Abstract
Pbx1 is the product of a proto-oncogene originally discovered at the site of chromosomal translocations in acute leukemias. It binds DNA as a complex with a broad subset of homeodomain proteins, but its contributions to hematopoiesis have not been established. This paper reports that Pbx1 is expressed in hematopoietic progenitors during murine embryonic development and that its absence results in severe anemia and embryonic lethality at embryonic day 15 (E15) or E16. Definitive myeloerythroid lineages are present inPbx1−/−fetal livers, but the total numbers of colony-forming cells are substantially reduced. Fetal liver hypoplasia reflects quantitative as well as qualitative defects in the most primitive multilineage progenitors and their lineage-restricted progeny. Hematopoietic stem cells from Pbx1−/−embryos have reduced colony-forming activity and are unable to establish multilineage hematopoiesis in competitive reconstitution experiments. Common myeloid progenitors (CMPs), the earliest known myeloerythroid-restricted progenitors, are markedly depleted inPbx1−/−embryos at E14 and display clonogenic defects in erythroid colony formation. Comparative cell-cycle indexes suggest that these defects result largely from insufficient proliferation. Megakaryocyte- and erythrocyte-committed progenitors are also reduced in number and show decreased erythroid colony-forming potential. Taken together, these data indicate that Pbx1 is essential for the function of hematopoietic progenitors with erythropoietic potential and that its loss creates a proliferative constriction at the level of the CMP. Thus, Pbx1 is required for the maintenance, but not the initiation, of definitive hematopoiesis and contributes to the mitotic amplifications of progenitor subsets through which mature erythrocytes are generated.
Introduction
Hematopoiesis involves the production of a diverse array of mature blood cells via a hierarchy of progenitors with progressively more limited differentiation and self-renewal potential and is orchestrated by a complex array of regulatory proteins (reviewed by Tenen et al1). Several of these regulatory proteins are transcription factors originally discovered as the products of proto-oncogenes targeted by chromosomal aberrations in hematologic malignancies. One of these is Pbx1, which was identified by virtue of its disruption in t(1;19) chromosomal translocations in a subset of pediatric acute lymphoblastic leukemias.2,3 The highly related genes Pbx2 and Pbx3 were subsequently discovered on the basis of their homology to Pbx1 but have yet to be implicated in neoplasia or other diseases.4 Pbx proteins are mammalian homologues of the extradenticle (Exd) protein, which functions as a cofactor for Hox transcription factors in Drosophila.5 Like Exd, Pbx proteins possess a divergent homeodomain DNA-binding motif and interact physically with a subset of Hox proteins to enhance their DNA-binding affinities and specificities. In addition, homeodomain proteins of the Meis family are prevalent intracellular partners for Pbx proteins, control their nuclear localization, and promote cooperative DNA binding.6-8 The t(1;19) rearrangement fuses the homeodomain of Pbx1 to the transcriptional activation domains of the immunoglobulin enhancer–binding protein E2a. The E2a-Pbx1 chimeric oncoprotein retains the ability to bind DNA as a complex with Hox, but not Meis, proteins6 and may contribute to leukemogenesis by aberrant activation of Pbx1/Hox target genes.
Recently, evidence has emerged implicating Hox transcription factors as master regulators of hematopoietic cell fate decisions and leukemogenesis (reviewed by Magli et al9). Hoxgene expression in subsets of CD34+ progenitor cells from normal human bone marrow is developmentally regulated in a stage-specific manner.10 Perturbations of Hoxgene expression in primary murine hematopoietic progenitors have pronounced effects on their proliferation and differentiation in vivo and in vitro, including leukemic transformation.11,12Deregulated Hox expression has also been implicated in human hematologic malignancies, providing circumstantial support for the importance of Hox-dependent transcriptional regulation in the normal development of the hematopoietic lineages (reviewed by Look13). Targeted disruption of select Hoxgenes in mice has demonstrated that the encoded homeodomain transcription factors are required for normal hematopoiesis.14-16 Taken together, these data suggest that the establishment and maintenance of definitive hematopoiesis in mammals depends on the transcriptional regulatory functions of several Hox proteins.
Given its role as a Hox DNA-binding cofactor and leukemic oncoprotein, Pbx1 is likely to be required for hematopoietic development; however, its expression patterns and specific contributions during hematopoiesis are unknown. Here we demonstrate that Pbx1 is expressed during embryonic hematopoiesis and that its targeted disruption leads to substantial defects in hematopoietic progenitors with erythroid-differentiation potential resulting in severe fetal anemia and death by embryonic day 16 (E16).
Materials and methods
Generation of Pbx1 knockout mice
Disruption of the Pbx1 gene was performed by homologous recombination in the TL1 embryonic stem (ES) cell line,40 resulting in the insertion of a PGK-neo cassette into exon 3. Homologous recombinant clones were identified by Southern blot analysis, and euploid clones were micro-injected into C57BL/6J host blastocysts. Chimeric male mice from 2 independently derived ES clones (38 and 176) passed the targeted Pbx1 allele through the germline. All phenotypes were analyzed in embryos derived from third- and fourth-backcross generations on a C57BL/6 background. Some phenotypes were also analyzed (methylcellulose assays, hematocrits) on a fully inbred 129/SvTer background. The homozygous (−/−) phenotypes were identical on both genetic backgrounds and in embryos derived from either of the 2 independently targeted ES clones. Mice derived from both clones have been used for these studies. Genotyping was performed by Southern blot analysis ofSspI-digested DNA from tail biopsies or yolk sacs by means of a probe (3′) external to the targeting construct or by polymerase chain reaction (probes and conditions available on request).
Hematocrit determination
Peripheral blood was obtained from viable E15 embryos by cardiac puncture with the use of 32 × 0.8-mm heparinized capillary tubes. Tubes were spun in a hematocrit centrifuge according to manufacturer's specifications, and the percentage of volume occupied by packed red cells was calculated.
Immunohistochemistry
Embryos were fixed in Bouin fixative and embedded in paraffin. After dewaxing and microwave antigen retrieval in 0.5 M Tris, pH 10, sections of embedded embryos were stained with a Pbx1b-specific monoclonal antibody (mAb).17 Secondary staining was performed with a biotinylated goat antimouse antibody followed by strepavidin-conjugated horseradish peroxidase (HRP).
Western blotting
Lysates were prepared from fetal liver (FL) cell suspensions or from fractionated FL cells by boiling in sodium dodecyl sulfate (SDS) sample buffer and shearing through a 28-gauge needle. Separate nuclear and cytoplasmic extracts were made by lysing FL cells in a hypotonic buffer with 0.1% NP-40 and pelleting nuclei at 6000g for 4 minutes. The supernatant solution was used as cytoplasmic extract. Nuclear proteins were extracted with 400 mM NaCl by rocking at 4°C for 20 minutes. Then, 15 to 30 μg protein from whole cell extracts or 5 to 10 μg nuclear or cytoplasmic protein was electrophoresed through a 10% SDS-polyacrylamide gel and transferred to an Immobilon-P membrane (Millipore, Bedford, MA) in tris-glycine buffer with 20% methanol. Membranes were probed with mAbs specific for Pbx1b and Pbx3a or for all 3 long isoforms of Pbx. After probing with HRP-conjugated anti–immunoglobulin (Ig) G mAb, complexes were detected by enhanced chemiluminescence (Amersham, Buckinghamshire, England).
Fluorescence-activated cell sorting and analysis
FLs were dissected from E14.5 embryos, drawn through a 25-gauge needle, and passed through fine mesh to generate a single-cell suspension. Erythrocytes were lysed in ammonium chloride potassium buffer. Cells were stained with phycoerythrin-conjugated anti-FcγR (2.4G2) fluorescein isothiocyanate (FITC)–conjugated CD34 (RAM34) (Pharmingen, San Diego, CA), Texas red–conjugated anti–Sca-1 (E13-161-7), allophycocyanin-conjugated anti–c-Kit (2B8), and biotinylated mAbs specific for Ter119 and AA4.1. FL hematopoietic stem cell (HSC) populations were stained and sorted as described by Morrison et al.18 For experiments requiring the sorting of HSCs or myeloid progenitors, Ter119+ cells were partially removed with sheep anti–rat IgG-conjugated magnetic beads (Dynabeads M-450) (Dynal, Oslo, Norway), and the remaining cells were stained with streptavidin-RED670 (Gibco BRL, Bethesda, MD). All cell populations were sorted or analyzed by means of a highly modified triple-laser (488-nm argon laser, 599-nm dye laser, and UV laser) FACS Vantage (Becton Dickinson Immunocytometry Systems, Mountain View, CA). Progenitors were purified by sorting and then re-sorting to obtain precise numbers of cells that were essentially pure for the indicated surface-marker phenotype. In single-cell clonogenic assays, the re-sort was performed by means of a carefully calibrated automatic cell deposition unit (ACDU) system (Becton Dickinson). This system deposited a specific number of purified cells onto methylcellulose medium in 96-well plates.
Myeloid colony assays
For colony-forming cell (CFC) assays, single-cell suspensions were prepared from E14 FL as described above. We mixed 20 000 cells from each liver in 1 mL 0.9% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) in Iscoves modified Dulbecco medium supplemented with 20 ng/mL stem cell factor (SCF), 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN), 5 ng/mL granuloctye CSF, and 5000 U/L erythropoeitin (Epo) (Amgen, Thousand Oaks, CA). Triplicate cultures from each FL sample (0.75 mL per well) were plated in 24-well plates. Colonies were counted after 7 days (3 days for erythroid colony forming units [CFU-E]) on the basis of standard morphological criteria. For clonal analysis of sorted myeloid progenitors, cells were cultured in an Iscove's-based methylcellulose medium (Methocult H4100) (Stem Cell Technologies) that was supplemented with 20% fetal bovine serum, 1% bovine serum albumin, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, and 10% FL-conditioned medium. Cytokines such as mouse SCF (20 ng/mL) (R&D Systems), mouse interleukin-3 (IL-3; 30 ng/mL) (Genzyme, Cambridge, MA), mouse IL-11 (10 ng/mL) (R&D Systems), mouse GM-CSF (10 ng/mL), mouse thrombopoietin (10 ng/mL) (R&D Systems), mouse Flt-3 ligand (10 ng/mL) (R&D Systems), and human erythropoietin (1000 U/L) (R&D Systems) were added at the start of the culture. Colonies were enumerated under an inverted microscope consecutively from day 5 to day 12. Mixed colonies containing both erythroid and myelomonocytic cells (CFU-Mix)—such as granulocyte, erythrocyte, megakaryocyte, macrophage CFU; granulocyte, erythrocyte, megakaryocyte CFU; and granulocyte, erythrocyte, megakaryocyte CFU—were determined by Giemsa staining of cells that were picked from individual colonies by means of fine drawn-out Pasteur pipettes. All cultures were incubated at 37°C in a humidified chamber under 7% CO2.
Transplantation
C57BL/6 mice were irradiated to 950 cGy from an X-ray source. For competitive reconstitution, 2.5 × 105 cells fromPbx1−/− or Pbx1+/−cells were mixed with an equal number of cells from a normal C57BL/6K embryo and injected into the tail veins of irradiated recipients. The congenic strains of mice, C57BL/Ka-Thy1.1 (Ly5.1) and C57BL/Ka-Thy1.1 (Ly5.2), were used as described by Kondo et al.19 For radioprotection and spleen CFU (CFU-S) experiments, cells were administered to anesthetized recipients by retro-orbital injection. Donor (Pbx1−/−) cell contribution in long-term survivors of radioprotection experiments was determined by Southern blot analysis by means of a probe (3′) external to the targeting construct on SspI-digested DNA isolated from whole bone marrow cells.
Proliferation and apoptosis indices
Pregnant mice resulting from Pbx1+/−intercrosses were injected retro-orbitally with bromodeoxyuridine (BrdU) in 0.9% saline at 14 dpc. At 90 minutes after injection, the mice were killed and the livers dissected from the embryos. Common myeloid progenitors (CMPs) were sorted from FL-cell suspensions as described above, and cytospin preparations from the sorted progenitors were air-dried and fixed in methanol at −20°C for BrdU staining or in a 1:1 mixture of methanol and acetone at room temperature for proliferating cell nuclear antigen (PCNA) staining. BrdU staining was performed with FITC-conjugated anti-BrdU (Becton Dickinson, San Jose, CA) according to the manufacturer's specification. Cells were counterstained with 4 μg/mL propidium iodide. PCNA staining was performed as described by Hall et al.20 Percentages were derived by counting 100 to 200 cells. Terminal deoxynucleotidyl transferase–mediated dUTP-biotin end labeling of fragmented DNA (TUNEL) assays were performed by means of an in situ cell death detection kit (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's recommendations.
Results
Pbx1 is expressed in hematopoietic progenitors during embryonic hematopoiesis
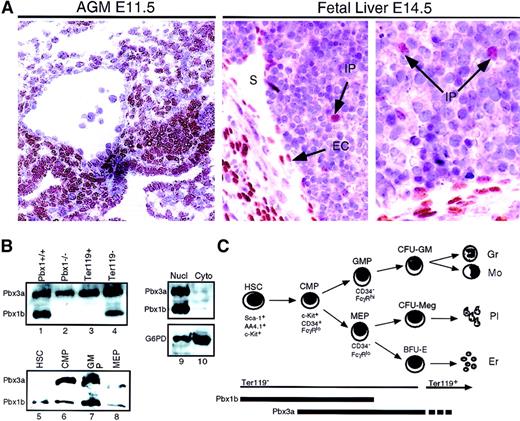
Pbx1 is expressed in the mesenchyme throughout the mid-gestation embryo.21 Its specific expression in the murine fetal hematopoietic compartments was examined here by immunostaining with highly specific mAbs developed in our laboratory.17 Strong nuclear staining for the Pbx1b, but not the Pbx1a, isoform was observed in the aorta-gonad-mesonephros (AGM), the earliest intra-embryonic site of hematopoiesis, at E11.5 (Figure 1A). This staining included cells that are enriched for hematopoietic progenitors in the mesonephric mesenchyme as well as occasional cells lining the dorsal aorta.22 At E14.5, Pbx1b+cells were much less frequent in the FL than in the AGM, comprising primarily cells of the liver capsule, sinusoidal endothelium, and occasional intraparenchymal cells (Figure 1A). By Western blotting, expression of Pbx1b (38 kd) and Pbx3a (46 to 47 kd) was detected in extracts prepared from FL cells at E14.5 (Figure 1B). Both of these Pbx proteins were detected exclusively in the nuclear fraction (lanes 9 and 10). Three other Pbx proteins (Pbx1a, Pbx2, and Pbx3b) were not detected by Western blot in FL during this stage of hematopoietic differentiation (data not shown).
Pbx expression in embryonic hematopoietic tissues.
(A) Immunoperoxidase staining of AGM (left panel) and FL (2 right panels) with a Pbx1b-specific mAb. Nuclear staining includes mesonephric mesenchyme and some cells lining the dorsal aorta in the AGM, as well as endothelial cells (ECs) lining hepatic sinusoids (S) and rare intraparenchymal (IP) cells in the fetal liver. (B) Western blotting with Pbx1b and Pbx long-form–specific mAbs. Lanes 1 and 2, whole cell extract from wild-type (wt) andPbx1−/−E14 FL, respectively; lanes 3 through 8, whole cell extracts from populations of cells enriched for the indicated progenitors by immunomagnetic beads or flow cytometry; lanes 9 and 10, nuclear and cytoplasmic extracts from wt FL at E14, respectively. (C) Schematic depiction of myeloid differentiation from HSCs to CMPs to granulocyte/monocyte progenitors (GMPs) and megakaryocyte/erythrocyte progenitors (MEPs) as described by Akashi et al.24
Pbx expression in embryonic hematopoietic tissues.
(A) Immunoperoxidase staining of AGM (left panel) and FL (2 right panels) with a Pbx1b-specific mAb. Nuclear staining includes mesonephric mesenchyme and some cells lining the dorsal aorta in the AGM, as well as endothelial cells (ECs) lining hepatic sinusoids (S) and rare intraparenchymal (IP) cells in the fetal liver. (B) Western blotting with Pbx1b and Pbx long-form–specific mAbs. Lanes 1 and 2, whole cell extract from wild-type (wt) andPbx1−/−E14 FL, respectively; lanes 3 through 8, whole cell extracts from populations of cells enriched for the indicated progenitors by immunomagnetic beads or flow cytometry; lanes 9 and 10, nuclear and cytoplasmic extracts from wt FL at E14, respectively. (C) Schematic depiction of myeloid differentiation from HSCs to CMPs to granulocyte/monocyte progenitors (GMPs) and megakaryocyte/erythrocyte progenitors (MEPs) as described by Akashi et al.24
To identify the subsets of FL cells expressing Pbx1b and Pbx3a, cells were purified on the basis of their surface phenotypes by means of immunomagnetic enrichment and/or flourescence-activated cell sorter (FACS). Ter119+ erythroid cells (Figure1C), which compose approximately 70% of the FL population at E14.5, expressed Pbx3a but not Pbx1b (Figure 1B). A population of cells expressing the lin−c-Kit+Sca-1+AA4.1+surface phenotype includes all of the long-term repopulating cells in FL at E1418,23 and will be referred to as HSC-enriched for the purpose of describing the experiments in this report. The lin−Sca-1−c-Kit+ fraction of bone marrow (BM) and FL can be subdivided into 3 progenitor populations with distinct lineage potentials on the basis of the differential expression of CD34 and FcγR (Figure 1C)24 (Traver et al,39 accompanying article, page 627). CD34−/FcγRlomegakaryocyte/erythrocyte-restricted progenitors (MEPs) give rise exclusively to erythrocytes and megakaryocytes whereas CD34+/FcγRhigranulocyte/macrophage-restricted progenitors (GMPs) produce only granulocytes and monocytes. Cells in the CD34+/FcγRlo fraction are precursors of both MEPs and GMPs and are thus designated CMPs. By Western blot analysis, all of these phenotypically defined progenitor populations expressed Pbx1b and Pbx3a (lanes 6-8), and immunofluorescent staining confirmed that Pbx1b was localized exclusively in the nuclear compartment (not shown).
Pbx1 deficiency results in severe fetal anemia
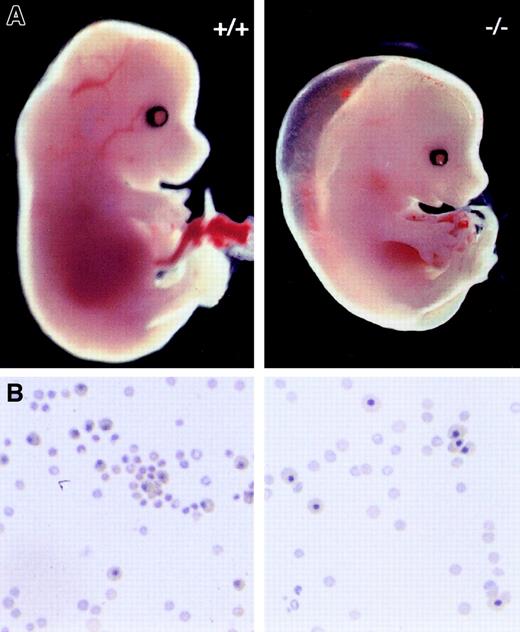
Pbx1-deficient mice were created by gene targeting of the Pbx1 transcriptional unit whose disruption was shown to result in a null allele.40 As expected, no Pbx1 protein was detected by Western blot inPbx1−/− FL at E14.5 (Figure 1B, lane 2).Pbx1+/− mice were born at the expected Mendelian ratio, were fertile, and exhibited no gross abnormalities except for significantly smaller size. Pbx1−/−embryos, however, died between E15.5 and E16.5 and exhibited patterning defects of the skeleton, severe hypoplasia of abdominal and thoracic organs, and splenic aplasia.40 Among the most visually obvious features of Pbx1−/− embryos were their striking pallor and subcutaneous edema (Figure2A). These features, which first became evident at E12.5, were associated with profound anemia. While the size and morphology of circulating erythrocytes did not differ significantly between Pbx1−/− and wild-type (wt) blood (Figure 2B), mean hematocrits were 5% forPbx1−/− embryos at E15.5, which contrasted with 38% for Pbx1+/− and wt littermates (Figure 3A). The approximately 8-fold decrease in hematocrit reflected a similar reduction in the number of circulating erythrocytes and is of a sufficient magnitude to contribute to subcutaneous edema and death between E15.5 and E16.5.25
Gross and microscopic features of anemia in Pbx1−/− embryos.
(A) Appearance of wt (+/+) and Pbx1−/−embryos at E14.5. (B) Cytospins of peripheral blood showing nucleated and nonnucleated erythrocytes in circulation at E15. Equivalent numbers of cells were spun down for each sample.
Gross and microscopic features of anemia in Pbx1−/− embryos.
(A) Appearance of wt (+/+) and Pbx1−/−embryos at E14.5. (B) Cytospins of peripheral blood showing nucleated and nonnucleated erythrocytes in circulation at E15. Equivalent numbers of cells were spun down for each sample.
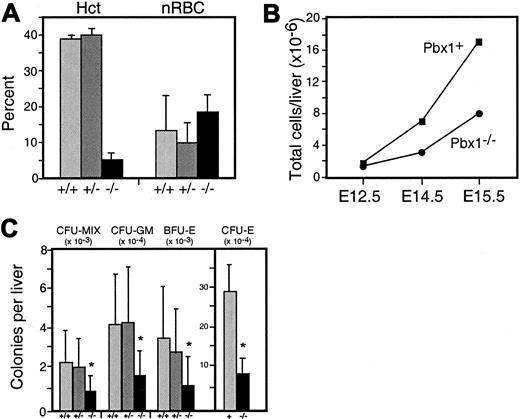
Anemia and reduced CFCs inPbx1−/− embryos.
(A) Hematocrits (Hcts) and percentage of nucleated red blood cells (nRBCs) in E15 embryos; number of embryos were 6 (+/+), 9 (+/−), and 5 (−/−). (B) Fetal liver cell counts at E12.5, E14.5, and E15.5 (15 or more FLs used for each data point).Pbx1+ shows combined wt and heterozygous embryos. P < .05 for differences at E14.5 and E15.5. (C) Colonies of each type scored by morphological criteria per liver on the basis of FL total cell counts. Asterisk denotes P < .05 for Pbx1−/−compared with wt. Pbx1+ denotes data pooled from wt and heterozygous cells. Data are the the means and SDs obtained from 16 (−/−), 15 (+/+), and 27 (+/−) FLs of each genotype, respectively.
Anemia and reduced CFCs inPbx1−/− embryos.
(A) Hematocrits (Hcts) and percentage of nucleated red blood cells (nRBCs) in E15 embryos; number of embryos were 6 (+/+), 9 (+/−), and 5 (−/−). (B) Fetal liver cell counts at E12.5, E14.5, and E15.5 (15 or more FLs used for each data point).Pbx1+ shows combined wt and heterozygous embryos. P < .05 for differences at E14.5 and E15.5. (C) Colonies of each type scored by morphological criteria per liver on the basis of FL total cell counts. Asterisk denotes P < .05 for Pbx1−/−compared with wt. Pbx1+ denotes data pooled from wt and heterozygous cells. Data are the the means and SDs obtained from 16 (−/−), 15 (+/+), and 27 (+/−) FLs of each genotype, respectively.
Anemia in Pbx1−/− embryos was associated with a significant decrease (2- to 3-fold) in the number of cells per FL (Figure 3B), most of which are normally erythrocyte precursors between E12 and E16. Although the absolute number of Ter119+ cells per liver was reduced in Pbx1−/− embryos, the relative percentage of Ter119+ cells inPbx1−/− FL at E14.5 was similar to wt FL (68% ± 8% vs 75% ± 1.7%, respectively) and not significantly reduced as a percentage of total FL cells. Thus, FL hypoplasia inPbx1−/− embryos reflected a proportional decrease in erythroid as well as nonerythroid lineages including, presumably, multipotent hematopoietic progenitors.
Definitive hematopoiesis initiates in Pbx1−/−embryos
Although these data showed that Pbx1 was required for normal embryonic hematopoiesis, it was unlikely to be essential for primitive hematopoiesis, since the survival of Pbx1−/−embryos up to E16.5 exceeded the duration of significant numbers of yolk sac–derived erythrocytes. Moreover, examination of the peripheral blood of Pbx1−/− embryos at E15.5 revealed substantial numbers of nonnucleated erythrocytes (Figure 2B) likely to be of definitive origin. The proportion of residual nucleated erythrocytes at E15.5 was not significantly higher inPbx1−/− embryos than in their wt littermates (Figure 3A). This suggested not only that primitive erythropoiesis was spared by the absence of Pbx1, but that definitive erythropoiesis in the FL was initiated at the expected stage of development. Accordingly, myelomonocytic cells were always observed in FLs ofPbx1−/− embryos (not shown). Despite their extreme pallor, no hemorrhages were observed in the skin, abdominal cavity, or central nervous systems of Pbx1−/−embryos, suggesting that platelet production was sufficient to prevent spontaneous bleeding (Figure 2A and not shown).
Total myeloerythroid CFCs are reduced inPbx1−/−FL
Myeloerythroid CFCs in FL were quantified at E14.5. Granulocyte-macrophage CFUs, erythroid burst forming units (BFU-E) and CFU-Mix were scored on the basis of standard morphological criteria after 7 days of culture in methylcellulose supplemented with cytokines. Examination of cells within the colonies revealed them to have normal morphology. The total numbers of CFCs per liver inPbx1−/− embryos were reduced by 2- to 3-fold (Figure 3C). Furthermore, enumeration of erythroid colonies after 3 days in methylcellulose cultures revealed a reduction in absolute numbers of CFU-E per liver (4-fold) that was even more pronounced than the decrease in other CFCs (Figure 3C). Addition of a 10-fold excess of Epo to the culture medium did not alter these findings, suggesting that insensitivity to Epo was not responsible for the decrease in CFU-E growth. Culture of several thousand Ter119+ cells from wt or Pbx1−/− FL exhibited no colony-forming potential (not shown), indicating that the observed reductions were specific to progenitor populations that normally express Pbx1.
Pbx1−/−hematopoietic progenitors show decreased colony-forming potentials in vitro and in vivo
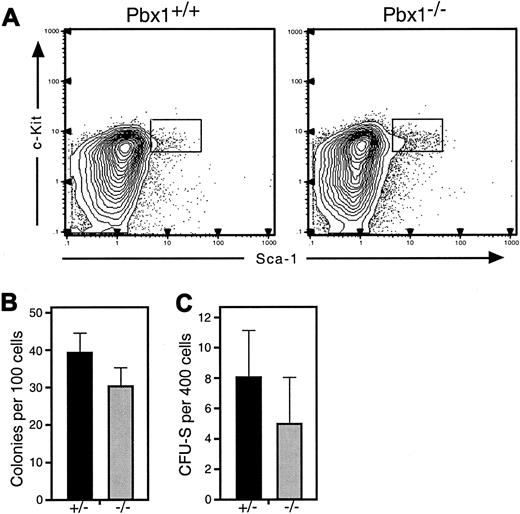
To determine whether pluripotent progenitors were decreased inPbx1−/− FL, their phenotypic frequency was examined at E14. Flow cytometry showed that the absolute numbers of lin−Sca-1+c-Kit+AA4.1+cells that normally contain all of the multilineage long-term repopulating activity in FL were modestly decreased inPbx1−/− embryos (Figure4A), but this did not achieve statistical significance (P = .07). Cells expressing this surface phenotype were sorted individually into single wells containing methylcellulose medium. In these assays,Pbx1−/− cells showed normal colony distributions while displaying a slight decrease in the number of colonies generated on a per-cell basis (Figure 4B).
Reduced pluripotent progenitor numbers and clonogenic potential in Pbx1−/− FL.
(A) Representative FACS profiles of wt (left) andPbx1−/−(right) FL at E14.5 show a relative increase in frequency of lin−Sca-1+c-Kit+ cells inPbx1−/−FL. Note that the absolute numbers of lin−Sca-1+c-Kit+AA4.1+cells per FL, however, are reduced (wt = 2.8 × 104cells per FL; Pbx1−/−= 1.6 × 104 cells per FL). Results are shown for single animals of each genotype. (B) In vitro clonogenic potential is shown for HSC-enriched pluripotent progenitors, which were purified by 2 rounds of FACS analysis of cells from 4 pooled FLs of each genotype by means of the FACS gates shown in panel A. (C) Day-8 CFU-S frequency for purified Pbx1−/−andPbx1+/−lin−Sca-1+c-Kit+ cells injected into irradiated wt recipients. Data were obtained from a single experiment (representative of 2) on cells (with 4 or more pooled FLs of each genotype) purified by 2 rounds of FACS sorting by means of the gates shown in panel A.
Reduced pluripotent progenitor numbers and clonogenic potential in Pbx1−/− FL.
(A) Representative FACS profiles of wt (left) andPbx1−/−(right) FL at E14.5 show a relative increase in frequency of lin−Sca-1+c-Kit+ cells inPbx1−/−FL. Note that the absolute numbers of lin−Sca-1+c-Kit+AA4.1+cells per FL, however, are reduced (wt = 2.8 × 104cells per FL; Pbx1−/−= 1.6 × 104 cells per FL). Results are shown for single animals of each genotype. (B) In vitro clonogenic potential is shown for HSC-enriched pluripotent progenitors, which were purified by 2 rounds of FACS analysis of cells from 4 pooled FLs of each genotype by means of the FACS gates shown in panel A. (C) Day-8 CFU-S frequency for purified Pbx1−/−andPbx1+/−lin−Sca-1+c-Kit+ cells injected into irradiated wt recipients. Data were obtained from a single experiment (representative of 2) on cells (with 4 or more pooled FLs of each genotype) purified by 2 rounds of FACS sorting by means of the gates shown in panel A.
To determine whether the in vivo clonogenic potential ofPbx1−/− pluripotent progenitors was also impaired, we transplanted graded numbers of purified cells from the HSC-enriched (lin−Sca-1+c-Kit+AA4.1+) population into lethally irradiated mice and assayed the number of colonies that developed 8 days later in the spleen (CFU-S). Whereas day-8 CFU-S activity in adult BM lies within committed myeloerythroid progenitors downstream of HSCs,26-28 day-8 activity in FL arises almost exclusively from the HSC-enriched lin−Sca-1+c-Kit+AA4.1+population.39Lin−Sca-1+c-Kit+AA4.1+cells from Pbx1−/− FL generated approximately 30% to 40% fewer spleen colonies than their wt counterparts (Figure4C), a reduction comparable to that observed in their plating efficiency. A similar reduction in day-8 CFU-S was observed for unfractionated whole FL cells from Pbx1−/−embryos on a per-cell basis (data not shown), indicating that populations other than the tested HSC-enriched fraction did not contain appreciable CFU-S activity.
Pbx1−/−FL pluripotent progenitors exhibit defective multilineage hematopoietic reconstitution
To further determine whether pluripotent hematopoietic progenitors were functionally impaired by Pbx1 deficiency, competitive reconstitution experiments were performed. FL cells (2.5 × 105) from E14 Pbx1−/−embryos or their heterozygous littermates (Ly5.1+) were injected into lethally irradiated F1 mice created by intercrossing strains expressing either of the codominant Ly5.1 or Ly5.2 surface markers. An equal number of competitor FL cells (Ly5.2+) from a normal E14 embryo were injected simultaneously. Engraftment of recipient mice was monitored over time by FACS analysis of peripheral blood cells for expression of Ly5 allotypes. The ratio of Ly5.1- to Ly5.2-derived cells was determined after gating out residual recipient-derived Ly5.1/Ly5.2 double-positive cells. In addition, the Ly5.1/Ly5.2 ratio was analyzed in specific lineages by means of myeloid and lymphoid surface antigens. At the earliest time point analyzed, Pbx1−/−-derived peripheral blood cells were noticeably rarer thanPbx1+/−-derived cells (Figure5A). By 4 months,Pbx1+/−-derived progenitors had engrafted and were contributing to myeloid and lymphoid reconstitution equally with wt progenitors, suggesting no heterozygous deficit inPbx1+/− HSCs as tested by this functional assay. Pbx1−/− progenitors, in contrast, contributed to fewer than 2% of engrafted hematopoietic cells. Myeloid and lymphoid lineages in the latter animals were derived almost exclusively from the Ly5.2-expressing (ie,Pbx1+/+) competitor cells (Figure 5B).
Relative defects in multilineage hematopoietic repopulation by
Pbx1−/− FL cells. (A) Competitive repopulation assays show thatPbx1+/−(Ly5.1+) FL cells contributed equally with wt (Ly5.2+) competitor cells at 18 weeks after transplantation while Pbx1−/−(Ly5.1+) FL cells contributed to fewer than 5% of peripheral blood leukocytes. Data points represent the mean values for 10 recipient animals in each cohort transplanted with FL cells pooled from at least 4 embryos of each genotype. (B) Analysis ofPbx1+/−and Pbx1−/−FL cell contribution to myeloid (Gr-1, Mac-1) and lymphoid (B220, CD3) competitive reconstitution at the 18-week time point. (C) Radioprotection assays show the percentage of mice surviving at least 30 days following lethal irradiation. Data are expressed as a function of transplanted cell dose for Pbx1+ (pooled wt and Pbx1+/−) and Pbx1−/−FL donor cells. Data are from representative experiments replicated at least twice with the use of FL cells pooled from 4 or more embryos of each genotype. (D) Southern blots of genomic DNA from whole BM of wt mice radioprotected by Pbx1+ orPbx1−/−FL cells showing wt and disrupted (knockout [ko]) Pbx1 alleles. Pbx1+denotes data pooled from wt and heterozygous cells. The probe consisted of a DNA fragment flanking (3′) and external to the Pbx1targeting construct. The endogenous wt band served as an internal single-copy control signal. The presence of ko allele in BM of mice reconstituted with Pbx1−/−FL cells indicates that they contributed to long-term reconstitution. Data are shown for 2 representative recipient mice at 3 different concentrations of injected FL cells pooled from at least 4 FLs of each genotype.
Relative defects in multilineage hematopoietic repopulation by
Pbx1−/− FL cells. (A) Competitive repopulation assays show thatPbx1+/−(Ly5.1+) FL cells contributed equally with wt (Ly5.2+) competitor cells at 18 weeks after transplantation while Pbx1−/−(Ly5.1+) FL cells contributed to fewer than 5% of peripheral blood leukocytes. Data points represent the mean values for 10 recipient animals in each cohort transplanted with FL cells pooled from at least 4 embryos of each genotype. (B) Analysis ofPbx1+/−and Pbx1−/−FL cell contribution to myeloid (Gr-1, Mac-1) and lymphoid (B220, CD3) competitive reconstitution at the 18-week time point. (C) Radioprotection assays show the percentage of mice surviving at least 30 days following lethal irradiation. Data are expressed as a function of transplanted cell dose for Pbx1+ (pooled wt and Pbx1+/−) and Pbx1−/−FL donor cells. Data are from representative experiments replicated at least twice with the use of FL cells pooled from 4 or more embryos of each genotype. (D) Southern blots of genomic DNA from whole BM of wt mice radioprotected by Pbx1+ orPbx1−/−FL cells showing wt and disrupted (knockout [ko]) Pbx1 alleles. Pbx1+denotes data pooled from wt and heterozygous cells. The probe consisted of a DNA fragment flanking (3′) and external to the Pbx1targeting construct. The endogenous wt band served as an internal single-copy control signal. The presence of ko allele in BM of mice reconstituted with Pbx1−/−FL cells indicates that they contributed to long-term reconstitution. Data are shown for 2 representative recipient mice at 3 different concentrations of injected FL cells pooled from at least 4 FLs of each genotype.
The autonomous reconstitution potential ofPbx1−/− FL progenitors was also tested in radioprotection experiments. Lethally irradiated wt recipient mice were transplanted with graded doses of Pbx1−/− andPbx1+/− whole FL cells and assayed for survival of at least 30 days (radioprotection). At doses ranging from 5 × 104 to 5 × 105 cells per animal,Pbx1−/− FL cells were only half as effective at radioprotection as Pbx1+ cells (pooled from heterozygous and wt embryos) (Figure 5C). As expected, mice that did not receive transplants died within 2 weeks of irradiation owing to hematopoietic failure (not shown).
All of the radioprotected mice survived 6 months after transplantation, allowing an evaluation of the long-term repopulating capacity ofPbx1−/− HSCs in noncompetitive transplantation conditions. Analysis of peripheral blood at 6 months showed no significant differences between recipients ofPbx1−/− and Pbx1+grafts in terms of hemoglobin content, leukocyte counts, or platelet counts (data not shown). By Southern blot analysis, the disruptedPbx1 allele was readily detected in genomic DNA from the BM of transplant recipients. In recipients of Pbx1−/−FL, the intensity of the knockout band relative to the wt band suggested that as many as half of the cells in each BM sample were derived from Pbx1−/− progenitors (Figure5D). Taken together, these data suggested that althoughPbx1−/− progenitors failed to compete with wt progenitors in a transplantation setting, the impairment of function was not absolute when large doses of Pbx1−/−cells were transplanted alone.
Absolute and relative numbers of CMP are reduced inPbx1−/−FL
We next examined recently identified myeloerythroid-committed progenitor subsets downstream of HSCs in E14 FL.39 The relative frequencies of each population inPbx1−/− and wt FL were examined by flow cytometry. In wt or Pbx1+/− FL, CMPs (CD34+/FcγRlo) composed approximately 1% of whole FL cells (Figure 6A). In contrast,Pbx1−/− CMPs were substantially reduced in frequency to approximately 0.4%, on average. This decrease in relative numbers corresponded to an approximate 5-fold decrease in their absolute numbers per Pbx1−/− liver (Figure6B). MEPs and GMPs were present at roughly normal relative frequencies within Pbx1−/− FL (Figure 6A). The absolute numbers of MEPs and GMPs, however, were decreased approximately 2-fold in direct proportion to the overall liver size (Figure 6B) and paralleled reductions in erythroid and GM-CFCs (Figure 3C).
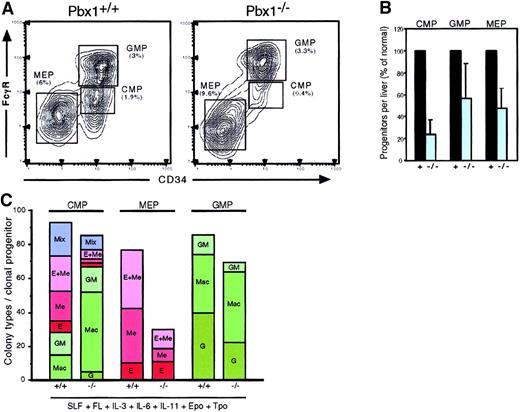
Reduced frequency and altered lineage potentials of common myeloid progenitors.
(A) Flow cytometric analysis of hematopoietic progenitor populations in wt (left) and Pbx1−/−(right) FL at E14.5. Percentages for each subset are calculated from the lin−c-Kit+Sca-1− population. (B) Total numbers of progenitors of each type per FL are expressed as percentage of wild type. (C) Cumulative colony types are shown for purified progenitors that were plated as single cells in methylcellulose cultures. CMPs (FcγRlo/CD34+) gave rise to macrophage (Mac), granulocyte/macrophage (GM), erythroid (E), erythroid/megakaryocytic (E + Me), and mixed colonies. MEPs (FcγRlo/CD34−) produced only E, Me, and E + Me colonies, but fewer than 25% ofPbx1−/−MEPs produced colonies. Pbx1+ denotes data pooled from wt and heterozygous cells.
Reduced frequency and altered lineage potentials of common myeloid progenitors.
(A) Flow cytometric analysis of hematopoietic progenitor populations in wt (left) and Pbx1−/−(right) FL at E14.5. Percentages for each subset are calculated from the lin−c-Kit+Sca-1− population. (B) Total numbers of progenitors of each type per FL are expressed as percentage of wild type. (C) Cumulative colony types are shown for purified progenitors that were plated as single cells in methylcellulose cultures. CMPs (FcγRlo/CD34+) gave rise to macrophage (Mac), granulocyte/macrophage (GM), erythroid (E), erythroid/megakaryocytic (E + Me), and mixed colonies. MEPs (FcγRlo/CD34−) produced only E, Me, and E + Me colonies, but fewer than 25% ofPbx1−/−MEPs produced colonies. Pbx1+ denotes data pooled from wt and heterozygous cells.
Absence of Pbx1 leads to deficiencies in the clonogenic potentials of CMPs and MEPs
The erythroid colony–forming potentials of CMPs and MEPs were assayed since these subsets appear to be the major erythropoietic progenitors in FL.39 These progenitors, as well as GMPs, were enriched to greater than 99% purity by 2 successive rounds of flow cytometry, and cells were individually sorted into single wells containing methylcellulose media as described by Akashi et al.24 Even though sorted singlePbx1−/− CMPs had normal overall plating efficiency, they produced approximately 3-fold fewer erythroid, megakaryocytic, and mixed erythroid/ megakaryocytic colonies than did their wt counterparts (Figure 6C). The skewed in vitro differentiation of Pbx1−/− CMPs toward the myelomonocytic pathway suggested that Pbx1 was required for the efficient production of erythroid colonies. Furthermore, only 30% ofPbx1−/− MEPs produced a visible colony after 7 days in culture, as compared with 80% of wt MEPs (Figure 6C). The colonies that were produced by Pbx1−/− MEPs were comparable in size to the wt MEP colonies and contained morphologically normal-appearing erythroid and megakaryocytic cells (not shown). These data suggested that the reduced ability ofPbx1−/− MEPs and CMPs to produce erythroid-committed progeny directly underlies the anemia inPbx1−/− embryos. As expected, no colony-forming activity was observed in the lin+ or c-Kit− fractions of whole FL (data not shown), indicating that the observed reductions were not a consequence of altered cell surface phenotypes in Pbx1−/− progenitors.
Numerical decrease in Pbx1−/−FL CMPs is associated with reduced proliferation
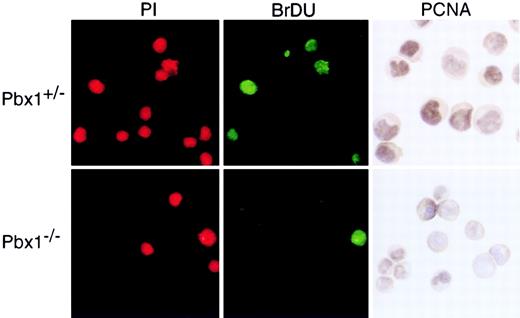
We determined the proliferative and apoptotic indices of purified CMPs to evaluate the basis for their markedly reduced numbers inPbx1−/− FL. Cytospin preparations of the c-Kit+CD34+FcγRlo populations were fixed and stained for PCNA or used for TUNEL assays. The latter showed that less than 5% of each progenitor population was apoptotic and revealed no differences between wt and Pbx1−/−progenitor populations (data not shown). Approximately 80% of CMPs from wt embryos exhibited nuclear PCNA staining (Figure7), consistent with their high proliferative index (D.T., unpublished results, 2000). In contrast, only 20% to 25% of the Pbx1−/−CMPs showed similar staining. Comparison of GMP and MEP populations stained for PCNA revealed no significant differences betweenPbx1−/− and wt (not shown). To confirm these results, in vivo BrdU-labeling studies were performed. Cytospin preparations of flow-sorted CMPs stained for BrdU incorporation showed reductions in S-phase cells that paralleled the decreased fraction ofPbx1−/− CMPs observed by PCNA staining (Figure7). These data indicated that FL CMPs normally constitute a highly proliferative subset and suggested that reduced cycling ofPbx1−/− CMPs probably contributed to their proportionally decreased numbers within the progenitor compartment at E14.
Proliferative indices of common myeloid progenitors.
CMPs were purified by flow cytometry, and proliferation was measured by BrdU incorporation and PCNA staining of cytospin preparations. ForPbx1+/−CMPs, 40% of cells that stained with propidium iodide showed incorporation of BrdU into their DNA during the 90-minute labeling period, and 80% showed nuclear punctate PCNA staining. Equivalent staining of Pbx1−/−CMPs was 20% for BrdU and 25% for PCNA.
Proliferative indices of common myeloid progenitors.
CMPs were purified by flow cytometry, and proliferation was measured by BrdU incorporation and PCNA staining of cytospin preparations. ForPbx1+/−CMPs, 40% of cells that stained with propidium iodide showed incorporation of BrdU into their DNA during the 90-minute labeling period, and 80% showed nuclear punctate PCNA staining. Equivalent staining of Pbx1−/−CMPs was 20% for BrdU and 25% for PCNA.
Discussion
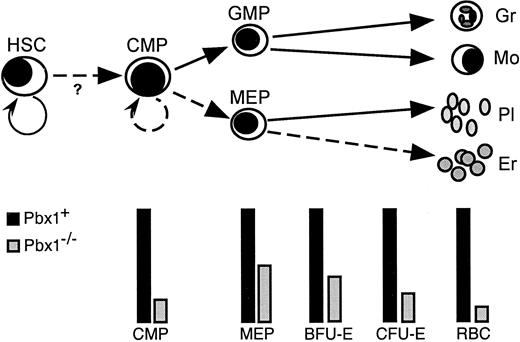
The studies in this report demonstrate that the Hox cofactor and proto-oncoprotein Pbx1 is essential for the adequate maintenance of definitive hematopoiesis. This role is consistent with our finding of Pbx1 expression in pluripotent and lineage-restricted progenitors at sites of embryonic hematopoiesis. Lack of Pbx1 expression in these tissues results in FL hypoplasia and anemia, associated with lethality in late gestation. Impaired function of Pbx1−/−cells along the hematopoietic developmental pathway from HSCs to MEPs suggests that additive defects underlie the profound anemia in these mice. The defects manifest as reduced numbers and/or impaired functions of HSCs, CMPs, and MEPs, including reduced differentiation toward erythroid cells (Figure 8). In addition to diminished production of erythroid colonies by CMPs and MEPs, decreased proliferation of CMPs further limits the production of erythrocytes. The association of Pbx1 with the extent of progenitor expansion has important implications for its mutational activation in a subset of acute leukemias.
Schematic model of myeloid differentiation showing alterations induced by lack of Pbx1.
Semicircular arrows represent self-renewal divisions. Solid lines represent developmental pathways spared by absence of Pbx1 while dashed lines denote affected pathways. Bars beneath the schematic indicate relative levels of various progenitor populations. The lineal relationships of BFU-E and CFU-E with FL MEP have not been experimentally determined and may not be linear as shown.39 Gr indicates granulocyte; Mo, monocyte; Pl, platelet; Er, erythrocyte; RBC, red blood cell; ?, uncertain effect on transition of HSCs to CMPs.
Schematic model of myeloid differentiation showing alterations induced by lack of Pbx1.
Semicircular arrows represent self-renewal divisions. Solid lines represent developmental pathways spared by absence of Pbx1 while dashed lines denote affected pathways. Bars beneath the schematic indicate relative levels of various progenitor populations. The lineal relationships of BFU-E and CFU-E with FL MEP have not been experimentally determined and may not be linear as shown.39 Gr indicates granulocyte; Mo, monocyte; Pl, platelet; Er, erythrocyte; RBC, red blood cell; ?, uncertain effect on transition of HSCs to CMPs.
The hematopoietic failure in Pbx1−/− embryos is distinctly different from that of previously reported knockout mice. The survival of Pbx1−/− embryos to E15 and E16 and the presence of nucleated erythrocytes indicate thatPbx1 does not play an essential role at the yolk sac stage of hematopoiesis. Mutations affecting primitive hematopoiesis typically result in death by E10.5.29-32 Furthermore, the relatively abundant enucleate red blood cells in Pbx1−/−embryos at E15.5 indicate that transition to definitive hematopoiesis has occurred, since primitive erythrocytes do not extrude their nuclei. These features contrast with murine embryonic phenotypes associated with targeted disruption of genes essential for the transition from primitive to definitive hematopoiesis. For example, the peripheral blood of embryos nullizygous for the Aml1proto-oncogene, is composed almost exclusively of nucleated erythrocytes prior to death at E12.5.33 Similarly, the majority of circulating erythrocytes in embryos lackingc-Myb or Rb are of primitive origin as late as E15.34-36 The presence of extensive hemorrhages inAml1, c-Myb, and Rb knockout embryos also distinguishes them from Pbx1−/− embryos, implying that the production of platelets as well as definitive erythrocytes is more efficient in the latter. Thus, definitive hematopoiesis is clearly initiated in Pbx1−/−embryos, but it fails to keep pace with the rapid growth of the fetus, in contrast to the more severe abrogations of definitive hematopoiesis reported in previous knockout models.
The failure to adequately maintain definitive hematopoiesis inPbx1−/− embryos appears to be the cumulative result of both quantitative and qualitative defects at several stages in the development of multilineage hematopoietic progenitors.Pbx1−/− HSCs exhibit decreased clonogenic potential in vitro and in vivo. The 5-fold decrease in numbers of CMPs in Pbx1−/− FL imposes a further limitation on the production of erythroid progeny by Pbx1−/−HSCs. Normally, the majority of erythroid-colony activity in FL appears to reside in CMPs as evidenced by their ability to give rise to numerous robust BFU-E, which contrasts with the greater erythropoietic role for MEPs in adult BM.39Pbx1−/− CMPs, however, produce predominantly GM colonies, in contrast to normal FL CMPs, which typically display even greater megakaryocyte-erythroid production (2-fold) when compared with adult CMPs. Thus, the reduced frequency and skewed differentiation capacity of Pbx1−/− CMPs are likely to have a substantially greater impact on erythropoiesis than might be expected from the more modestly reduced numbers of FL MEPs. Finally, Pbx1−/− MEPs themselves show a marked decrease in clonogenic potential. The summation of these incremental deficiencies manifests as substantial reductions in FL CFCs and severe anemia. Although the loss of Pbx1 could potentially alter the phenotypes of functionally defined FL progenitors to account for our observations, several lines of evidence indicate that this is not the case. First, no significant colony-forming activity was detected in the lin+ or c-Kit− populations. Thus, in the testing of essentially all phenotypic populations in the FL, virtually all of the colony-forming activity was within the c-Kit+fraction, as is the case in wt mice. Furthermore, each of the CMP, MEP, and GMP populations showed appropriate lineage restriction in the in vitro clonogenic assays, supporting our conclusions that the respective populations were similar in wt and Pbx1−/−FLs, with the latter showing functional deficits.
An additive phenotype is consistent with the proposed role of Pbx1 as a cofactor for multiple homeodomain proteins during hematopoietic development. Accordingly, while mice deficient for individualHox genes such as HoxA9, HoxB6, orHoxC8 exhibit some hematopoietic abnormalities,14-16 the abnormalities are not as severe as those we have described in Pbx1−/− embryos. By comparison, mice deficient for MLL, a protein required for the maintenance of expression of multiple Hox genes, exhibit complete failure of FL hematopoiesis.31 37 Thus, while the loss of individual Hox genes may be compatible with life, the simultaneous disruption of multiple Hox-dependent pathways in hematopoietic development produces catastrophic defects. The severity of the hematopoietic defects in Pbx1−/− mice argues that Pbx1, like MLL, may be required for the regulation of multiple Hox-dependent developmental pathways.
While we observed defects in multiple subsets ofPbx1−/− hematopoietic progenitors, the single most drastic progenitor perturbation was a reduction in the absolute number (5-fold) of FL CMPs. The bottleneck created by this reduction in CMPs is more likely to impair multilineage hematopoiesis than the modest decreases in HSC clonogenic potential. Limited production of downstream progenitors due to inadequate numbers of CMPs could account for the inability of Pbx1−/− HSCs to compete with wt HSCs, yet allow for the establishment of long-term hematopoiesis under noncompetitive conditions. The failure ofPbx1−/− HSCs to contribute to T- or B-cell reconstitution in competitive transplantations may imply a similar role for Pbx1 at the level of a common lymphoid progenitor.19
The substantial reduction in the proliferative index ofPbx1−/− CMPs suggests that their insufficient expansion accounts for most if not all of the reduction in their numbers. It is not clear whether this decreased proliferation results from the absence of Pbx1 in the CMPs themselves or from micro-environmental defects in Pbx1−/− FL. However, the impaired in vitro clonogenic properties of purified CMPs as well as MEPs demonstrate at least partial cell-autonomous contributions. Our observations implicate Pbx1 transcriptional complexes in the mitotic expansion of the CMP hematopoietic progenitor subset. The suggestion that proliferative expansion of immature cells requires Pbx1 is not limited to the hematopoietic system. Most organs are either hypoplastic or aplastic in Pbx1−/−embryos. Furthermore, analysis of skeletal defects inPbx1−/− embryos40 suggests that chondrocyte proliferation is markedly diminished while chondrocyte maturation is accelerated. The significance of these findings in understanding the mechanism of lymphoid leukemogenesis by E2a-Pbx1 is underlined by the observation that expression of this fusion protein in murine myeloid progenitors leads to enhanced self-renewal (K. Smith and M.L.C., unpublished data, 1999) and myeloid leukemia.38 By indiscriminately activating Pbx1 target genes, this oncoprotein may similarly lead to excessive self-renewal of lymphoid progenitors, eventually resulting in acute leukemia.
The authors thank Carmencita Nicholas and Eva Pfendt for expert technical assistance, Phil Verzola for photographic assistance, Ramesh A. Shivdasani for instruction in the determination of fetal hematocrits, Koichi Akashi for assistance with the ACDU, and Len Zon for critical review of the paper.
Supported by grants from the National Cancer Institute (CA70404, CA42971, and CA42551). J.F.D. was supported by Public Health Service grant 5T32-CA09151 awarded by the National Cancer Institute, and D.T. was supported by National Institute of Allergy and Infectious Diseases grant 5T32 AI-07290.
J.F.D., L.S., and D.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael L. Cleary, Department of Pathology, Stanford University School of Medicine, Stanford, CA 94305; e-mail: mcleary@stanford.edu.

![Fig. 5. Relative defects in multilineage hematopoietic repopulation by. / Pbx1−/− FL cells. (A) Competitive repopulation assays show thatPbx1+/− (Ly5.1+) FL cells contributed equally with wt (Ly5.2+) competitor cells at 18 weeks after transplantation while Pbx1−/−(Ly5.1+) FL cells contributed to fewer than 5% of peripheral blood leukocytes. Data points represent the mean values for 10 recipient animals in each cohort transplanted with FL cells pooled from at least 4 embryos of each genotype. (B) Analysis ofPbx1+/− and Pbx1−/− FL cell contribution to myeloid (Gr-1, Mac-1) and lymphoid (B220, CD3) competitive reconstitution at the 18-week time point. (C) Radioprotection assays show the percentage of mice surviving at least 30 days following lethal irradiation. Data are expressed as a function of transplanted cell dose for Pbx1+ (pooled wt and Pbx1+/−) and Pbx1−/−FL donor cells. Data are from representative experiments replicated at least twice with the use of FL cells pooled from 4 or more embryos of each genotype. (D) Southern blots of genomic DNA from whole BM of wt mice radioprotected by Pbx1+ orPbx1−/− FL cells showing wt and disrupted (knockout [ko]) Pbx1 alleles. Pbx1+denotes data pooled from wt and heterozygous cells. The probe consisted of a DNA fragment flanking (3′) and external to the Pbx1targeting construct. The endogenous wt band served as an internal single-copy control signal. The presence of ko allele in BM of mice reconstituted with Pbx1−/− FL cells indicates that they contributed to long-term reconstitution. Data are shown for 2 representative recipient mice at 3 different concentrations of injected FL cells pooled from at least 4 FLs of each genotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/3/10.1182_blood.v98.3.618/5/m_h81511358005.jpeg?Expires=1766020442&Signature=JfY9jZvsP~x~0eL65nxNRqRnLjieZEAbvaG51w04YYEIGpDuTYpDLAzbSHc7ItWgd67GZs6jKL5UAjqmCXgjKwqAaqgyj6q25lkDDLhvWIM4bCmxOTU-sTm1F21AymiorZcmOP~PPju4Fb00FGasSRgDolV5b7HnR2m-R6hElJ0u8nwG65NNUhxWQIxS2XqzfDDSrNXekFiwazyJHCpN6YOHhNpYqRUg72D4289VmTJ1pzcEo1CwiMplgm-38V5o3dtYLlzyYzJrDAYFWTu80UOsoeHF1-wDD93jhmKMkd0iwqqh2HQ~dFR2CJdWRF4eXbKe1TqbRLVhPNZkshEkuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)