Abstract
Cytokine-mobilized peripheral blood hematopoietic stem cells (MPB HSC) are widely used for transplantation in the treatment of malignancies, but the mechanism of HSC mobilization is unclear. Although many HSC in bone marrow (BM) cycle rapidly and expand their numbers in response to cytoreductive agents, such as cyclophosphamide (CY), and cytokines, such as granulocyte colony-stimulating factor (G-CSF), MPB HSC are almost all in the G0 or G1phase of the cell cycle. This has raised the question of whether a subset of noncycling BM HSC is selectively released, or whether cycling BM HSC are mobilized after M phase, but before the next S phase of the cell cycle. To distinguish between these possibilities, mice were treated with one dose of CY followed by daily doses of G-CSF, and dividing cells were marked by administration of bromodeoxyuridine (BrdU) during the interval that BM HSC are expanding. After CY and 4 days of G-CSF, 98.5% of the 2n DNA content long-term repopulating MPB (LT)-HSC stained positively for BrdU, and therefore derived from cells that divided during the treatment interval. Next, LT-HSC from mice previously treated with a single dose of CY, which kills cycling cells, and 3 daily doses of G-CSF, were nearly all killed by a second dose of CY, suggesting that CY/G-CSF causes virtually all LT-HSC to cycle. Analysis of cyclin D2 messenger RNA (mRNA) expression and total RNA content of MPB HSC suggests that these cells are mostly in G1 phase. After CY/G-CSF treatment, virtually all BM LT-HSC enter the cell cycle; some of these HSC then migrate into the blood, specifically after M phase, and are rapidly recruited to particular hematopoietic organs.
Introduction
In adult mammals, hematopoietic progenitors and hematopoietic stem cells (HSC) migrate from bone marrow (BM) to the periphery in response to several stimuli, including cytoreductive drugs, such as cyclophosphamide (CY),1,2 and cytokines, such as granulocyte colony-stimulating factor (G-CSF).3-5G-CSF is widely used clinically to mobilize progenitors and HSC for collection and transplantation, often in combination with CY, which augments its effect.1 2 Despite common use, the mechanisms of cytokine mobilization of HSC are poorly understood.
Combined administration of CY and G-CSF is associated with expansion of the HSC pool.6 Following a single dose of CY and subsequent daily doses of G-CSF to mice, a large fraction of HSC in the BM entered the cell cycle, leading to a more than 12-fold expansion in long-term (LT) repopulating HSC in the BM after CY and 2 days of G-CSF treatment. On the day after the dramatic 3-day expansion, the number of BM LT-HSC began to decline, concomitant with a precipitous increase in the number of LT-HSC in the blood and spleen. The rapid expansion of LT-HSC in BM following administration of CY and G-CSF suggested that most of these cells had entered the cell cycle.6 The question of whether all LT-HSC entered the cell cycle after CY/G-CSF, however, or whether a subset of quiescent HSC escaped stimulation, was not addressed.
Intriguingly, despite the fact that HSC in the BM were cycling rapidly at the time of mobilization, HSC released into the blood after treatment with CY/G-CSF were either in the G0 or G1 phase of the cell cycle.6 In addition, hematopoietic progenitor and stem cells that appear in the blood following treatment of mice or humans with various mobilizing cytoreductive drugs or cytokines are in either the G0 or G1 phase.7-14 At least 3 hypotheses could explain the strong bias in favor of G0/G1 HSC in the blood7: (1) HSC may be released from the BM in all phases of the cell cycle, but S/G2/M-phase HSC might be preferentially cleared, leaving G0/G1 cells overrepresented in the blood; (2) alternatively, there could be a preferential release of noncycling (G0) HSC into the blood, leaving cycling cells behind in the BM; and (3) it is also possible that all HSC enter the cell cycle in response to CY/G-CSF, and that actively cycling cells migrate selectively to blood after M phase and prior to the next S phase, thus placing them in G0 (if they were to exit the cell cycle on mobilization) or in G1phase.
We have previously shown that nearly all of the reconstituting activity of mobilized HSC isolated from CY/G-CSF–treated C57BL/Ka-Thy-1.1 mice is contained in 2 phenotypically defined populations: Thy-1.1loSca-1+Lineage−(Lin−)c-Kit+Mac-1−cells with mostly long-term self-renewing multipotent progenitor activity (LT-HSC), and Thy-1.1loSca-1+Lin−c-Kit+Mac-1locells with mostly transiently self-renewing multipotent progenitor activity.6 In the present study, we focus primarily on LT-HSC. We provide evidence for hypothesis 3, that there is a selective migration of actively cycling postmitotic HSC from BM to blood during mobilization. In addition, we demonstrate directly that the transit time of mobilized peripheral blood (MPB) HSC in the blood is extremely brief, and that mobilized progenitor cells show enhanced migration to the spleen and liver, as opposed to BM, at early time points following transplantation.
Materials and methods
Mice
Six- to 12-week-old C57BLKa-Thy-1.1 mice were bred and maintained at the Stanford University Laboratory Animal Facility and allowed to freely imbibe acidified water (pH 2.5). β-Actin/enhanced green fluorescent protein (eGFP) transgenic mice were generated using the pCXEGFP vector, generously provided by Dr Jun-ichi Miyazaki. The construction of the pCXEGFP vector and its use in generating eGFP transgenic mice have been described previously.15 16 To generate eGFP transgenic mice, pCXEGFP was linearized withBamHI and SalI and the fragment containing the cytomegalovirus–immediate early (CMV-IE) enhancer, chicken β-actin promoter, eGFP complementary DNA (cDNA), and β-globin polyadenylation sequences was gel purified using the Qiaquick gel extraction kit (Qiagen, Valencia, CA). Purified, linearized DNA was injected into zygotes from crosses of F1 (C57BL/Ka-Thy1.1 × C3H) mice. Founder mice were screened by FACS analysis of peripheral blood leukocytes, and eGFP+ animals were back-crossed to C57BL/Ka-Thy1.1 mice. Mice used for stem/progenitor cell isolation had been back-crossed for at least 5 generations.
HSCmobilization
Treatment protocols.
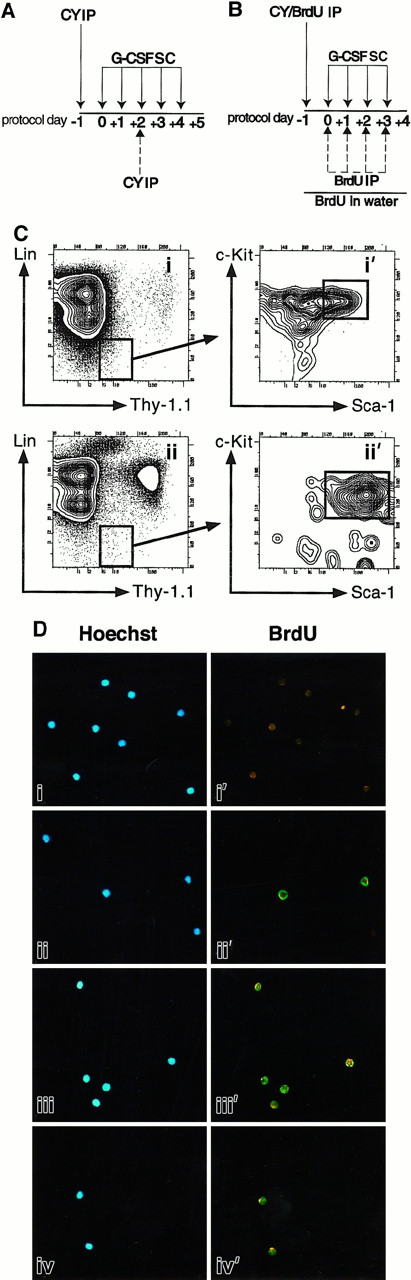
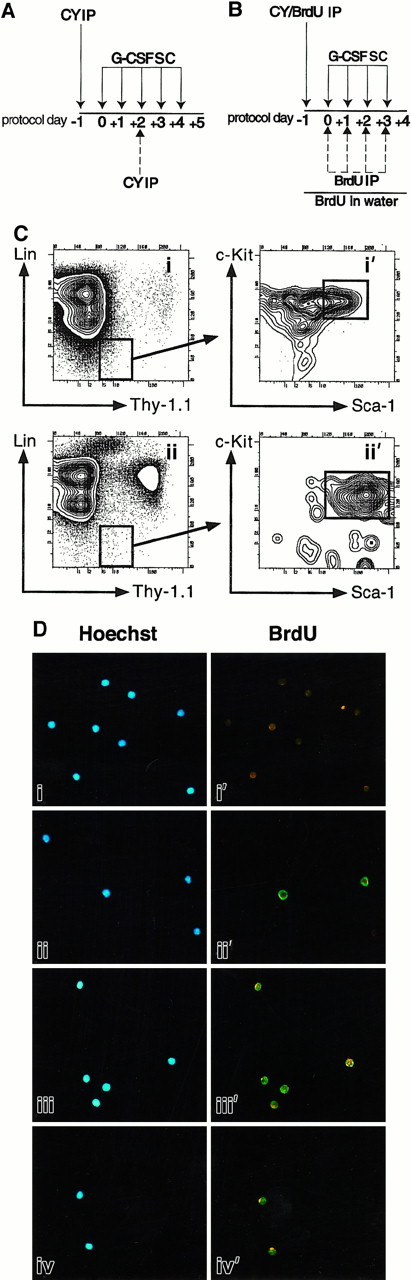
(A) CY/G-CSF dosing. Mice were injected intraperitoneally (IP) with a single dose of CY (4 mg) and injected subcutaneously (SC) on successive days with recombinant human G-CSF (5 μg) as shown. In one experiment, mice were also injected with a second dose of CY (4 mg) on day +2 (dashed arrow). (B) BrdU protocol. Mice were injected with CY and G-CSF as in panel A. In addition, they were given 4 mg BrdU as an intraperitoneal injection on day −1, and placed on BrdU-containing water for the duration of treatment. In one experiment (Table 1, experiment 1), mice also received daily intraperitoneal injections of BrdU on days 0, +1, +2, and +3 as shown (dashed arrows). These daily intraperitoneal injections were subsequently found not to increase BrdU labeling, and discontinued. (C) Flow cytometry plots of CY/G-CSF day +4 bone marrow (i, i′), and blood (ii, ii′) nucleated cells. Plots i and i′ and plots ii and ii′ are of the same BM and blood samples, respectively. Plots i′ and ii′ show the Sca-1 and c-Kit staining profiles of the Thy-1.1loLin− cells that are boxed in i and ii, respectively. Boxes represent FACS gates used to isolate LT-HSC. All plots show cells after gating out of dead cells. The units of all plots (both axes) are log10 fluorescence intensity. (D) Representative photomicrographs of control thymocytes and of LT-HSC from BrdU-treated mice. In each case, the left and right panels depict the same field, viewed either through a UV filter for visualization of Hoechst 33342 staining of all nuclei (blue staining), or a FITC/Texas Red filter for visualization of anti-BrdU antibodies (green staining), respectively. Thymocytes from untreated mice (i, i′), or BrdU-treated mice (ii, ii′), were double sorted and stained with anti-BrdU antibodies. Thy-1.1loSca-1+Lin−c-Kit+Mac-1−cells were isolated from BM (iii, iii′) and peripheral blood (iv, iv′) after treatment of mice with BrdU and CY/G-CSF (day +4) (original magnification × 125).
Treatment protocols.
(A) CY/G-CSF dosing. Mice were injected intraperitoneally (IP) with a single dose of CY (4 mg) and injected subcutaneously (SC) on successive days with recombinant human G-CSF (5 μg) as shown. In one experiment, mice were also injected with a second dose of CY (4 mg) on day +2 (dashed arrow). (B) BrdU protocol. Mice were injected with CY and G-CSF as in panel A. In addition, they were given 4 mg BrdU as an intraperitoneal injection on day −1, and placed on BrdU-containing water for the duration of treatment. In one experiment (Table 1, experiment 1), mice also received daily intraperitoneal injections of BrdU on days 0, +1, +2, and +3 as shown (dashed arrows). These daily intraperitoneal injections were subsequently found not to increase BrdU labeling, and discontinued. (C) Flow cytometry plots of CY/G-CSF day +4 bone marrow (i, i′), and blood (ii, ii′) nucleated cells. Plots i and i′ and plots ii and ii′ are of the same BM and blood samples, respectively. Plots i′ and ii′ show the Sca-1 and c-Kit staining profiles of the Thy-1.1loLin− cells that are boxed in i and ii, respectively. Boxes represent FACS gates used to isolate LT-HSC. All plots show cells after gating out of dead cells. The units of all plots (both axes) are log10 fluorescence intensity. (D) Representative photomicrographs of control thymocytes and of LT-HSC from BrdU-treated mice. In each case, the left and right panels depict the same field, viewed either through a UV filter for visualization of Hoechst 33342 staining of all nuclei (blue staining), or a FITC/Texas Red filter for visualization of anti-BrdU antibodies (green staining), respectively. Thymocytes from untreated mice (i, i′), or BrdU-treated mice (ii, ii′), were double sorted and stained with anti-BrdU antibodies. Thy-1.1loSca-1+Lin−c-Kit+Mac-1−cells were isolated from BM (iii, iii′) and peripheral blood (iv, iv′) after treatment of mice with BrdU and CY/G-CSF (day +4) (original magnification × 125).
Bromodeoxyuridinetreatment
Bromodeoxyuridine (BrdU) was administered as previously described17 (Figure 1B). Mice treated with CY/G-CSF and BrdU showed changes in HSC frequency, absolute numbers, and tissue distribution (data not shown) characteristic of treatment with CY/G-CSF alone.6 No alterations in peripheral blood counts or in HSC function, frequency, absolute numbers, tissue distribution, or cell cycle status were observed following treatment with BrdU alone.17
Tissue preparation and HSC isolation
Tissue processing.
Single-cell suspensions of BM and spleen cells were prepared as previously described.6 In a typical BrdU experiment, BM was pooled from 5 mice, and blood was pooled from the same 5 mice and from 5 to 15 additional mice.
Antibodies.
Monoclonal antibodies (mAbs) used in immunofluorescence staining were prepared from hybridomas and included 19XE5 (anti–Thy-1.1), 2B8 (anti–c-Kit), E13 (anti–Sca-1, Ly6A/E). Lineage marker Abs included KT31.1 (anti-CD3), GK1.5 (anti-CD4), 53-7.3 (anti-CD5), 53-6.7 (anti-CD8), Ter119 (antierythrocyte-specific antigen), 6B2 (anti-B220), 8C5 (anti–Gr-1), and M1/70 (anti–Mac-1). The mAbs BU-1 (anti-BrdU, Caltag, South San Francisco, CA) and fluorescein isothiocyanate (FITC)-conjugated antimouse polyclonal Ab (Caltag) were used in BrdU analyses. Biotinylated 3C11 (anti–c-Kit), was sometimes used for positive selection of HSC. 2B8 and 3C11 recognize nonoverlapping epitopes on c-Kit.
HSC isolation.
The HSC were isolated as described previously.18Sca-1+ or c-Kit+ cells were sometimes enriched by positive selection using MACS (Miltenyi Biotec, Sunnyvale, CA) streptavidin-conjugated magnetic beads according to the manufacturer's instructions. Figure 1C shows staining profiles and approximate sorting gates used for isolation of CY/G-CSF day +4 BM (i, i′) and blood (ii, ii′) LT-HSC.
Cellcycle analyses
Cell cycle analyses were performed as described.17Briefly, double-sorted HSC were stained with Hoechst 33342 (Molecular Probes, Eugene, OR) at 10 μg/mL for 45 minutes at 37°C in Hoechst medium.19 Pyronine Y (PY) was then added to 1.0 μg/mL, and the cells were incubated for an additional 15 minutes, prior to washing and analysis by flow cytometry.
Quantitation of BrdU incorporation by HSC
Cells were stained with BrdU and visualized by fluorescence microscopy as previously described.17 To obtain percentages of BrdU+ HSC, cell nuclei (stained blue with Hoechst 33342) were identified using the Hoechst filter, then BrdU+ (green) nuclei in the same field were counted using the FITC/Texas Red filter (Figure 1D). In each experiment, whole thymocytes sorted from mice not exposed to BrdU were used as negative controls and were always 100% negative (Figure 1D, i, i′, and data not shown). Thymocytes from mice exposed to BrdU were used to control for possible nonspecific staining. Consistent with expectations,20 a significant fraction of thymocytes sorted from mice treated with BrdU alone for 5 days were BrdU+ (ii, ii′, and data not shown). LT-HSC isolated from BM (iii, iii′) or blood (iv, iv′) of BrdU/CY/G-CSF–treated animals showed the characteristic BrdU punctate green nuclear staining.21 To rule out the possibility that residual staining from the FITC-conjugated anti–Thy-1.1 could yield false-positive staining, Thy-1.1lo BM cells were sorted directly onto a slide and observed to have no staining (data not shown).
Reverse transcriptase–polymerase chain reaction analysis of cyclin D2 expression
Nested reverse transcriptase–polymerase chain reaction (RT-PCR) on double-sorted LT-HSC (5 cells/sample) was performed as described.17 Primer sequences are as follows: sense primers, 5′AGA GAC CAT CCC GCT GAC TGC3′; 5′GAA AAG CTG TGC ATT TAC ACC3′. Antisense primers, 5′GCA GGC TGT TCA GCA GCA GAG3′; 5′GCT CAG TCA GGG CAT CAC ACG3′. RT primers, 5′TCC CGC ACG TCT GTA GGG GTG3′.
Isolation and PKH-26-labeling of murine erythrocytes
Red blood cells (RBC) from untreated C57BL/Ka-Thy1.1 mice were isolated by density centrifugation over a Ficoll cushion (Sigma, St Louis, MO). Purified RBC (108) were then labeled with the fluorochrome PKH-26, according to the manufacturer's recommendations (Sigma).
Hematopoietic progenitor short-term homing assays
Hematopoietic progenitor cells enriched for HSC (Lin−/loSca-1+c-kit+) were isolated from day +4 MPB of β-actin/eGFP transgenic mice. The eGFP+ progenitor cells (45 000) and 150 000 PKH-26–labeled erythrocytes were sorted and injected together into the retrorbital sinus of anethetized day +4 CY/G-CSF C57BL/Ka-Thy1.1 recipients. Samples of peripheral blood were collected from the tail vein at the indicated time points (from 30 seconds to 3 hours). After 3 hours, mice were killed and tissues and organs were collected, as indicated, for analysis. Quantitation of PKH-26+ RBC or eGFP+ progenitors in blood and tissue samples was performed by flow cytometry. Preliminary experiments demonstrated that samples from uninjected control mice did not generate signals in the PKH-26+ or eGFP+ gates.
Statistical analysis
The Student t test was used to compute the Pvalues shown in Figure 2B.
Second dose of CY.
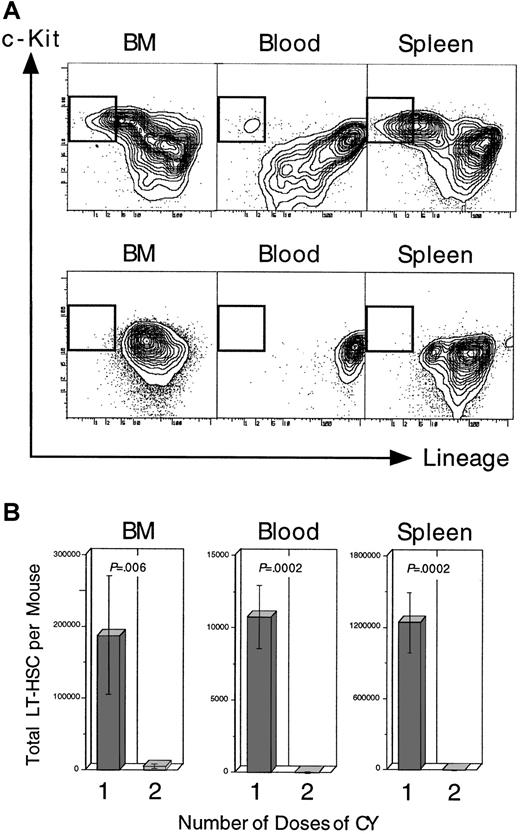
Administration of a second dose of CY, given on day +2 of the standard CY/G-CSF protocol (Figure 1A; top), resulted in the disappearance of nearly all phenotypically defined LT-HSC from the BM on day +4, and the failed appearance of LT-HSC in the blood and spleen at day +4. Panel A shows the fluorescence profile of Thy-1.1loSca-1+–gated BM, blood, or spleen cells for lineage and c-Kit staining. The gates for LT-HSC (Lin− c-Kit+) are boxed. Cells in panel A (top) were isolated from a representative control mouse receiving the standard CY/G-CSF regimen (Figure 1A; top). Cells in panel A (bottom) were from a representative mouse that also received a second dose of CY on day +2 of the protocol. Mice in both groups were killed on day +4. The units of all plots (both axes) are log10fluorescence intensity. Panel B shows the estimated number of LT-HSC per mouse for control animals, and for those that received the additional dose of CY on day +2 (control, n = 3 mice; 2nd dose CY, n = 4 mice). The number of LT-HSC was calculated by multiplying the obtained frequencies by the number of nucleated cells in each tissue, assuming that, mice BM recovered from the 4 long bones represents 15% of the total BM, and blood volume in milliliters is 10% of the animal's weight in grams.53
Second dose of CY.
Administration of a second dose of CY, given on day +2 of the standard CY/G-CSF protocol (Figure 1A; top), resulted in the disappearance of nearly all phenotypically defined LT-HSC from the BM on day +4, and the failed appearance of LT-HSC in the blood and spleen at day +4. Panel A shows the fluorescence profile of Thy-1.1loSca-1+–gated BM, blood, or spleen cells for lineage and c-Kit staining. The gates for LT-HSC (Lin− c-Kit+) are boxed. Cells in panel A (top) were isolated from a representative control mouse receiving the standard CY/G-CSF regimen (Figure 1A; top). Cells in panel A (bottom) were from a representative mouse that also received a second dose of CY on day +2 of the protocol. Mice in both groups were killed on day +4. The units of all plots (both axes) are log10fluorescence intensity. Panel B shows the estimated number of LT-HSC per mouse for control animals, and for those that received the additional dose of CY on day +2 (control, n = 3 mice; 2nd dose CY, n = 4 mice). The number of LT-HSC was calculated by multiplying the obtained frequencies by the number of nucleated cells in each tissue, assuming that, mice BM recovered from the 4 long bones represents 15% of the total BM, and blood volume in milliliters is 10% of the animal's weight in grams.53
Results
MPB HSC are derived from dividing precursors
To test whether G0/G1 HSC appearing in the blood following mobilization derive from noncycling (G0) BM LT-HSC that are selectively released into the blood, or are instead LT-HSC that after actively cycling in the BM are released into the blood after M phase of the cell cycle, mice were treated simultaneously with CY/G-CSF and with the thymidine analog BrdU, which is incorporated during DNA synthesis (Figure 1B,D). Virtually all (99.1 ± 0.5% SD) Thy-1.1loSca-1+Lin−c-Kit+Mac-1−cells isolated from the BM of day +4 CY/BrdU/G-CSF–treated mice stained positively for BrdU (Table 1). Thy-1.1loSca-1+Lin−c-Kit+Mac-1−cells from MPB of CY/BrdU/G-CSF–treated mice were 98.5% BrdU+ (Table 1, experiment 2), and Thy-1.1loSca-1+Lin−/loc-Kit+cells (this population includes the Thy-1.1loSca-1+c-Kit+Mac-1lotransiently self-renewing HSC) were 97.8% BrdU+ (Table 1, experiment 3). Thus, nearly all HSC isolated from MPB had divided at least once during BrdU exposure, even though only a small percentage of such cells are typically in the S, G2, or M phases of the cell cycle at the time of their isolation6 (data not shown). Given that the vast preponderance of HSC are in the BM until day +3, and these are dividing,6 the most likely explanation for the presence of BrdU+, G0/G1 phase HSC in the blood is that HSC divided in the BM and migrated to the blood prior to their next S phase.
If the MPB and splenic HSC are derived from dividing LT-HSC following CY/G-CSF, then giving these mice a second dose of CY on day +2 of the protocol should eliminate them, because CY preferentially kills dividing cells.22 As reported previously, the initial dose of CY on day −1 did not result in a reduction in the frequency or the absolute number of LT-HSC in the BM on day 0.6 However, as shown in Figure 2, BM, blood, and spleens from mice given the second dose of CY on day +2 contained only a small fraction of LT-HSC on day +4 compared to control mice given the standard regimen. These data suggested that at day +2 of the CY/G-CSF regimen, nearly all of the LT-HSC that could give rise to MPB HSC (or BM or splenic HSC) were cycling.
LT-HSC isolated from the blood at CY/G-CSF day +4 express cyclin D2 messenger RNA
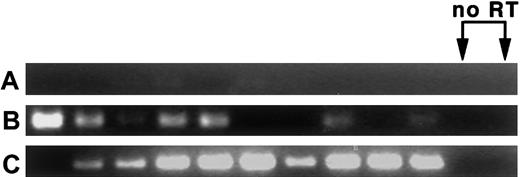
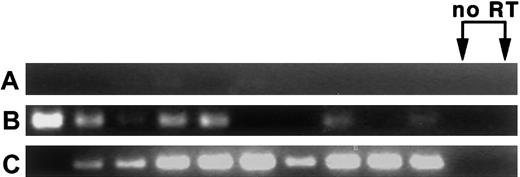
MPB LT-HSC are found primarily in the G0/G1 fraction of the cell cycle,7-14 and appear to migrate into the bloodstream following completion of M phase. To determine whether MPB HSC remain in cycle in the bloodstream, or if entry into the bloodstream is accompanied by exit from the cell cycle (ie, entry into G0phase), the expression of messenger RNA (mRNA) for cyclin D2, a marker for cycling cells,23 was assayed. In 2 experiments, nested RT-PCR performed on 5 cells per sample revealed that most CY/G-CSF day +4 BM and blood LT-HSC express cyclin D2 mRNA. Data from one of the experiments is shown in Figure 3. In this experiment, 7 of 10 BM and 9 of 10 blood LT-HSC samples were cyclin D2+. Little cyclin D2 mRNA from control splenic T cells was detected. In the second experiment (not shown), 12 of 12 BM and 12 of 12 blood samples were positive. Because cyclin D2 mRNA is rapidly degraded following exit from the cell cycle,24 25 these results are consistent with the notion that HSC isolated from blood are in G1 phase.
LT-HSC from BM and blood of CY/G-CSF–treated animals express cyclin D2 mRNA.
(A) Splenic T cells, (B) CY/G-CSF day +4 LT-HSC from BM, and (C) CY/G-CSF day +4 MPB LT-HSC. Cells were double sorted into lysis buffer in 96-well plates at 5 cells/well, followed by RT and nested PCR for cyclin D2 (35 cycles per primer pair; see “Materials and methods”). PCR products of the predicted length (331 bp) were obtained. Reverse transcriptase (RT) was omitted from samples in lanes 11 and 12.
LT-HSC from BM and blood of CY/G-CSF–treated animals express cyclin D2 mRNA.
(A) Splenic T cells, (B) CY/G-CSF day +4 LT-HSC from BM, and (C) CY/G-CSF day +4 MPB LT-HSC. Cells were double sorted into lysis buffer in 96-well plates at 5 cells/well, followed by RT and nested PCR for cyclin D2 (35 cycles per primer pair; see “Materials and methods”). PCR products of the predicted length (331 bp) were obtained. Reverse transcriptase (RT) was omitted from samples in lanes 11 and 12.
HSC recovered from the BM at CY/G-CSF day +3, or from the blood or spleen at CY/G-CSF day +4, have elevated levels of total RNA compared to HSC isolated from BM of untreated animals
To characterize further the cell cycle status of HSC appearing in the periphery following treatment with CY/G-CSF, double-sorted HSC were stained with Hoechst 33342 and PY (see “Materials and methods”), allowing simultaneous assessment of the fraction of cells with more than 2n DNA content (Hoechst 33342), as well as relative levels of total double-stranded RNA (PY26). As cells progress from G0 to G1, and from G1 to S, G2, and M phases, they accumulate RNA, mostly in the form of double-stranded ribosomal RNA,26 and so it is reasonable to propose that PY high cells have elevated protein synthesis. As shown in Figure 4 and Table2, total RNA levels of G0/G1 and S/G2/M HSC isolated from CY/G-CSF–treated animals (day +3 to day +5) were always higher than RNA levels of corresponding populations from untreated mice in all tissues examined. In 3 experiments, LT-HSC from blood of CY/G-CSF day +4 and +5 animals had 50%, 13%, and 24% (mean = 29% ± 19% SD) higher PY staining than HSC from BM of untreated mice (Table 2), suggesting that MPB LT-HSC are in a more activated state than LT-HSC from BM of untreated mice.
RNA levels of MPB HSC.
Mobilized G0/G1 and S/G2/M HSC isolated from BM or blood of day +5 CY/G-CSF–treated mice have higher average levels of total RNA than the corresponding populations from BM of untreated mice. LT-HSC were double sorted and then stained with the RNA-staining dye PY and the DNA-staining dye Hoechst 33342 as described in “Materials and methods.” The horizontal line passing through all plots is a reference line approximating the G0/G1 boundary of HSC from BM of untreated mice. Vertical lines separate G0/G1 from S/G2/M HSC. The numbers in the corners of the plots indicate the percentage of HSC in each quadrant. Heavy bars indicate the mean fluorescence intensity (MFI) in the phycoerythrin (PE) channel, on which emitted fluorescence from PY was recorded (PE MFI data from all experiments are normalized and presented in Table 2). Note the small fraction (2.3%) of MPB HSC with more than 2n DNA content. The representative data presented are from experiment 1 of Table 2. PY indicates pyronine Y; CV, coefficient of variation (of the indicated means).
RNA levels of MPB HSC.
Mobilized G0/G1 and S/G2/M HSC isolated from BM or blood of day +5 CY/G-CSF–treated mice have higher average levels of total RNA than the corresponding populations from BM of untreated mice. LT-HSC were double sorted and then stained with the RNA-staining dye PY and the DNA-staining dye Hoechst 33342 as described in “Materials and methods.” The horizontal line passing through all plots is a reference line approximating the G0/G1 boundary of HSC from BM of untreated mice. Vertical lines separate G0/G1 from S/G2/M HSC. The numbers in the corners of the plots indicate the percentage of HSC in each quadrant. Heavy bars indicate the mean fluorescence intensity (MFI) in the phycoerythrin (PE) channel, on which emitted fluorescence from PY was recorded (PE MFI data from all experiments are normalized and presented in Table 2). Note the small fraction (2.3%) of MPB HSC with more than 2n DNA content. The representative data presented are from experiment 1 of Table 2. PY indicates pyronine Y; CV, coefficient of variation (of the indicated means).
Mobilized stem and progenitor cells exhibit extremely short transit times in the blood and migrate to specific hematopoietic organs
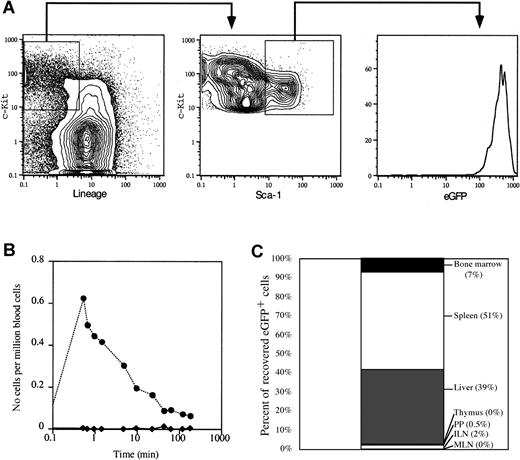
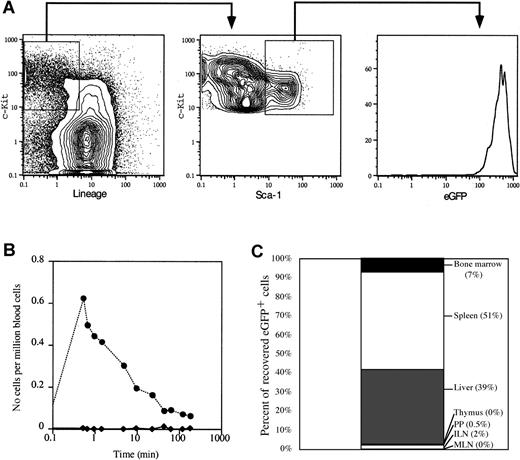
If HSC are selectively mobilized following M phase, but are found in the blood primarily in G1 phase, then transit of MPB HSC in the blood must be relatively brief (ie, shorter than progression from G1 to S phase). To test directly the half-life of MPB HSC and progenitor cells in the bloodstream, we purified Lin−/lockit+Sca-1+ hematopoietic progenitor cells from MPB of mice transgenically expressing eGFP driven by the β-actin promoter. These mice express high levels of eGFP in all cells,16 including HSC (Figure5A), a fact that can be exploited to track the in vivo migration of transplanted eGFP+ cells in a wild-type host. FACS-sorted MPB eGFP+ hematopoietic progenitors from day +4 CY/G-CSF–treated animals were injected intravenously into wild-type recipients similarly treated with CY/G-CSF. To control for the injection, normal erythrocytes, fluorescently tagged with the red fluorochrome PKH-26 were coinjected with the eGFP+ cells. Whereas PKH-26+erythrocytes were easily detectable in the blood of the recipient at the early time points (< 1 minute), eGFP+ progenitor cells were not detected (Figure 5B). In fact, although PKH-26+RBC were detectable in the bloodstream throughout the experiment, eGFP+ progenitors cells were never observed in the blood. Importantly, eGFP+ progenitor cells were easily identified in the spleen, liver, and BM when recipient mice were killed 3 hours after intravenous injection (Figure 5C). In addition, the migration pattern of transplanted MPB HSC was consistent with previous studies showing that mobilization initiates migration of BM HSC to the spleen through the blood.6 The eGFP+ stem and progenitor cells were found primarily in the liver and spleen, and only a small fraction of cells migrated to BM. The eGFP+ cells were not detected in lymph nodes, Peyer patches, or thymus. Taken together, these data demonstrate that the transit time of mobilized stem and progenitor cells in the blood is very brief and suggest that migration of HSC into spleen and liver may contribute to the rapid clearance of HSC from the circulation.
Mobilized hematopoietic progenitor cells are rapidly cleared from the bloodstream.
(A) Representative FACS plots showing the gates used to isolate eGFP+ progenitors (Lin−/loc-kit+Sca-1+) from day +4 CY/G-CSF–treated eGFP transgenic mice. The sorted populations are boxed. The right panel shows the high level of expression of eGFP in sorted progenitor cells. Data are expressed as contour or histogram plots representing fluorescence intensity for the indicated marker. (B) Clearance from the blood of eGFP+ progenitors (♦) and PKH-26+ RBC (●). Day +4 CY/G-CSF–treated wild-type mice were anesthetized and injected intravenously with sorted eGFP+ progenitor cells and PKH-26–labeled erythrocytes, as described in “Materials and methods.” Samples of peripheral blood were obtained from the recipient mouse at the indicated time points. The frequency of eGFP+ or PKH-26+ at each time point was determined by flow cytometry. Data are expressed as the number of eGFP+ or PKH-26+ cells per 106 total nucleated blood cells. (C) In vivo homing of MPB stem and progenitor cells. eGFP+ progenitor cells were infused into anesthetized day +4 CY/G-CSF–treated wild-type recipients, as in panel B. After 3 hours, animals were killed and the number of eGFP+ cells in each organ was determined by flow cytometry. Data are presented as the percent of total eGFP+ cells recovered from each organ, as indicated. ILN indicates inguinal lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer patches.
Mobilized hematopoietic progenitor cells are rapidly cleared from the bloodstream.
(A) Representative FACS plots showing the gates used to isolate eGFP+ progenitors (Lin−/loc-kit+Sca-1+) from day +4 CY/G-CSF–treated eGFP transgenic mice. The sorted populations are boxed. The right panel shows the high level of expression of eGFP in sorted progenitor cells. Data are expressed as contour or histogram plots representing fluorescence intensity for the indicated marker. (B) Clearance from the blood of eGFP+ progenitors (♦) and PKH-26+ RBC (●). Day +4 CY/G-CSF–treated wild-type mice were anesthetized and injected intravenously with sorted eGFP+ progenitor cells and PKH-26–labeled erythrocytes, as described in “Materials and methods.” Samples of peripheral blood were obtained from the recipient mouse at the indicated time points. The frequency of eGFP+ or PKH-26+ at each time point was determined by flow cytometry. Data are expressed as the number of eGFP+ or PKH-26+ cells per 106 total nucleated blood cells. (C) In vivo homing of MPB stem and progenitor cells. eGFP+ progenitor cells were infused into anesthetized day +4 CY/G-CSF–treated wild-type recipients, as in panel B. After 3 hours, animals were killed and the number of eGFP+ cells in each organ was determined by flow cytometry. Data are presented as the percent of total eGFP+ cells recovered from each organ, as indicated. ILN indicates inguinal lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer patches.
Discussion
We examined the relationship between LT-HSC expansion in the BM and mobilization from BM to blood. By administering BrdU to mice undergoing a mobilizing regimen of CY/G-CSF and then isolating BM and MPB LT-HSC, the proliferation history of LT-HSC in both compartments was determined. As previously reported, in otherwise untreated mice placed on continuous oral BrdU for 5 days, about 40% of Thy-1.1lo Sca-1+ Lin−c-Kit+ Mac-1− cells isolated from the BM stained positively for BrdU.17 Similarly, less than 30% of rhodamine 123lo/Hoechst 33342lo primitive HSC isolated from the BM of mice on continuous oral BrdU for 5 days stained positively for BrdU.27 In contrast, virtually all HSC isolated from the BM of day +4 CY/G-CSF–treated mice were BrdU+, as were nearly all MPB HSC from the same mice. Because appreciable DNA synthesis does not occur while LT-HSC are in the blood, we concluded that BrdU+ MPB LT-HSC underwent cell division elsewhere. Because significant numbers of LT-HSC do not appear in the blood until day +3, it is not possible that all of the cells could have cycled in the blood prior to day +4, when the cells were obtained, even if the cell cycle time was short (8-10 hours), and even if all LT-HSC that entered the blood remained there. The most likely place for HSC division to have occurred is the BM. The near total susceptibility of BM LT-HSC from CY/G-CSF–treated mice to a second dose of CY confirms that most or all of these cells were cycling on day +2. This result is consistent with the increased sensitivity to 5-fluorouracil (5-FU) of splenic HSC stimulated to enter the cell cycle with Steel factor,28 and of BM HSC stimulated to cycle with 5-FU,29 as well as with the observation that the HSC compartment is significantly damaged by the administration of alternating doses of cytotoxic agents and G-CSF to mice.30The fact that 99% of MPB LT-HSC had previously cycled in the BM, and that 95% of these cells were in G0/G1phase,6 indicates that LT-HSC egress from BM to blood occurs after M phase and before the next S phase. Thus, it is not the case that a quiescent subset of G0-phase LT-HSC is selectively mobilized in CY/G-CSF–treated mice. Rather, all BM LT-HSC enter the cell cycle, and some of these cycling cells then migrate into the blood, by a process that appears tightly coordinated with the cell cycle.
It remained formally possible that HSC may be released from the BM in all phases of the cell cycle, but S/G2/M-phase HSC are preferentially cleared, leaving G0/G1 cells overrepresented in the blood. If this were true, the earliest HSC appearing in the spleens of mice following mobilization with CY/G-CSF would be expected to have an S/G2/M fraction as high or higher than that of the BM, because the spleen is a primary destination for circulating HSC in mice.6,11,31-33 However, on the day that significant numbers of HSC abruptly appear in the blood and spleen following CY/G-CSF, only about one third as many splenic HSC as BM HSC are in S/G2/M.6 Further, if this hypothesis were true, one would expect that MPB HSC from splenectomized mice would have a higher percentage of S/G2/M cells than MPB HSC from intact mice, which has been shown not to be the case.7Finally, the demonstration that MPB progenitors, the majority of which are in G1 phase of the cell cycle, are rapidly and uniformly cleared from circulation argues against selective clearance of S/G2/M-phase cells from the blood.
The association of LT-HSC expansion with mobilization begs the question of whether mobilization follows cell division in all instances in which HSC migration is observed. In normal prenatal development, sequential migrations of pluripotent HSC are thought to be associated with expansion of the HSC pool.34,35 Following treatment of adult animals with some cytokines, however, mobilization can occur very rapidly. Within 30 minutes of administration, interleukin 8 (IL-8) causes mobilization of hematopoietic progenitors and LT-HSC in mice,36 and of hematopoietic progenitors in primates,37 albeit in numbers much lower than at the peak of CY/G-CSF mobilization.6 Thus, it might appear that IL-8 causes mobilization by a different pathway than CY/G-CSF or that it acts by bypassing the early steps of the CY/G-CSF pathway. However, it may be that only BM HSC that have recently undergone cell division are mobilized by IL-8. On the order of 8% of the approximately 80 000 LT-HSC in adult mouse BM6 enter the cell cycle each day.17,27 If 8% of these LT-HSC also complete M phase each day, and if half of these cells are rapidly mobilized by IL-8 treatment, MPB would contain a maximum of about 1.5 LT-HSC/μL blood after IL-8 (assuming a blood volume of 2 mL). This estimate appears consistent with published data on mobilization induced by IL-8.36 Therefore, even though there are clearly differences between mobilization induced by IL-8 and CY/G-CSF, postmitotic, G1-phase cells may migrate in both cases.
About 60 000 LT-HSC appear in the spleen between days +2 and +3 of the CY/G-CSF treatment protocol (an ∼100-fold increase), even though the number of LT-HSC in the blood at any moment during this interval averages fewer than 10 000.6 At a minimum, it would appear that the equivalent of the entire complement of LT-HSC in the blood would have to be delivered to the spleen 6 times in 24 hours. The percentage of cycling cells in the BM or spleen is insufficient to account for more than a small fraction of the ∼100-fold increase in the number of splenic cells.6 Thus, the most likely explanation is that LT-HSC arrive in the spleen from the BM through the blood at a high rate of flux. This hypothesis was confirmed by directly measuring the clearance of mobilized stem and progenitor cells from MPB. Transferred eGFP+ progenitor cells were removed from the circulation of day +4 CY/G-CSF–treated wild-type recipients in less than 1 minute, whereas control PKH-26–labeled RBC were observed in the blood for at least 3 hours after injection, the observation interval. Importantly, the rapid clearance of mobilized eGFP+ progenitor cells from the circulation cannot be explained entirely by nonspecific clearance mechanisms, because significant migration of eGFP+ cells into the spleen and liver of the mobilized recipients was observed within 3 hours of transfer. Taken together, the above data suggest that MPB HSC are actively cycling G1-phase cells that migrate rapidly from the blood into specific hematopoietic organs. These data suggest that MPB HSC are in the circulation for only one or 2 passes prior to migration to other sites, whereas RBC are fated to circulate in the bloodstream without homing.
Alternatively, LT-HSC may cycle in the BM, pass through M phase, and enter G0 phase before or on arriving in the blood. A recent study of human MPB CD34+ cells,12 only a fraction of which are HSC,38 supports this view, whereas another group concluded that human MPB CD34+ cells were in G1 phase.13 It has been shown that human CD34+Thy-1+Lin− cells sorted from MPB were about 12 hours slower to enter S phase when placed in culture under cytokine stimulation than their counterparts isolated from BM of untreated volunteers.11 This was reasonably interpreted to suggest that mobilized HSC were in a relatively quiescent state. In light of the current study, however, some of this difference may be due to the fact that the process of mobilization gives rise to a population of HSC in the blood that is synchronized in early G1 phase, whereas G1 phase HSC isolated from BM of untreated mice are distributed throughout the entire G1 phase, with some cells very close to entry into S phase. Thus, HSC isolated from normal BM may have a head start in relation to entry into S phase. The cyclin D2 and PY data are consistent with the notion that HSC entering the blood are mostly in G1 phase or are in a state of activation intermediate between the G0 and G1 phases. (The fact that MPB HSC are largely cyclin D2+ and have increased PY staining also is strong evidence that the BrdU incorporation resulted from authentic S-phase synthesis and not from BrdU-induced unscheduled DNA repair, as these are independent markers of cells in the mitotic cycle.)
One might imagine that an early step in physiologic or drug-induced HSC migration would involve weakening or detachment of specific adhesive interactions between HSC and surrounding stromal cells and ECM elements. There is reason to assume that BM LT-HSC, which express high levels of c-Kit,18 α4-integrin, and CD44 (D.E.W., S.J.M., I.L.W., unpublished data, October 1996), have interactions of significant avidity with stromal membrane-bound Steel factor, vascular cell adhesion molecule 1 (VCAM-1), and hyaluronate, respectively, as each of these ligands is ubiquitous in the BM.39-43 The α4-integrin–VCAM-1 interaction is believed to play a role in retention of HSC and progenitors in the BM.44-48 Detachment from substrate can occur in association with cell division.49,50 This could be accomplished either by modulation of expression levels of adhesion molecules such as α4β1 integrin, or by alteration of the affinity of receptors such as integrins (reviewed in reference 51) or CD4452 for their ligands. It has long been observed that cultured adherent cells loosen their attachment to the substrate and “round-up” during M phase.49 Similarly, dividing HSC may detach from stromal elements during M phase, and in the process become competent to migrate, given the appropriate secondary signals. This model is consistent with the observed appearance in the blood of postmitotic HSC that have recently cycled.
We thank Libuse Jerabek for superb laboratory management, Veronica Braunstein for antibody production, Koichi Akashi and Dennise Dalma-Weiszhausz for careful review of the manuscript, David Parks for advice on flow cytometry, Stanley Tamaki and Nobuko Uchida for advice on DNA/RNA staining for flow cytometry, Allen Smith for advice on DNA repair, Jos Domen for helpful discussions, and Diana J. Laird for statistical advice.
Supported by National Institutes of Health grant 5R01 HL-58770 to I.L.W. D.E.W. was supported by National Institute of Allergy and Infectious Diseases Training grant 5T32 AI-07290. S.H.C. is supported by the Medical Scientist Training Program grant 5T32 GM-07365 at Stanford University School of Medicine. A.J.W. is supported by the American Cancer Society, grant PF-00-017-01-LBC.
I.L.W. has declared a financial interest in Amgen and Systemix.
D.E.W. and S.H.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Irving L. Weissman, Department of Pathology, Beckman B257, Stanford University School of Medicine, Stanford, CA 94305; e-mail: irv@stanford.edu.