Abstract
Is peripheral stem cell mobilization followed by autologous stem cell transplantation (ASCT) feasible in patients with human immunodeficiency virus (HIV)– associated lymphoma (HIV-L)? Studies have demonstrated that, in the HIV- negative (HIV−) setting, ASCT may improve lymphoma-free survival in high-risk non-Hodgkin lymphoma (NHL) or relapsed Hodgkin disease (HD) and NHL. Given the poor prognosis of HIV-L with conventional chemotherapy, this dose-intensive approach was explored. Nine patients with HIV-HD or NHL mobilized a median of 10.6 × 106 CD34+ cells/kg and engrafted after ASCT. CD4 counts recovered to pretransplantation levels and HIV viral loads were controlled in patients compliant with antiretroviral therapy. Seven of 9 patients remain in remission from their lymphoma at a median of 19 months after transplantation. Thus, patients with HIV-L on antiretroviral therapy can engraft following ASCT. Prolonged lymphoma remissions, without significant compromise of immune function, can be seen, suggesting that ASCT can be used in selected patients with HIV-L.
Introduction
Highly active antiretroviral therapy (HAART) has changed the natural history of human immunodeficiency virus (HIV) infection by improving immune function in HIV-infected individuals.18 However, multiple studies have shown no impact of HAART on the incidence of acquired immunodeficiency syndrome (AIDS)–related non-Hodgkin lymphoma (NHL) or Hodgkin disease (HD).1-6
Earlier therapeutic approaches to HIV-associated lymphoma (HIV-L) focused on reduction of chemotherapy dose intensity due to fears of further worsening the immune function in these patients.7In the era of HAART, more aggressive approaches are being explored.8-11 In HIV-negative (HIV−) patients with relapsed aggressive NHL and HD, high-dose chemotherapy with autologous stem cell transplantation (ASCT) is the optimal therapy.12 This modality has also been explored in patients with NHL in first remission who are considered to be at increased risk for relapse with conventional-dose chemotherapy.13 14 We report here on our initial experience using ASCT in patients with HIV-L receiving HAART.
Study design
Between March 1998 and May 2000, 9 HIV-L patients on HAART received myeloablative chemotherapy followed by ASCT at our institution. Informed consent was obtained from all patients in accordance with the City of Hope Medical Center institutional review board guidelines. The median age was 41.6 years (range, 11.4-54.3 years). Seven patients had NHL and 2 had HD (Table1). The NHL patients who were in first remission had high-risk features as defined by the international prognostic index (IPI).15 19
All patients were on HAART (Table 1) at the time of stem cell collection before transplantation and had undetectable HIV viral loads (VLs). Stem cells were mobilized with a combination of chemotherapy and 10 μg/kg granulocyte colony-stimulating factor (G-CSF) and collected as previously described.10 A median of 10.6 × 106 CD34+ cells/kg was collected. Five patients had additional stem cells collected as part of a gene therapy trial, which was reported separately.16
High-dose chemotherapy with the CBV regimen (cyclophosphamide 100 mg/kg ideal body weight, 1,3-bis-(2-chloroethyl)1-nitrosourea (BCNU) 450 mg/m2, etoposide 60 mg/kg adjusted ideal body weight) was administered to all patients.13,17 Autologous stem cells were reinfused on transplantation day 0. Five patients, who were also on the gene therapy trial, had genetically modified stem cells (0.5-2.0 × 106 CD34+ cells/kg) reinfused after the infusion of unmanipulated stem cells.16Twenty-four hours after stem cell infusion, G-CSF (5 μg/kg) was started and continued until engraftment. Routine anti-infective prophylaxis was administered to all patients as previously described.10 Most patients were able to continue HAART through the transplantation. Three patients missed one dose, one missed 3 doses, and only one patient missed all doses due to persistent nausea.
Results and discussion
Engraftment occurred in all 9 patients. White cell engraftment, defined as an absolute neutrophil count greater than 500/μL, occurred at a median of 11 days (range, 9-12). Platelet engraftment, defined as platelet transfusion independence and a platelet count greater than 20 × 109/μL, occurred at a median of 10 days (range, 7-15).
Infectious complications during the period of neutropenia up to the day of engraftment were seen in 6 patients. These included coagulase-negative Staphylococcus orStreptococcus pneumoniae bacteremia in 4 patients. One patient developed a dental abscess. On transplantation day +11 one patient developed culture-negative sepsis, manifest as hypotension, gastrointestinal bleeding, and skin erythema. Infectious complications after engraftment included Clostridium difficile colitis in one patient at day +38. Two patients developed an interstitial pneumonia between day +40 and day +60. One of these patients also developed a lobar pneumonia in year 2 after transplantation as well as Staphylococcus aureus bacteremia and recurrent sinusitis. One patient developed a bacterial pneumonia, presumably after influenza, at +21 months for which he required temporary ventilatory support. All patients responded to antimicrobials.
Opportunistic infections were seen in 3 patients—an uncomplicated disseminated varicella zoster infection at 2 months after transplantation in one patient, cytomegalovirus viremia and fever at 37 days in another patient, and Pneumocystis cariniipneumonia (PCP) at 8 months and cytomegalovirus retinitis at 17 months after transplantation in one patient who stopped both PCP prophylaxis and antiretrovirals. All patients responded to treatment.
Conditioning regimen-related complications included grade 2 mucositis in 6 patients and grade 2 hyperbilirubinemia in one patient. One of these patients also had BCNU pneumonitis, presenting as interstitial infiltrates at day +55; this responded well to oral prednisone. All toxicity was graded according to common toxicity grading criteria version 2.0.
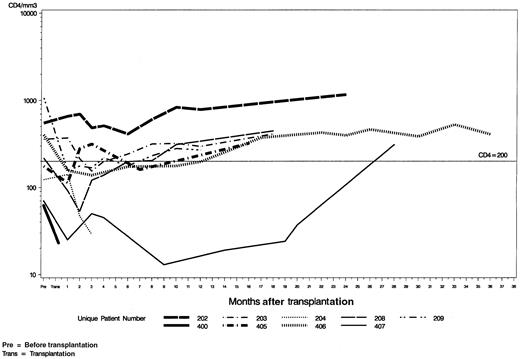
The CD4 counts gradually decreased after transplantation in all 9 patients and reached a nadir of 138/μL (range, 25-411) at a median of 2 months in 7 evaluable patients (Figure1). Two nonevaluable patients died at approximately 4 months after transplantation with relapsed lymphoma. Six of these 7 evaluable patients recovered CD4 counts to pretransplantation levels by a median of 14 months (range, 2-28). One patient (UPN 209) had the highest CD4 counts before transplantation (> 1000/μL), which may account for the failure to return to this baseline.
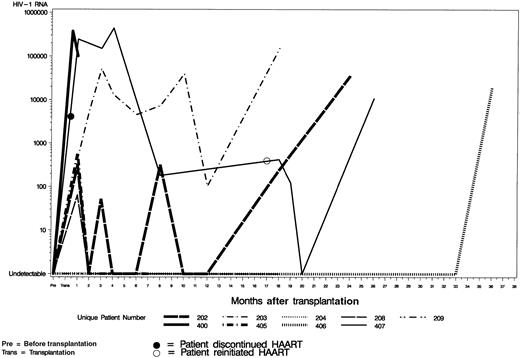
Six patients had a rise in their HIV VL in the first month following the transplantation, whereas 3 patients maintained an undetectable VL (Figure 2). In 5 of the 7 patients evaluable at 12 months, the VL was at undetectable levels (b-DNA assay or RNA polymerase chain reaction Roche Amplicor assay). One patient (UPN202), had a significant rise at 18 months and one (UPN406) at 36 months due to noncompliance with HAART. Two patients (UPN203, UPN407) had a persistently high VL after transplantation. UPN203 required multiple changes in his regimen. He was compliant with HAART, hence his high VL may reflect the inability of HAART to eradicate the latent HIV reservoir. Patient UPN407 stopped all antiretroviral therapy for a prolonged period after transplantation for psychosocial reasons, which explains the continued high VL.
We report here on the largest single institution series of patients with HIV-L on HAART treated with ASCT. All patients received the same high-dose chemotherapy conditioning and achieved long-term engraftment with stem cell rescue in a time course similar to HIV−patients. Pre-engraftment infectious complications were similar to the HIV− setting with bacterial infections predominating.12 Conditioning regimen-related complications were also similar to the HIV− setting in that there was no increase in mucositis or hepatotoxicity associated with combining HAART and high-dose chemotherapy. After transplantation, the several opportunistic infections that occurred responded well to treatment. In the majority of patients we have seen recovery of immune function as manifest by low VL and rising CD4 counts. This is similar to changes in immune parameters in HIV-L patients treated with CHOP and concomitant antiretroviral therapy.20 Future studies will address the pharmacokinetic effects of myeloablative therapy in combination with HAART and the reconstitution of immune function after ASCT. The effects of this approach on lymphoma-free survival remain to be seen. The surviving patients have been followed for a median of 19.0 months (range, 12-36 months). Two patients relapsed with disease in the bone marrow at 40 days and 95 days, respectively, and died. All other patients remain in remission.
The advent of effective antiviral therapy now allows us to administer dose-intensive and potentially curative chemotherapy to HIV-infected individuals with lymphoma. Infectious complications can be managed through a combination of prophylaxis and close monitoring after transplantation. Hence, HIV status should no longer by itself preclude ASCT for treatment of lymphoma.
The authors thank Sarah Cole and Celina Acedo for biostatistical support and Diana Garcia for secretarial support.
Supported in part by United States Public Health Service grants CA30206, CA33572, and AI 38592 and grant M01 RR-43 from the General Clinical Research Center branch of the National Center for Research Resources, National Institutes of Health. A.K. is the recipient of a Lymphoma Research Foundation of America Fellowship Award. A.M. is the recipient of an American Cancer Society Clinical Oncology Career Development Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Amrita Krishnan, Division of Hematology and Bone Marrow Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: akrishnan@coh.org.