1 × 103Lin−c-Kit+CD34high, CD34low, and CD34− cells obtained from 1-week-old mouse BM cells, 8 of 8, 5 of 8, and 0 of 8 mice, respectively, showed successful engraftment, indicating that HSC were most enriched in Lin−c-Kit+CD34highfraction. The recipients transplanted with Lin−c-Kit+CD34high and Lin−c-Kit+CD34low cells sorted from 4-week-old mouse BM cells revealed a similar engraftment rate. Transplantation using 8-week-old mouse BM cells showed that, although most HSC still expressed CD34, they were more enriched in Lin−c-Kit+CD34low cells than in Lin−c-Kit+CD34high cells. In contrast to results with BM cells obtained from mice younger than 8 weeks, HSC were found in Lin−c-Kit+CD34− in addition to Lin−c-Kit+CD34low cell fractions in BM from 10- to 16-week-old mice. When 1 × 105Ly-5.2+Lin−c-Kit+CD34highcells in 10- to 16-week-old mouse BM, which was the largest population in Lin−c-Kit+ cells, were transplanted into 4 recipients, none was successfully engrafted with Ly-5.2+cells. By contrast, only 1 to 5 × 102Ly-5.2+Lin−c-Kit+CD34−cells could repopulate in 5 of 8 recipients. Because the number of Lin−c-Kit+CD34− cells was only one twentieth that of Lin−c-Kit+CD34high cells in 10- to 16-week-old mouse BM, the result indicates that most hematopoietic repopulating ability is present in Lin−c-Kit+CD34− cell fraction in 10- to 16-week-old mice. Thus, CD34 expression on murine HSC decreases with aging.
CD34 and c-Kit expression in BM of mice at various ages.
Expression of c-Kit and CD34 on Lin− BM cells of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2), and the percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T)- and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (1-5 × 102[●], 1-5 × 103 [○], 1-2 × 104[■] and 1 × 105 cells [▵]) sorted from the BM of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of 1-, 4-, 8-, and 16-week-old mice (n = 5, 3, 7, and 10) are shown in each window in the flow cytometry graphs.
CD34 and c-Kit expression in BM of mice at various ages.
Expression of c-Kit and CD34 on Lin− BM cells of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2), and the percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T)- and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (1-5 × 102[●], 1-5 × 103 [○], 1-2 × 104[■] and 1 × 105 cells [▵]) sorted from the BM of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of 1-, 4-, 8-, and 16-week-old mice (n = 5, 3, 7, and 10) are shown in each window in the flow cytometry graphs.
Second transplantation of donor-derived HSC from the primary recipients
To confirm that adult Lin−c-Kit+CD34− HSC are the progeny of neonatal CD34-expressing HSC, we carried out a second transplantation of Ly-5.2+ cells sorted from BM of Ly-5.1 primary recipients who were transplanted with 1 × 103 BM Lin−c-Kit+CD34high cells from Ly-5.2 neonates. CD34 expression on Ly-5.2+Lin−c-Kit+ BM cells of the primary recipients 4 and 16 weeks after the first transplantation of Lin−c-Kit+CD34high cells from Ly-5.2 neonatal BM was similar to that for 4- and 16-week-old mice, respectively (Figures 4A and 5A, and compare Figure 3). We then sorted Ly-5.2+Lin−c-Kit+CD34high, CD34low, and CD34− cells from the primary recipients and transplanted them into Ly-5.1 secondary recipients. As shown in Figure 4B, most of the stem cell activity of Ly-5.2+ cells resided in the CD34-expressing cell fraction in BM cells of the primary recipients 4 weeks after the first transplantation. Figure 4C shows a representative PB profile of a second recipient transplanted with 2 × 103Ly-5.2+Lin−c-Kit+CD34highcells (R3) from a first recipient. As shown in Figure 5B, however, the stem cell activity was found in the Lin−c-Kit+CD34− cell fraction in BM cells of the primary recipient 16 weeks after transplantation. A representative PB profile of a mouse transplanted with 2 × 103Ly-5.2+Lin−c-Kit+CD34−cells (R1) in the primary recipient BM 16 weeks after transplantation is shown in Figure 5C. These results indicate that neonatal Lin−c-Kit+CD34high HSC generate Lin−c-Kit+CD34− HSC, and that the decrease in CD34 expression on HSC of young donors occurs even in aged congeneic recipients with a time course similar to that seen in the donor.
Second transplantation of donor-derived HSC in first recipients (4 weeks).
(A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the first recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 4 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 second recipient PB 8 to 10 weeks after the second transplantation. The second recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (2 × 103 cells for each) in BM of the first recipients (Ly-5.1). (C) A representative PB profile of a second recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in all B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
Second transplantation of donor-derived HSC in first recipients (4 weeks).
(A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the first recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 4 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 second recipient PB 8 to 10 weeks after the second transplantation. The second recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (2 × 103 cells for each) in BM of the first recipients (Ly-5.1). (C) A representative PB profile of a second recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in all B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
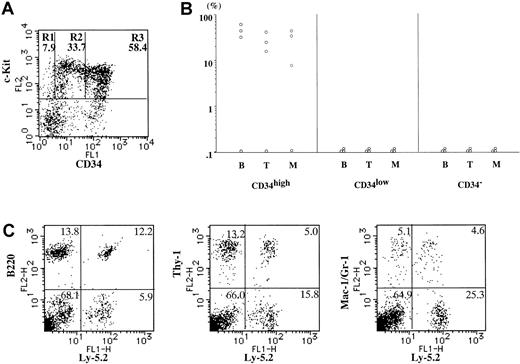
Second transplantation of donor-derived HSC in first recipients (16 weeks).
(A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the primary recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 16 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 secondary recipient PB 8 to 10 weeks after the second transplantation. The secondary recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (2 × 103[○] and 1 × 104 cells [■]) from BM of the primary recipients (Ly-5.1). (C) A representative PB profile of a secondary recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34− cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
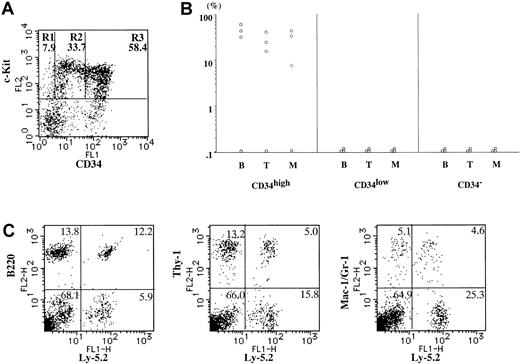
Second transplantation of donor-derived HSC in first recipients (16 weeks).
(A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the primary recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 16 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 secondary recipient PB 8 to 10 weeks after the second transplantation. The secondary recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (2 × 103[○] and 1 × 104 cells [■]) from BM of the primary recipients (Ly-5.1). (C) A representative PB profile of a secondary recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34− cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.
CD34 expression on colony-forming cells
Finally, we examined CD34 expression on murine HPC, using a methylcellulose clonal culture assay. Lin−c-Kit+CD34high, CD34low, and CD34− cells (2.5 × 102 cells) sorted from BM cells of mice at various developmental stages (18 dpc to 15 weeks old) were cultured in the presence of SCF, IL-3, IL-6, G-CSF, Epo, and Tpo. As shown in Table1, Lin−c-Kit+CD34high cells produced the largest number of hematopoietic colonies, whereas no colonies were generated from Lin−c-Kit+CD34−cells at any developmental stage, indicating that HPC, unlike HSC, continue to express CD34 throughout development.
Discussion
Despite the clinical importance of CD34 antigen as a marker for primitive hematopoietic cells for HSC transplantation or gene therapy, it has been controversial whether or not all HSC express CD34 in mice or humans. We here demonstrated developmental changes in CD34 expression on murine HSC. In fetal, neonatal, and younger adult hematopoietic tissues, most of Lin−c-Kit+ HSC expressed CD34. However, the CD34 expression on HSC decreased with aging, and HSC were relatively enriched in the Lin−c-Kit+CD34−cell fraction in mice over 10 weeks of age. The evidence for a decrease of CD34 expression on HSC during development was strengthened by the second transplantation experiments showing that donor-type HSC were present in the CD34− cell fraction in recipients engrafted with neonatal Lin−c-Kit+CD34high HSC 16 weeks after the first transplantation. This developmental change in CD34 expression on murine HSC may explain the contradictory data in previous reports regarding CD34 expression. Although Morel and coworkers8 noted the presence of CD34 antigen on HSC in 4- to 6-week-old mice, Osawa and colleagues9 showed, using older mice, that Lin−c-Kit+Sca-1+CD34low/−cells rather than Lin−c-Kit+Sca-1+CD34highcells possessed stem cell activity. There remains a possibility that CD34 varies among different strains of mice, since we, as well as Morel and associates and Osawa and colleagues, used C57BL/6 mice.
The function of CD34 in hematopoiesis has been elusive, although potential adhesive functions of CD34 have been reported.34,35 Recently, 2 groups of investigators reported on hematopoiesis in CD34-deficient mice.36,37 One group noted a decreased number of HPC in 10.5 dpc yolk sac, 14.5 dpc fetal liver, adult BM, spleen, and PB, and a poor response of adult HPC to cytokines, which suggested the involvement of CD34 in fetal and adult hematopoiesis.36 The present observation that HPC, unlike HSC, expressed CD34 throughout murine development from fetus to adult is consistent with their results. However, neither group discussed the biologic activity of long-term repopulating HSC of CD34-deficient mice. Therefore, the function of CD34 on HSC still remains unclear. An analysis of differences in biologic activities between neonatal Lin−c-Kit+CD34high HSC and adult Lin−c-Kit+CD34− HSC should be instructive to define the function of CD34 on HSC.
We found a difference in differentiation potential between neonatal Lin−c-Kit+CD34high cells and adult Lin−c-Kit+CD34− cells. Mice transplanted with neonatal Lin−c-Kit+CD34high cells showed a predominant reconstitution of B lymphocytes, compared to adult Lin−c-Kit+CD34− cells. The predominant reconstitution of B lymphocytes was also found in engraftment of Lin−c-Kit+CD34highcells from fetal liver. A similar observation was made in the mice reconstituted by fetal liver Thy-1lowSca-1+Lin− Mac-1+CD4−c-Kit+HSC.38 Therefore, the predominant reconstitution of B lymphocytes may result from a strong B-lymphoid potential of HSC from fetal, neonatal, and younger adult cells.
We found no difference in proliferation potentials between neonatal Lin−c-Kit+CD34high HSC and adult Lin−c-Kit+CD34− HSC. Although our experiments were not designed to evaluate the proliferative potential of single HSC, stable chimerism was maintained for over 6 months in all recipients engrafted with the respective HSC phenotypes. Morrison and coworkers38 noted indistinguishable proliferation activities of single HSC between young and old mice, yet it was also reported that the proliferation potential of HSC isolated from fetal liver is higher than that from adult BM.24 39 Further studies are needed to clarify the difference in proliferation potentials between neonatal Lin−c-Kit+CD34high HSC and adult Lin−c-Kit+CD34− HSC.
Aside from the evidence for developmental changes in CD34 expression on HSC, the present study has important implications for the clinical application of HSC. Because CD34-enrichment procedures are used to prevent graft-versus-host disease or for the purging of tumor/leukemic cells in therapeutic HSC transplantation, and are also used as a target cell population for gene therapy, it is an extremely crucial issue whether human HSC express CD34. Based on the present findings, we consider that more attention should be directed to age of the donor in discussing this issue. In addition, the present findings also suggest that fetal and neonatal HSC have characteristics different from those of adult HSC. Accordingly, more detailed characterization of umbilical cord blood HSC may contribute to further development of cord blood transplantation, which is now increasingly used as an alternative to BM transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kohichiro Tsuji, Department of Clinical Oncology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: tsujik@ims.u-tokyo.ac.jp.

![Fig. 1. Flow cytometric analysis and transplantation results. / (A) Expression of c-Kit and CD34 on Lin− BM cells of a neonatal mouse (Ly-5.2). Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 4) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●] 1 × 103 [○] and 5 × 103 cells [▪]) in BM of neonatal mice (Ly-5.2). (C) A representative PB profile of a recipient mouse (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from Ly-5.2 mouse BM. Ly-5.2+ cells were present in all of B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598001.jpeg?Expires=1770139135&Signature=qnjDX74Ys7GLMo7c4tIf2M5SuwdX9rncuO37WHtFa78z0jRF4YRLBKstu3FcB6e3xGz-2KhJ7zGj8CrIsVyRTA~cMGdFrmpQ1DlC~vWPPQCo41hPJuczH3eBS3OHkZIYDnXbVHZ9Vx9uThMMmz-1NuHSpBfVx9vXctE3uwChxhf8vV4sylVLC97KsgClcOvMx5iY6yXVbc5608mv-FF77t2yicP6EXiG9s7aJBhSc4V7OwoJ9H4GPFYldhkLlM3dPwLbyFI2~53xo4TcrjNY1oah0L93Hj65vBB8gPEBafGGWfCGYGosi6cWl77LVPdyDy-tQuTRcB34Xs~F2fMyBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. CD34 expression on HSC in fetal and neonatal liver and spleen. / Expression of c-Kit and CD34 on Lin− BM cells of neonatal liver, neonatal spleen, and fetal liver cells (16 dpc) of Ly-5.2 mice, and the percentages of Ly-5.2+ cells in B220+cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●], 1 × 103 [○], 2 × 103 [▪], 1 × 104 [■] and 2 × 104 cells [⧫]) in Ly-5.2 neonatal liver, neonatal spleen, and fetal liver cells (14-18 dpc). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of neonatal spleen, neonatal liver, and fetal liver cells (n = 2, 2, and 4) are shown in each window in the flow cytometry graphs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598002.jpeg?Expires=1770139135&Signature=B0ruVU~iaxxRCZeee1cYLu7YiEkjToruAzEIHhrRPJNMckrSnrsHLwrz88r8hjxTTbMmp4KpetTjI~4T3gDEAUI5GPUyUbOidgvrk6DWh22eoeVhmOrm3gRbkB1~xSSZp1qnv0uOHJpoxA48IiVgAHCdU6mr~hkg4Z5uEiPMuuQsvl0mbI4~dO3MagEVstkQ7E1hglYh4r96JflxZ0FchM5sGsgF3WmRhlNtMo08HstaE6KgszmKbZEb1KwIObbyXLcRJ-aD2vvNfvidog6uKda5PByoCQ~mwcMqJ1~RGlsIA-ENhVuT1sQxjFRmLr61rvbkfBpTkCIVKsLJAnLL3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD34 and c-Kit expression in BM of mice at various ages. / Expression of c-Kit and CD34 on Lin− BM cells of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2), and the percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T)- and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (1-5 × 102[●], 1-5 × 103 [○], 1-2 × 104[■] and 1 × 105 cells [▵]) sorted from the BM of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of 1-, 4-, 8-, and 16-week-old mice (n = 5, 3, 7, and 10) are shown in each window in the flow cytometry graphs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598003.jpeg?Expires=1770139135&Signature=aQPdUP9z7A1lbBN5mT8NYbGpMq66HdeVCA8PVjjKi~5nHtJEaPjb65P-Juhe1YSNdILjYhqYZ9VyPGVUqppT~yyfvOFjdU3275WZZbLzE0SysAfeovTbziqlVI31GQhbn~czepoXyAyLE5ziKlFuKYYytxaqEL6bkT078WNSFvcVvi2BHfRODXY5ZpaeXIi-3UCppU7udcm3WGYfgLEYMBp6T0WpeHN8Nq8ys4jnlWDAoM3d0H0hPvP85EvOks3lmgk2ZyHSNEb89s0cRfct7hdjaS5bHp134UNLOnRu92n1Mvn8NkwdXdL1u7kJRrPNTy2P5pgrVr1ybEBMWOnwMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Second transplantation of donor-derived HSC in first recipients (16 weeks). / (A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the primary recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 16 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 secondary recipient PB 8 to 10 weeks after the second transplantation. The secondary recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (2 × 103[○] and 1 × 104 cells [■]) from BM of the primary recipients (Ly-5.1). (C) A representative PB profile of a secondary recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34− cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598005.jpeg?Expires=1770139135&Signature=hTSAR4tvyVmjcT0sAZ5z6wtv5ynNfp5DW6jQGabH2-~U9yWRVfXJQJxofQ~-5XqOfv21NSm5rT82Ghtwrmts7YHDVGI2BkYDNHBLdfWWVwefcwucV~Nbsj-MAOAW49M8kXeeYNAOgMve5yBaTJmavWOx5SMBOpFGOyt202dMyAxNB4ZYd2uExsLD5zIywXMy3vQBuRqTJuA~c~38ylmKEsdb1bUWnh~3eZHdiqdHiO8xkeKaQL9hmtRe0RirsHy2sxP9nIb~XWjA~CDSLuVV1Y4X1Tshu1GzaijKYXBtOO2~cWE8QYyiLWu6LqtIOD7foP4-KFwSGGEJZwEh-Outew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Flow cytometric analysis and transplantation results. / (A) Expression of c-Kit and CD34 on Lin− BM cells of a neonatal mouse (Ly-5.2). Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 4) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●] 1 × 103 [○] and 5 × 103 cells [▪]) in BM of neonatal mice (Ly-5.2). (C) A representative PB profile of a recipient mouse (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34high cells (R3) sorted from Ly-5.2 mouse BM. Ly-5.2+ cells were present in all of B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598001.jpeg?Expires=1770139136&Signature=hXrrg0-QZ4kMt3s3z58WIChkyd3y7NIWJlisLj6acHKHQ7YL0fmE6yJCtl4BWYRaL-asK39q-rYmXtPQScoCoBONXHs5ljj29iMBfNRDbSm9yeQT6V8dikH7o7-xJFnlLNDaqqUGI4LpNhS1~MBeKOaTZWSqRQBjv52G4ELNl-WIqOOu5mh51hpv1KRAsW9BS~T2j53jFL6n5a7B3kyQo~1wpuf6JTSX9vbphJiJDPOxqETOtYEHoD00Tpmu3qBCcOucBkip0Zn7rqBUhZxH2nZTkgw3tjZ9WUz9YrLqkZARyw3FAe7QL0A5tOTXHEIJu1fLfDSUxRaNZ8w43cWmSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. CD34 expression on HSC in fetal and neonatal liver and spleen. / Expression of c-Kit and CD34 on Lin− BM cells of neonatal liver, neonatal spleen, and fetal liver cells (16 dpc) of Ly-5.2 mice, and the percentages of Ly-5.2+ cells in B220+cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low (R2), and CD34high (R3) cells (1 × 102 [●], 1 × 103 [○], 2 × 103 [▪], 1 × 104 [■] and 2 × 104 cells [⧫]) in Ly-5.2 neonatal liver, neonatal spleen, and fetal liver cells (14-18 dpc). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of neonatal spleen, neonatal liver, and fetal liver cells (n = 2, 2, and 4) are shown in each window in the flow cytometry graphs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598002.jpeg?Expires=1770139136&Signature=1K48BA8lsJn8CXxIX5OztkcztGwEDNyPsb-AlWlrdX~l-5rvK1aKLegrIwyH2tx1kWj62Dmc~kFWXEq8No6QCuNHVduAi0hzY5vbdtS3HYzvM0wAe4iRu34XTcqY4mBikwD13MBnPUDrrqYFA55afQ3Qowtcapg7KF67wray2C289hdEZMKjjK0GWhqjOIBOQc2ftHq11p4qEr-u4fNIXz58wVR-PVxor~XSFAN1QCz-y4gWD6b6H~4LLcI97~-JXwNgvbki0Yv8MUee97-vCVTffLKMKA9hcPbodNlqVU-XakRJ8FbufIx5EemmGdTNcks8iCEVdmPUaTHFHfEN0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD34 and c-Kit expression in BM of mice at various ages. / Expression of c-Kit and CD34 on Lin− BM cells of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2), and the percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T)- and Gr-1/Mac-1+ cells (M) in Ly-5.1 recipient PB 8 to 10 weeks after transplantation. The recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (1-5 × 102[●], 1-5 × 103 [○], 1-2 × 104[■] and 1 × 105 cells [▵]) sorted from the BM of 1-, 4-, 8-, and 16-week-old mice (Ly-5.2). The averages of the proportions of the 3 fractions in Lin−c-Kit+cells of 1-, 4-, 8-, and 16-week-old mice (n = 5, 3, 7, and 10) are shown in each window in the flow cytometry graphs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598003.jpeg?Expires=1770139136&Signature=47Hsqt-Zc2q1V9JtSHgh~PMLqnz4dOAUX8ykTMXQ5ezozaFRAB9FjUOvUapj973-4CDF4qXz5VYW3FcAEjnWcQd1Yw4sTx810lBGWEnqUszcDi8Ds99PIU4wW2SjiEw-wAaFiz3R84cl2N1V8uC5kdTaNZXpC7s01mWbX9rjYOV8p0UMX4EQ4o2dbX4z0DzYhTEeVSBCqJNH5C1GxlEkZVfA97Yt-bYjmqgmXUmMY1bH3llCKgTpEkR42PJ7Mk34jDPcvo1f7Qigm~V79rd4QtY07Xw7uZ4-GJwLtmWzdlZm6H5Sb~QQLXujNhJL2~qq0arq4~LN4t4aZa9MWrlIiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Second transplantation of donor-derived HSC in first recipients (16 weeks). / (A) Expression of c-Kit and CD34 on Ly-5.2+Lin− BM cells of the primary recipient mice (Ly-5.1) engrafted with 1 × 103 neonatal Lin−c-Kit+CD34high BM cells (Ly-5.2) 16 weeks after transplantation. Lin−c-Kit+ cells were fractionated into CD34− (R1), CD34low (R2), and CD34high (R3) cells on the basis of CD34 expression. The averages of the proportions of the 3 fractions in Lin−c-Kit+ cells (n = 3) are shown in each window. (B) The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) in Ly-5.1 secondary recipient PB 8 to 10 weeks after the second transplantation. The secondary recipients were transplanted with CD34− (R1), CD34low(R2), and CD34high (R3) cells (2 × 103[○] and 1 × 104 cells [■]) from BM of the primary recipients (Ly-5.1). (C) A representative PB profile of a secondary recipient (Ly-5.1) engrafted with 1 × 103Lin−c-Kit+CD34− cells (R3) sorted from the first recipient. Ly-5.2+ cells were present in B220+ cells, Thy-1+ cells, and Gr-1/Mac-1+ cells in the PB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.419/5/m_h80210598005.jpeg?Expires=1770139136&Signature=FgsdYfMz6C1rJOchMgIchpYsdw9XaXoi24TU7yk6mbqxrDyvkoz9A-sczsF~PyEgmL6TsDn37TMkchnWHEJy~musIi0DYr0~gmPyBQBByGUekCneYT5CDCy3U5ztHWOLxB8ERlymrGwfs7NzU7j7A0CVots3UuDQeaFiYf-1BlaovLaX1uk8v2vho8aVK3koZ-D56iK-WNglV7ju7l1681yCXAP8c3jWuLaNK7zr3Y7SazUPtII-05Jzw7Psz5IkdqUYENhjmOa6~4ZBCFqvwwNJqnFg3phmJUzWuiPnDOKiNK2orUeWH2Id~1K8EiFv8sdFOWzhPWt3J59lLWRefg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)