The study of megakaryocytopoiesis has been based largely on in vitro assays. We characterize an in vivo model of megakaryocyte and platelet development in which human peripheral blood stem cells (PBSCs) differentiate along megakaryocytic as well as myeloid/lymphoid lineages in sublethally irradiated nonobese diabetic/severe combined immunodeficient (NOD-SCID) mice. Human hematopoiesis preferentially occurs in the bone marrow of the murine recipients, and engraftment is independent of exogenous cytokines. Human colony-forming units–megakaryocyte (CFU-MK) develop predominantly in the bone marrow, and their presence correlates with the overall degree of human cell engraftment. Using a sensitive and specific flow cytometric assay, human platelets are detected in the peripheral blood from weeks 1 to 8 after transplantation. The number of circulating human platelets peaks at week 3 with a mean of 20 × 109/L. These human platelets are functional as assessed by CD62P expression in response to thrombin stimulation in vitro. Exogenous cytokines have a detrimental effect on CFU-MK production after 2 weeks, and animals treated with these cytokines have no circulating platelets 8 weeks after transplantation. Although cytokine stimulation of human PBSCs ex vivo led to a significant increase in CFU-MK, CD34+/41+, and CD41+ cells, these ex vivo expanded cells provided only delayed and transient platelet production in vivo, and no CFU-MK developed in vivo after transplantation. In conclusion, xenogeneic transplantation of human PBSCs into NOD/SCID mice provides an excellent in vivo model to study human megakaryocytopoiesis and platelet production.
Introduction
Megakaryocytopoiesis is a complex process that involves proliferation of megakaryocyte (MK) precursors followed by differentiation events. The final stage of thrombocytopoiesis involves transendothelial cytoplasmic fragmentation to produce platelets.1-3 A hierarchy of human MK progenitor cells has been defined based on in vitro clonogenic assays (high proliferative potential cell–MK,4 burst-forming unit–MK,5 and colony-forming unit–MK6[CFU-MK]) that have expanded our knowledge of cellular phenotypes and the cytokines required for colony formation. Suspension cultures have been used to further explore growth factor control of MK proliferation and differentiation.7,8 Ex vivo conditions have been optimized to generate cell populations enriched in progenitors, precursors, and mature MKs7,9 as well as functional platelets.10 11
The relationship between the cells defined by in vitro assays and those responsible for in vivo hematopoietic reconstitution remains unclear. At present, bone marrow (BM) and peripheral blood stem cell (PBSC) transplantation in people is the only assay to test the in vivo repopulating capacity of human hematopoietic stem cells (HSCs). Factors predicting stem cell engraftment have been postulated, but reliable markers of rapid and sustained megakaryocytic (MK) reconstitution after myeloablative chemotherapy remain undefined. The effects of posttransplantation stimulation of platelet production using human growth factors12-15 and the administration of ex vivo expanded clonogenic MKs are being explored in several clinical settings.16 17
In vivo assays developed to quantify true human pluripotent HSCs and study cellular phenotypes during hematopoiesis outside of clinical transplantation have used xenogeneic systems. Immunodeficient mice, including nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice,18 provide a small animal model with a microenvironment permissive for the engraftment of human HSCs. NOD/SCID mice, which consistently show extensive and reproducible engraftment of human HSCs,19-22 have multiple defects in innate and adaptive immunity. These animals are homozygous for a nonsense mutation in the scid gene Prkdcscid, which encodes a DNA-dependent protein kinase23 required for DNA repair, and thus these mice lack functional lymphoid cells. The NOD phenotype, because of multiple genes, confers to the mice a lack of complement activity and decreased function of both macrophages and natural killer cells. Multiple strategies have been pursued to optimize engraftment of human HSCs in these and other immunodeficient mice, including injection of recombinant human growth factors,24,25 introduction of transgenes for human cytokines,26 cotransplantation of human fetal tissues,27,28 and stromal cells engineered to produce human interleukin-3 (IL-3).29 Using these murine models for human HSC engraftment, the frequency of repopulating cells within the graft can be estimated, and their proliferation and differentiation potential can be determined.
There is a great need for an in vivo animal model in which human megakaryocytopoiesis and thrombopoiesis can be studied. Cashman et al reported that human cord blood (107 light-density fraction cells) is able to generate human MK progenitors (CFU-MK) and their CD41+ progeny in NOD-SCID mice. When BM cells are used, fewer CD41+ cells and no CFU-MKs are identified.20 Evidence of MK development and terminal differentiation into human platelets with human CD34+ PBSCs in these xenotransplant models is still lacking.
In the present study, we report that human CD34+ PBSCs can generate human CFU-MK and CD41+ cells in engrafted NOD/SCID mice. Platelet production, the final event of MK development, is also supported in this murine microenvironment. In addition, we assess the engraftment potential of human cells after ex vivo cytokine stimulation to optimize MK growth and development. This preclinical mouse model provides an in vivo system to study the cellular basis of MK reconstitution and the feasibility of stem cell manipulation to improve posttransplantation platelet recovery.
Materials and methods
Human stem cells
PBSCs harvested from patients and donors for autologous stem cell transplantation were positively selected for CD34+cells using immunomagnetic beads (Dynal, Lake Success, NY) and anti-CD34 monoclonal antibody (HPCA 1) on a Nexell Isolex 300i Device. CD34+ cells were enriched to 96% to 98% purity. Small aliquots (10 × 106-25 × 106CD34+) were cryopreserved until used for this study. Use of human cells was approved by the Yale University Institutional Review Board.
Ex vivo expansion of human stem cells
CD34+ PBSCs were seeded at 50 × 103cells/mL in serum free X-vivo 10 medium (BioWhittaker, Rockland, ME) supplemented with 20 ng/mL recombinant human IL-3, IL-6, IL-11, FLT3-ligand (Fl3), thrombopoietin (TPO), and stem cell factor (SCF) (R&D Systems, Minneapolis, MN). Cultures were incubated at 37°C in a humidified atmosphere containing 5% carbon dioxide. The cell count was monitored daily, and cell concentration was readjusted by the addition of fresh medium, with no further cytokine supplementation, when the cell concentration exceeded 150 × 103 cells/mL.
Analysis of progenitor cells generated ex vivo
Cells grown in culture were analyzed by flow cytometry for phenotype (CD34 and CD41) and DNA content. Appearance of cultured cells was evaluated with Wright's stained cytospin slides. Evaluation of the MK clonogenic potential (CFU-MK) was performed at different times in a serum substitute medium supplemented with human recombinant IL-3, IL-6, and TPO using collagen as a gelling agent (MegaCult-C, Stem Cell Technologies, Vancouver, BC). After incubation for 10 days at 37°C in a humidified atmosphere with 5% carbon dioxide, the medium was dehydrated, and the colonies were fixed with methanol/acetone. Detection of CFU-MK was performed by staining the cells with a primary antibody to the human MK-specific antigen glycoprotein (GP)IIb/IIIa (CD41) linked to a secondary biotinylated antibody-alkaline phosphatase avidin conjugate detection system.
Transplantation of human cells in NOD/SCID mice
Five-week-old NOD/SCID mice (Jackson Laboratory, Bar Harbor, ME) were obtained and housed in microisolators under pathogen-free conditions and fed with autoclaved food and water. Mice received a sublethal dose of 300 to 375 cGy total body irradiation using a Gamma Cell 40 (Nordion International, Kanata, ON, Canada) equipped with 2 opposing 137Cs sources. Sulfatrim (Alpharma, Baltimore, MD) was added to the drinking water after mice were irradiated. In each experiment, a single donor was used. Human CD34+ PBSCs were thawed and resuspended in RPMI (Sigma, St Louis, MO) containing 5% fetal calf serum (FCS; Gemini Bio Products, Woodland, CA), and a cell dose from 0.2 × 106 to 1 × 106 cells/mouse was transplanted into mice by tail vein injection 4 hours after irradiation in a volume of 200 μL RPMI/5% FCS per mouse. In most of the experiments, to determine the engraftment effect of repeated exposure to exogenous growth factors, mice were randomized to receive phosphate-buffered saline (PBS) or one of several combinations of recombinant human TPO, SCF, IL-3, IL-6, IL-11, or Fl3 (0.2 μg/kg per dose per cytokine; R&D Systems) intraperitoneally 3 times per week for the duration of the studies. All animal experiments were performed in accordance with institutional guidelines approved by the Animal Care and Use Committee of Yale University School of Medicine.
Human platelet detection in NOD/SCID mice
Peripheral blood (PB) was obtained via retro-orbital bleeding at different times. Two-color flow cytometric analysis using directly conjugated monoclonal antibodies was performed with 5 μL ethylenediaminetetraacetic acid anticoagulated PB. Antibodies used were fluorescein isothiocyanate (FITC)-labeled antihuman CD41a (GPIIb-IIIa), FITC antihuman CD42b (GPIb), FITC-antimouse immunoglobulin G1 (Pharmingen, San Diego, CA), and phycoerythrin (PE)-labeled antimouse CD61 (Dako, Carpinteria, CA). Cells were incubated with the antibodies for 30 minutes at room temperature, washed, and diluted in 300 μL Tyrodes/4-(2-Hydroxethyl)-1-piperazineethane sulfonic acid (HEPES) buffer and analyzed. PB from untransplanted mice was analyzed as an additional negative control. A FACScan flow cytometer (Becton Dickinson, San Jose, CA) was used for acquisition of platelet data, and analysis was performed using CellQuest software (Becton Dickinson). Platelets were analyzed by gating on low forward and side scatter, and 50 000 to 100 000 events were acquired. A detection threshold of 1 human platelet for 10 000 mouse platelets (0.01%) was determined by in vitro dilutional analysis experiments (data not shown).
In vitro assay to study human platelet activation
Aliquots of mouse PB (10 μL) were mixed with 40 μL thrombin (50 U/mL) and incubated at room temperature for 10 minutes. Incubation with FITC-antihuman CD41a/PE-control and FITC–anti-CD41a/PE-antihuman CD62 was performed for 20 minutes at room temperature, and platelets were diluted in 250 μL Tyrodes/HEPES buffer. Live acquisition of 100 to 200 human platelet events was performed by gating on FITC+ events in the platelet size range.
Analysis of human engraftment in NOD/SCID mice
Under inhalation anesthesia, PB was obtained via heart puncture 1 to 8 weeks after xenotransplant, and mice were euthanized by cervical dislocation. Single-cell suspensions were prepared from BM cells derived from the femurs, tibiae, and iliac crests of each mouse flushed into Iscoves modified Dulbecco medium/5% FCS, and spleen cells were expressed from the splenic capsule into a small volume of medium by applying gentle pressure to the organ. Flow cytometric detection of human cells in murine tissues (BM and spleen) was performed directly with FITC- or PE-conjugated monoclonal antibodies against human CD45, CD3, CD19, CD41, CD33, and CD34 (Pharmingen). Approximately 1 × 106 cells were resuspended in PBS/1% albumin and incubated with monoclonal antibodies for 30 minutes at 4°C. After incubation, cells were washed twice with PBS/1% albumin and fixed with 2% formaldehyde. For each experiment, an aliquot of cells from a transplanted mouse was stained with isotype control mouse immunoglobulin G conjugated with FITC or PE, and the lack of cross-reactivity of human-specific antibodies with mouse cells was confirmed by staining BM and spleen cells of an untransplanted animal (additional negative control). A FACSCalibur flow cytometer (Becton Dickinson) was used for acquisition of data on nucleated cells. Genomic DNA was isolated from BM and spleen of transplanted mice using a QIAamp DNA mini kit (Qiagen, Valencia, CA). EcoRI digests (5 μg total DNA) were separated by agarose gel electrophoresis, transferred to nylon membrane, and probed using a 32P-labeled human chromosome 17–specific α-satellite probe (p17H8)30kindly provided by M. Bhatia (London, ON, Canada). The level of human cell engraftment was determined by comparing the density of the 2.7-kilobase band with that of control mixtures of human and mouse genomic DNA (limit of detection 0.1%, data not shown). A total of 2 × 106 cells from BM or spleen were assayed for CFU-MK using the MegaCult-C system.
Results
Engraftment and localization of human hematopoiesis in the BM and spleen of NOD/SCID mice after transplantation of human CD34+ PBSCs
Engraftment in NOD/SCID mice was defined as the presence of at least 0.5% human CD45+ cells of total nucleated cells in the murine microenvironment with evidence of multilineage reconstitution. For each animal, the result obtained by flow cytometry for human CD45 was close to that obtained using Southern blot analysis with a human chromosome 17–specific α-satellite probe (Figure1), confirming the validity of these assays.
Correlation of flow cytometry and Southern blot analysis to detect human engraftment in NOD/SCID mice.
Data are from 2 representative animals transplanted with 0.6 × 106 human CD34+ PBSCs and killed 8 weeks after transplantation. (A) Southern blot analysis of the BM and spleen of 2 mice. Human:mouse DNA controls are given as percent human DNA. (B) BM and spleen isotype controls for FITC (x-axis) and PE (Y-axis). (C) Evaluation of human cells labeled with antihuman CD19-PE and CD45-FITC monoclonal antibodies in the BM and spleen. The percentage of human cells is indicated.
Correlation of flow cytometry and Southern blot analysis to detect human engraftment in NOD/SCID mice.
Data are from 2 representative animals transplanted with 0.6 × 106 human CD34+ PBSCs and killed 8 weeks after transplantation. (A) Southern blot analysis of the BM and spleen of 2 mice. Human:mouse DNA controls are given as percent human DNA. (B) BM and spleen isotype controls for FITC (x-axis) and PE (Y-axis). (C) Evaluation of human cells labeled with antihuman CD19-PE and CD45-FITC monoclonal antibodies in the BM and spleen. The percentage of human cells is indicated.
Engraftment varied based on the human donor used. Thawed cells from 4 of 9 donors failed to engraft any of the transplanted mice. When transplantation was successful, 62 of 74 (84%) of the mice engrafted. The mean percentage of human CD45+ cells in the BM at week 8 after transplantation was 12% and 16% for animals irradiated with 300 cGy (n = 5) or 375 cGy (n = 6), respectively. However, there was a significant difference in early (10 days) postradiation morbidity (weight loss, pallor, and fur changes) and an increased mortality in the higher radiation dose group (30% vs 13%). A cell dose threshold of 0.6 × 106 human CD34+ PBSCs was necessary for consistent successful engraftment 3 weeks after transplantation. Higher doses (1 × 106 cells/mouse) did not yield an increase in the percentage of human cell engraftment. Based on these results, a radiation dose of 300 cGy with a cell dose of 0.6 × 106 cells/mouse was used in all subsequent experiments.
To determine the site of human hematopoiesis in NOD/SCID mice and evaluate the effect of exogenous human cytokines on engraftment, mice (n = 30) received intraperitoneal injections of human TPO, SCF, and IL-3 (3C group); TPO, SCF, IL-3, IL-6, IL-11, and Fl3 (6C group); or placebo (PBS group); and engraftment was analyzed in different organs after transplantation. Two weeks after transplantation, regardless of the cytokines used, human CD45+ cells were present in the spleen with percentages as high as 76% human CD45+ cells of total nucleated cells. In contrast, at weeks 4 and 8, human hematopoiesis was mainly in the BM in all groups (Figure2A). Thus, BM is the preferred site of long-term human hematopoiesis in NOD/SCID mice. This finding, confirmed by Southern analysis (Figure 2B), remained consistent in all experiments.
Localization of human hematopoiesis in NOD/SCID mice.
(A) Detection of human engraftment by flow cytometry of human CD45 in the BM and spleen of NOD/SCID mice (n = 30) transplanted with 0.6 × 106 CD34+ PBSCs and killed 2 ( ), 4 (
), 4 ( ), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.
), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.
Localization of human hematopoiesis in NOD/SCID mice.
(A) Detection of human engraftment by flow cytometry of human CD45 in the BM and spleen of NOD/SCID mice (n = 30) transplanted with 0.6 × 106 CD34+ PBSCs and killed 2 ( ), 4 (
), 4 ( ), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.
), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.
Role of exogenous human cytokines in engraftment of human CD34+ PBSCs in NOD/SCID mice
In a series of 6 experiments, in the PBS group, BM engraftment of human cells showed a gradual increase from week 2 to week 8 after transplantation. The mean values were 3.8% (n = 8), 5.9% (n = 11), and 16.7% (n = 10) human CD45+ cells at weeks 2, 4, and 8, respectively. There was a statistically significant difference between weeks 2 and 8 after transplantation (P = .03). In the 6C group, mean values of 2% (n = 6), 3.3% (n = 8), and 5.4% (n = 7) human CD45+ cells were identified at weeks 2, 4, and 8, respectively. In the 3C group, in contrast, engraftment decreased over time from 21% (n = 2), to 5.4% (n = 3), to 2.1% (n = 7) CD45+ cells at weeks 2, 4, and 8. The highest percentage of engraftment (50%) occurred in animals that received no human cytokines (PBS) 8 weeks after transplantation (Figure 3). The lower level of human engraftment in the 3C and 6C groups compared with the PBS group 8 weeks after transplantation suggests that administration of these human cytokines decreases human stem cell survival.
Effect of cytokine treatment on human CD34+PBSC engraftment in the BM of NOD/SCID mice.
Human engraftment of 59 mice transplanted with 0.6 × 106CD34+ PBSCs and treated with TPO, SCF, and IL-3 (3C, n = 10); TPO, SCF, IL-3, IL-6, IL-11, and Fl3 (6C, n = 21); or without cytokines (PBS, n = 29) until time of sacrifice. The percentage of CD45+ human cells was determined by flow cytometry 2 (○), 4 (■), and 8 (●) weeks after transplantation. Each dot represents the level of human cells detected in the BM of a single engrafted (CD45+ cells > 0.5%) mouse. The engraftment rate was 80% ± 9% for the 3 groups. R = statistically significant difference (P = .03) in engraftment in the PBS group between weeks 2 and 8.
Effect of cytokine treatment on human CD34+PBSC engraftment in the BM of NOD/SCID mice.
Human engraftment of 59 mice transplanted with 0.6 × 106CD34+ PBSCs and treated with TPO, SCF, and IL-3 (3C, n = 10); TPO, SCF, IL-3, IL-6, IL-11, and Fl3 (6C, n = 21); or without cytokines (PBS, n = 29) until time of sacrifice. The percentage of CD45+ human cells was determined by flow cytometry 2 (○), 4 (■), and 8 (●) weeks after transplantation. Each dot represents the level of human cells detected in the BM of a single engrafted (CD45+ cells > 0.5%) mouse. The engraftment rate was 80% ± 9% for the 3 groups. R = statistically significant difference (P = .03) in engraftment in the PBS group between weeks 2 and 8.
Conservation of stem cells (CD34) in NOD/SCID mice after transplantation of human CD34+ PBSCs
To investigate whether human CD34+ cells are conserved in NOD/SCID mice, the expression of human CD34 was assessed on BM cells. Two cohorts of mice were analyzed (6C or PBS group) and, in both, 50% to 60% of all mice that were positive for the human pan-leukocyte marker CD45 (chimeric mice) maintained a subpopulation of human CD34+ cells. At week 4, there was no difference between the PBS (n = 10) and the 6C groups (n = 6). However, 8 weeks after transplantation, a mean of 3.5% (n = 10) of the human cells expressed CD34 in the PBS group and a mean of 0.85% (n = 7) in the 6C group, a difference that was statistically significant (P = .03). These findings suggest that a detrimental effect of in vivo cytokines on maintenance of stem cells takes place between weeks 4 and 8 after transplantation.
Lymphoid, myeloid, and MK differentiation of human CD34+ PBSCs in NOD/SCID mice
Multilineage human engraftment was confirmed by flow cytometry for B (CD19), T (CD3), myeloid (CD33), and MK (CD41) cell specific markers (Figure 4). The phenotypic profile of engrafted CD45+ cells in murine BM in the 3C and 6C groups (n = 17) was shifted toward the B lymphoid series (CD19) at weeks 4 and 8 after transplantation. However, in the PBS group at week 4, most mice (7 of 11) did not express mature lymphoid markers (CD3 or CD19), and at week 8 these mice expressed a predominance of either T cells (CD3+, 3 of 8 mice) or B cells (CD19, 3 of 8 mice).
Human multilineage engraftment in the BM of chimeric NOD/SCID mice.
Representative flow cytometric analysis of BM cells from a mouse transplanted with 0.6 × 106 human CD34+PBSCs and killed 8 weeks after transplantation. (A) Isotype controls. (B) Immunophenotypes with the indicated FITC- or PE-conjugated antibodies, shown on the x-axis and y-axis, respectively.
Human multilineage engraftment in the BM of chimeric NOD/SCID mice.
Representative flow cytometric analysis of BM cells from a mouse transplanted with 0.6 × 106 human CD34+PBSCs and killed 8 weeks after transplantation. (A) Isotype controls. (B) Immunophenotypes with the indicated FITC- or PE-conjugated antibodies, shown on the x-axis and y-axis, respectively.
BM cells were evaluated for MK surface markers using antibodies specific for human GPIIb/IIIa (CD41a). For mice in the PBS group, 40% (4 of 10), 44% (4 of 9), and 20% (2 of 10) had human CD41+ BM cells at weeks 2, 4, and 8, respectively. The mean percentage of CD34+CD41+ and CD34−CD41+ cells was 1.8% and 5.1%, respectively. Based on the total number of BM cells in a mouse,31 this represents approximately 7 × 106 CD34+CD41+ cells in the marrow when only about 5 × 103CD34+CD41+ cells were present in the injected cell population. This represents a 1200-fold increase in the number of CD34+CD41+ cells. No CD34−CD41+ cells were injected, and the number in the BM was approximately 20 × 106, highlighting the high degree of megakaryocytopoiesis occurring in vivo. In the 3C and 6C groups, human CD41+ cells were not detected at any time. These findings suggest that administration of human cytokines negatively affects megakaryocytopoiesis.
Presence of human CFU-MK in NOD/SCID hematopoietic organs
The process of megakaryocytopoiesis is divided into 3 developmental stages: progenitors, immature cells, and mature MKs. The committed MK progenitor cell is the CFU-MK. In an initial experiment, mice were randomized to receive TPO, SCF, and IL-3 (3C group, n = 5) or placebo (PBS group, n = 2) for the duration of the experiment. At week 8 after transplantation, CFU-MKs were present only in the PBS group and were localized predominantly in BM when compared with the spleen or PB. There were no CFU-MKs in any of the 5 mice that received intraperitoneal cytokines. Based on these findings, in further experiments we assessed the presence of CFU-MK only in the murine BM.
A series of 6 experiments was performed to determine the effect of exogenous administration of various growth factors on human CFU-MK. In the PBS group, 100% (7 of 7 mice), 36% (4 of 11 mice), and 70% (7 of 10 mice) had human CFU-MK in the BM at weeks 2, 4, and 8, respectively. There was a linear relationship between the degree of engraftment and the number of CFU-MK colonies; at later time points, CFU-MKs were present only in mice with higher percentages of engraftment (Figure5A). In the 6C group, 60% (3 of 5 mice) had human CFU-MK at week 2. Despite levels of human engraftment as high as 5% and 25% at weeks 4 and 8, respectively, no CFU-MKs were detected (Figure 5B). In addition to CFU-MK, myeloid colonies that did not stain with antihuman GPIIb-IIIa were detected (Figure6A). Large unifocal aggregates of small colonies (3-10 cells) (Figure 6B) or intermediate-size colonies (11-40 cells) mainly were present (Figure 6C) and, rarely, large (> 41 cells) (Figure 6D) or mixed colonies were seen. When cells from untransplanted mice were plated, no colonies formed. The presence of human CFU-MK in the BM indicates that functional HSCs involved in human megakaryocytopoiesis are present in chimeric mice. Exogenous human growth factors had a detrimental effect on CFU-MK at week 4 to 8 after transplantation, consistent with loss of human HSCs.
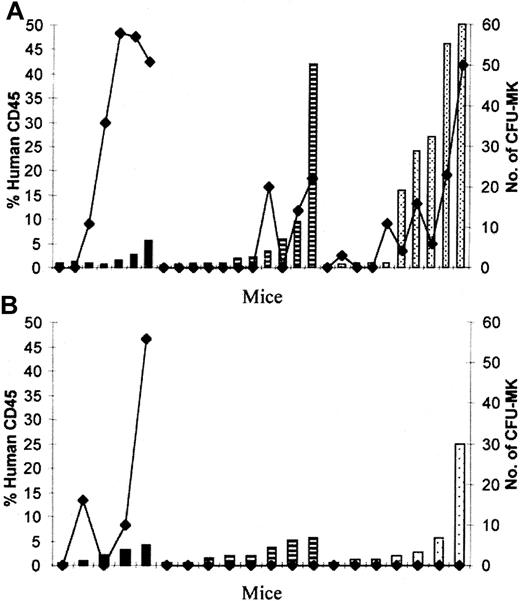
Demonstration of human CFU-MK in NOD/SCID hematopoietic organs.
Direct correlation between the percentage of human CD45+cells (columns) and the number of CFU-MK colonies (lines) generated from 2 × 106 BM cells from mice transplanted with 0.6 × 106 CD34+ PBSCs and analyzed 2 (black columns), 4 (striped columns), and 8 (dotted columns) weeks after transplantation. (A) PBS group. (B) 6C group.
Demonstration of human CFU-MK in NOD/SCID hematopoietic organs.
Direct correlation between the percentage of human CD45+cells (columns) and the number of CFU-MK colonies (lines) generated from 2 × 106 BM cells from mice transplanted with 0.6 × 106 CD34+ PBSCs and analyzed 2 (black columns), 4 (striped columns), and 8 (dotted columns) weeks after transplantation. (A) PBS group. (B) 6C group.
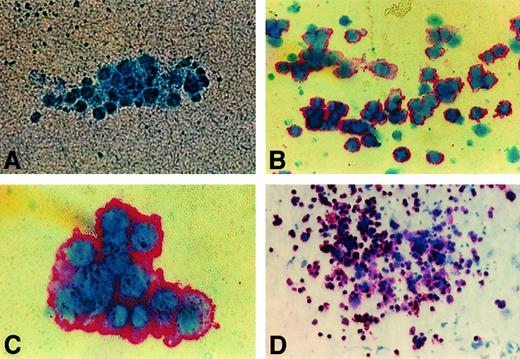
Immunocytochemistry stain of human GPIIb-IIIa (CD41a) on colonies from NOD/SCID BM.
Representative images of day 10 cultures of human colonies produced by BM cells from NOD/SCID mice transplanted with 0.6 × 106CD34+ PBSCs and killed 4 weeks after transplantation. (A) Negative colony (× 50). (B) Unifocal aggregate of small human CFU-MK (× 50). (C) Intermediate human CFU-MK (× 100). (D) Large human CFU-MK (× 10).
Immunocytochemistry stain of human GPIIb-IIIa (CD41a) on colonies from NOD/SCID BM.
Representative images of day 10 cultures of human colonies produced by BM cells from NOD/SCID mice transplanted with 0.6 × 106CD34+ PBSCs and killed 4 weeks after transplantation. (A) Negative colony (× 50). (B) Unifocal aggregate of small human CFU-MK (× 50). (C) Intermediate human CFU-MK (× 100). (D) Large human CFU-MK (× 10).
Human platelet production in NOD/SCID mice and correlation with human engraftment
Mice were bled weekly to monitor murine cell PB counts and human platelet production. Radiation caused severe pancytopenia with gradual recovery in the following 2 to 8 weeks. Murine platelet counts decreased from a mean of 1200 × 109/L to a mean of 250 × 109/L at week 2 and then gradually increased to 400 × 109/L at week 3, 700 × 109/L at week 4, to a normal count of 1089 × 109/L by week 8.
As shown in Figure 7, human platelets were detected by staining PB with both CD41a and CD42b monoclonal antibodies against human platelet surface GPIIb/IIIa and GPIb, respectively. In each experiment, human PB and untransplanted mouse PB were stained as positive and negative controls, respectively. A mean of 1.5% human platelets was detected at weeks 1 to 2 after transplantation. At week 3, human platelets peaked, reaching values as high as 14% in some mice (mean 6.5%). At week 4, human platelets decreased to a mean of 1% and further fell to a mean of 0.5% at week 8 after transplantation. The average absolute number of circulating human platelets at week 1 to 2 was 2.5 × 109/L, at week 3 was 20 × 109/L, and this fell by weeks 4 (7 × 109/L) and 8 (10 × 109/L) after transplantation.
Flow cytometric analysis of human platelets in PB of NOD/SCID mice.
PB from a representative transplanted mouse (A), human (B), and untransplanted mouse (C) was labeled with monoclonal antibodies against human CD41a, human CD42b, and mouse CD61. Mouse immunoglobulin G1 was used as an isotype control (top plots) for antihuman antibodies. Mouse platelets are found in the right lower quadrant of each blot. Human platelets are present in the upper left quadrant. Red cells and debris appear in the lower left quadrant.
Flow cytometric analysis of human platelets in PB of NOD/SCID mice.
PB from a representative transplanted mouse (A), human (B), and untransplanted mouse (C) was labeled with monoclonal antibodies against human CD41a, human CD42b, and mouse CD61. Mouse immunoglobulin G1 was used as an isotype control (top plots) for antihuman antibodies. Mouse platelets are found in the right lower quadrant of each blot. Human platelets are present in the upper left quadrant. Red cells and debris appear in the lower left quadrant.
In a series of 6 experiments, 36 (PBS group) and 29 (6C group) mice were bled between weeks 1 to 3. Most mice in both the PBS (72%) and 6C (82%) groups produced human platelets at these time points. There was a correlation between the extent of human engraftment in the BM and platelet production (Table 1). At week 4, 75% to 80% of the engrafted mice produced platelets in both groups (Table 1). However, the 2 groups differed at week 8, when 75% and 16.6% of engrafted mice produced platelets in the PBS and 6C groups, respectively. Human platelets were not present in mice that did not engraft at week 4 or 8.
Functional assessment of human platelets
To determine whether human platelets that develop in the mice are functional, platelet activation in response to in vitro activation with thrombin was tested. Thrombin induces platelet granule secretion, resulting in CD62P (P-selectin) expression on the platelets surface membrane.32,33 Using an antihuman CD62P monoclonal antibody, expression of CD62P on CD41a+ human platelets was increased after thrombin stimulation (Figure8). This suggests that this xenogeneic model of human megakaryocytopoiesis can be used to study the in vivo effects of drugs or other stressors on human MK and platelet development.
Thrombin activation of human platelets produced by engrafted NOD/SCID mice.
Shown are dot blots of human platelets from transplanted mice before and after thrombin stimulation. On the x-axis is FITC–anti-CD41a, which is present on all human platelets and not on murine platelets. The y-axis is PE–anti-CD62, which is expressed only on activated platelets.
Thrombin activation of human platelets produced by engrafted NOD/SCID mice.
Shown are dot blots of human platelets from transplanted mice before and after thrombin stimulation. On the x-axis is FITC–anti-CD41a, which is present on all human platelets and not on murine platelets. The y-axis is PE–anti-CD62, which is expressed only on activated platelets.
Ex vivo expansion of CD34+ PBSCs: in vivo human platelet production and loss of NOD/SCID repopulating cells
To determine whether direct administration of higher numbers of committed platelet progenitors (CFU-MK, CD34+/CD41+, and CD34−/CD41+ cells) in the graft improves human platelet development in this xenogeneic transplant model, ex vivo expanded cells were tested for their megakaryocytic engraftment capability. MK precursors were generated from CD34+ PBSCs in serum-free medium supplemented with human IL-3, IL-6, IL-11, Fl3, TPO, and SCF. The cell number increased 30-fold, and the number of CFU-MKs increased 27-fold by day 8. The percentage of CD34+/CD41+ cells increased from less than 2% to 13% by day 4. On day 8, however, only 0.5% of the total cells expressed CD34, and 17% of the cells were CD34−CD41+ (Figure9A). MKs were observed, and 4% of cells had a DNA content above 2N on day 7, consistent with the presence of MK.
Ex vivo expansion of human CD34+ PBSCs cultured in serum-free media in the presence of TPO, SCF, IL-3, IL-6, IL-11, and Fl3 and platelet production in NOD/SCID mice.
Human CD34+ PBSCs were transplanted before (day 0) and after 1, 3, 4, and 8 days of in vitro stimulation with TPO, SCF, IL-3, IL-6, IL-11, and Fl3. Mice were serially bled at weeks 2, 3, and 4 after transplantation. The transplant was performed without exogenous cytokines. BM and spleen cells were analyzed for human engraftment at week 4. (A) Uncultured (day 0) or cultured (days 3-8) human CD34+ PBSCs were analyzed using PE–anti-CD34 and FITC–anti-CD41. Isotype controls for each time point are shown in the upper panel. CD34/CD41 phenotypes are shown in the lower panel. The percentage of each subpopulation is indicated. (B) Human platelet production in 4 mice transplanted with human CD34+ PBSCs prior to expansion (day 0). Evidence of human engraftment at week 4 was only detected in mice 1 and 3. (C) Human platelet production in mice that received human CD34+ PBSCs that had been expanded for 1 (black), 3 (dotted), 4 (striped), or 8 (clear) days. Each column represents an individual mouse. No human platelets were detected at weeks 2 or 4.
Ex vivo expansion of human CD34+ PBSCs cultured in serum-free media in the presence of TPO, SCF, IL-3, IL-6, IL-11, and Fl3 and platelet production in NOD/SCID mice.
Human CD34+ PBSCs were transplanted before (day 0) and after 1, 3, 4, and 8 days of in vitro stimulation with TPO, SCF, IL-3, IL-6, IL-11, and Fl3. Mice were serially bled at weeks 2, 3, and 4 after transplantation. The transplant was performed without exogenous cytokines. BM and spleen cells were analyzed for human engraftment at week 4. (A) Uncultured (day 0) or cultured (days 3-8) human CD34+ PBSCs were analyzed using PE–anti-CD34 and FITC–anti-CD41. Isotype controls for each time point are shown in the upper panel. CD34/CD41 phenotypes are shown in the lower panel. The percentage of each subpopulation is indicated. (B) Human platelet production in 4 mice transplanted with human CD34+ PBSCs prior to expansion (day 0). Evidence of human engraftment at week 4 was only detected in mice 1 and 3. (C) Human platelet production in mice that received human CD34+ PBSCs that had been expanded for 1 (black), 3 (dotted), 4 (striped), or 8 (clear) days. Each column represents an individual mouse. No human platelets were detected at weeks 2 or 4.
In contrast to CD34+ cells transplanted with no ex vivo expansion (Figure 9B), mice transplanted with all of the cells derived from 0.6 × 106 starting cells that had been ex vivo expanded for 1 day (n = 3), 3 days (n = 3), or 4 days (n = 3) had circulating human platelets only at week 3 (Figure 9C). These expanded populations contained an average of 1.5 × 104, 1.5 × 105, and 1 × 106CD34+CD41+ cells on day 1, day 3, and day 4 of expansion, respectively. Human platelets were not identified in mice transplanted with cells expanded for 8 days (n = 3; Figure 9C). Mice transplanted with ex vivo expanded cells had no evidence of human engraftment at week 4. Therefore, despite the many-fold increase of CFU-MK, CD34+/CD41+, and CD34−/CD41+ cells achieved with ex vivo expansion, these cells allowed only delayed and transient human platelet production in NOD/SCID mice. The number of circulating human platelets at week 3 was 10-fold greater from control unexpanded cells than from the ex vivo expanded cells. These findings suggest that phenotypically mature MKs generated in vitro provide only transient platelet engraftment in vivo and do not engraft, suggesting the loss of NOD/SCID repopulating cells.
Discussion
In vivo repopulating assays are an excellent means to investigate human hematopoiesis and to characterize primitive cell populations.34 The study of human stem cell engraftment,35 growth patterns of normal36and neoplastic human hematopoietic cells,37-40 ex vivo expansion,41-43 mobilization,44 and interactions with stroma45 as well as testing gene transfer to human HSCs46 47 have been made possible using these animal models. We provide evidence that NOD/SCID mice provide a microenvironment that supports proliferation and differentiation of megakaryocytic progenitors from human CD34+ PBSCs, as evidenced by the presence of functional MK progenitors (CFU-MK) and their progeny (CD41+ cells). We also demonstrate that terminal differentiation into human platelets is supported, providing for the first time an in vivo system for the study of functional human platelets from adult stem cells. The ability to study MK engraftment kinetics and platelet production in vivo may be a useful model for preclinical testing of new approaches to the use of PBSCs for hematopoietic reconstitution in autologous and allogeneic transplantation after myeloablative chemoradiation therapy.
The in vivo megakaryocytopoietic (CFU-MK) potential of human CD34+ PBSCs correlates with the degree of human engraftment in NOD/SCID mice. These cells produce human CFU-MK throughout the engraftment period (2-8 weeks) when exogenous growth factors are not administered. It is likely that primitive human precursors continuously maintain MK and platelet production. In support of this assessment, more mature MK progenitors are not capable of maintaining MK or platelet production, as shown by the lack of engraftment following administration of ex vivo expanded cells that contained 27-fold more CFU-MK than control unexpanded cells.
Human cell engraftment occurred in the absence of exogenous human cytokines as previously described for PBSCs and cord blood stem cells transplanted into NOD/SCID44,48,49 and SCID mice.50,51 Either the murine microenvironment is satisfactory to support human cell differentiation due to cross-species activity of murine cytokines or the injected human cells produce the necessary human cytokines, as suggested previously.50Engraftment of human CD34+ PBSCs transplanted into NOD/SCID mice is decreased if exogenous cytokines are administered for more than 4 weeks. Most of the cells regenerated in NOD/SCID mice were of the B lineage, as previously reported with cord blood,49,52BM,20 and PBSC21,53 54 transplanted mice; this lymphoid development was more pronounced when the 6-cytokine cocktail was administered. Similarly, in megakaryocytopoiesis, administration of the human cytokine cocktails used herein negatively affected the presence of CFU-MK (week 4) and subsequent in vivo platelet production (week 8). This sequence of events suggests that human growth factors may induce MK stem cell differentiation with loss of self-renewal capacity and that late committed progenitors are responsible for the residual platelet production at week 4.
Human CD34+ PBSCs infused into NOD/SCID mice initially colonize the spleen in a fashion similar to that observed in clinical transplantation.55 Subsequently, cells in the BM initiate human hematopoiesis, indicating that they can respond to signals generated in the murine microenvironment. Murine BM is the primary site of human hematopoiesis because it contains the highest percentage of human cells and in vitro clonable progenitors.44,48,49This is consistent with syngeneic murine transplants in which injected cells that home to the spleen are not capable of long-term reconstitution while those that home to the BM are.56
Engraftment of human CD34+ PBSCs into immunocompromised mice requires a minimum dose of 0.6 × 106cells/mouse.57,50 In our experiments, the yield of human cells produced did not increase with higher cell doses (up to 1.2 × 106/mouse), consistent with previous findings.53 However, much higher doses, as studied by others44 (up to 50 × 106cells/mouse) were not used in our experiments.
We examined the possibility of accelerating human platelet development in NOD/SCID mice by increasing the number of CFU-MK, CD34+/CD41+, and CD41+ cells in the hematopoietic graft. Clonogenic MK precursors were generated ex vivo from CD34+ PBSCs in serum-free medium58 to provide a defined medium and to guarantee the absence of MK inhibitors such as platelet factor 4 and transforming growth factor, which can be present in serum.59,60 We used a combination of human TPO, SCF, IL-3, IL-6, IL-11, and Fl3, which yielded the highest CFU-MK and CD34+/CD41+ cell expansion when compared with other combinations used in our laboratory (data not shown) and those previously published.7,16,61,62 This lack of engraftment by expanded MK progenitors confirms that the MK and platelet development obtained in the xenogeneic mice for up to 8 weeks is derived from earlier precursor cells, perhaps even pluripotent stem cells, present in the CD34+ PBSC population injected. The NOD/SCID repopulating capacity of ex vivo stimulated human cells is impaired when multiple cytokine cocktails and stem cell sources are used.42,63,45 53
We present a definitive method for assessing the precise number of human platelets in the mice. In our study, we prove that we have a platelet gate, separate out human and murine platelets for assessment, and use 2 different antibodies directed against human platelets as confirmation of platelet identity. It has been reported previously that human platelets are present in the PB of murine recipients of cord blood.64 Here we show that the megakaryocytic potential and the ability to differentiate into platelets in mice are retained in adult CD34+ cells, and we characterize the kinetics of this development. Importantly, these human platelets retain their functional abilities with regard to thrombin activation. This represents the introduction of a new model system in which the effects in vivo of various drugs and other manipulations can be tested on human megakaryocytopoiesis and platelet function.
Based on the data presented herein, we propose that the NOD/SCID mouse can be used as a preclinical model for studies on MK and platelet development. For example, this in vivo model is the first that could be used to study the effect of drugs and their metabolites on human megakaryocytopoiesis and platelet function.
We thank Drs Joel Rappeport and Nancy Berliner for critically reviewing the manuscript; Drs Barbara Degar, Joe Sinning, and Arati Ghana-Gupta for assistance in Southern blot analysis; Dr John Basile for assistance in statistical analysis; and Dr Zhinnan Jin for assistance in FACS analysis.
Supported by a Leukemia and Lymphoma Society Translational Research Award, NIH grant HL 61223, and NIH grant T32-HL 07262.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diane S. Krause, Yale University School of Medicine, Department of Laboratory Medicine, 333 Cedar St, PO Box 208035, New Haven, CT 06510; e-mail: diane.krause@yale.edu.













 ), 4 (
), 4 ( ), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.
), and 8 (▪) weeks after transplantation. (B) Detection of human engraftment in the BM and spleen of NOD/SCID mice (n = 10) by Southern blot analysis at week 8 after transplantation. Each lane (1-10) represents a single mouse. Human:mouse DNA controls are given as percent human DNA.