Previous trials of allogeneic bone marrow transplantation (BMT) in patients with multiple myeloma (MM) have demonstrated high response rates but also high transplantation-related mortality (TRM) and high relapse rates. Exploitation of this strategy remains of interest because donor lymphocyte infusions (DLIs) can induce a potent graft-versus-myeloma (GVM) effect. CD6 T-cell–depleted allogeneic BMT was combined with prophylactic CD4+ DLI administered 6 to 9 months after BMT in an effort to reduce TRM and to induce a GVM response after BMT. Twenty-four patients with matched sibling donors and chemotherapy-sensitive disease underwent BMT. CD6 T-cell depletion of donor bone marrow was the sole method of graft-versus-host disease (GVHD) prophylaxis. GVHD after BMT was minimal, 1 (4%) grade III and 4 (17%) grade II GVHD. Fourteen patients received DLI, 3 in complete response and 11 with persistent disease after BMT. Significant GVM responses were noted after DLI in 10 patients with persistent disease, resulting in 6 complete responses and 4 partial responses. After DLI, 50% of patients developed acute (≥ II) or extensive chronic GVHD. Two-year estimated overall survival and current progression-free survival (PFS) for all 24 patients is 55% and 42%, respectively. The 14 patients receiving DLI had an improved 2-year current PFS (65%) when compared with a historical cohort of MM patients who underwent CD6-depleted BMT survived 6 months with no GVHD and did not receive DLI (41%) (P = .13). Although this study suggests that prophylactic DLI induces significant GVM responses after allogeneic BMT, only 58% of patients were able to receive DLI despite T-cell–depleted BMT. Therefore, less toxic transplantation strategies are needed to allow a higher proportion of patients to receive DLI and the benefit from the GVM effect after transplantation.
Introduction
Previous studies of allogeneic bone marrow transplantation (BMT) in patients with multiple myeloma (MM) have reported complete response (CR) rates of 36% to 58% after BMT, but only 10% to 20% of patients achieve long-term remission. The European Bone Marrow Transplant Registry (EBMTR) reports a 44% CR rate, but an overall survival (OS) of only 32% for patients 4 years after transplantation.1 Bensinger et al2 reported 36% CRs in patients with chemotherapy-refractory MM, but OS was 20%, and progression-free survival (PFS) was only 16% at 5 years. High treatment-related mortality (TRM), up to 50% in some studies, contributes to the poor survival. This high TRM is most commonly related to complications of acute graft-versus-host disease (GVHD) and infections after BMT. This high TRM and high relapse rates after allogeneic BMT have limited the effectiveness of this approach in patients with MM. Although T-cell depletion of donor bone marrow (BM) has been shown to reduce the incidence of acute GVHD as well as other transplantation-related complications in MM, T-cell depletion is associated with abrogation of the graft-versus-leukemia (GVL) effect after transplantation.3 4 Therefore, measures to minimize TRM while maintaining GVL are urgently needed.
Donor lymphocyte infusions (DLI) have emerged as an effective method to treat patients who have relapsed after allogeneic BMT. DLI is effective in inducing a GVL response in patients with chronic myelogenous leukemia (CML), and approximately 70% of patients with chronic phase CML who receive DLI will achieve a cytogenetic CR.5,6Several studies have also demonstrated significant responses, including CRs, in patients with MM treated with DLI for posttransplant relapse.5,7-9 Overall, there appears to be a 40% to 60% response rate in patients with relapsed MM treated with DLI.10 In some MM patients, remissions appear to be durable and sustained for up to 6 years after DLI. These clinical responses confirm the existence of an allogeneic graft-versus-myeloma (GVM) effect mediated by DLI. In CML, treatment with DLI early in relapse (ie, cytogenetic relapse) is associated with an increased response rate and with a reduced incidence of complications compared with treatment of patients in more advanced stages of relapse.11 However, the role and optimal timing of DLI after BMT in patients with MM, who are at high risk for relapse, remain to be defined.
In the present study, we explored the use of CD6 T-cell depletion at the time of allografting in MM to reduce the high incidence of treatment-related complications. In an attempt to induce a GVM effect, which may be compromised by the T-cell depletion procedure, prophylactic CD4+ DLI was administered 6 to 9 months after transplantation. Although patients who received T-depleted BMT followed by CD4+ DLI had improved current progression-free survival (PFS) when compared with a similar cohort of patients previously treated with BMT alone, transplantation-related toxicities precluded many patients from receiving DLI. Transplantation preparative regimens that establish donor chimerism without excessive toxicity are therefore necessary to allow for the majority of patients to receive DLI and to derive the benefit of the GVM effect.
Patients and methods
Patients included in this analysis included 24 adults with MM who underwent allogeneic BMT from HLA-identical sibling donors at our institution between January 1, 1996, and September 1, 1999, with follow-up of at least 6 months after BMT. All patients received CD6 T-cell–depleted BMT and were eligible to receive prophylactic CD4+ DLI 6 to 9 months after BMT. The median follow-up among those alive (n = 12) is 2.3 years (range, 1.2 to 3.6). Patients younger than 60 years with a histocompatible sibling donor were eligible to participate. All patients had evidence of chemotherapy-responsive disease prior to BMT that allowed them to achieve less than 40% plasma cells in the BM at the time of transplantation. Treatment protocols were approved by the Institutional Review Board of the Dana-Farber Cancer Institute. Written informed consent was obtained in all cases.
Treatment protocol
Twenty-one patients received high-dose cyclophosphamide and total body irradiation (TBI) as conditioning for transplantation. These patients received cyclophosphamide 60 mg/kg on 2 successive days, followed by 1400-cGy TBI administered twice daily in 7 equal 200-cGy fractions over 3 days. Lung shielding was used to provide uniform radiation dose distribution. All patients receiving TBI were treated on a dedicated facility at 10 cGy/min. Three patients who were unable to receive TBI because of prior local radiotherapy received oral busulfan 16 mg/kg (1 mg/kg every 6 hours for a total of 16 doses) and cyclophosphamide, as described above.
Donor BM was harvested on the last day of ablative therapy, and T-cell depletion was performed by using anti-CD6 monoclonal antibody and complement lysis, as previously described.13 14 CD6 depletion of donor BM was the sole method of GVHD prophylaxis, and no other immune-suppressive medications were administered after BMT. Marrow was infused on the same day it was harvested (day 0). All patients received granulocyte colony-stimulating factor (G-CSF) at 5 μg/kg/d beginning at day +1 and continuing until the absolute neutrophil count exceeded 1 × 103 cells/μL.
All patients were treated in high-efficiency particular air-filtered rooms by using standard reverse isolation procedures. Oral prophylactic antibiotics were administered to all patients. If fever developed, oral antibiotics were discontinued and intravenous broad-spectrum antibiotics were instituted. All blood components were leukoreduced before storage and irradiated (2500 Gy) to prevent transfusion-associated GVHD.
CD4+ donor lymphocyte infusion
All patients, including patients who obtained a CR after BMT, were eligible to receive DLI 6 to 9 months after BMT if there was no clinical or laboratory evidence of GVHD and if they were not receiving medication to either prevent or to treat GVHD. Donor lymphocytes were obtained from the original BM donor by apheresis using peripheral venous access. The collection and CD8 depletion to enrich for CD4+ DLI has been described previously.7 The first 11 patients received a single infusion of 3 × 107CD4+ cells/kg, and 3 patients received a single infusion of 1 × 107 CD4+ cells/kg. No other immune-modulating therapy was given after DLI, and no GVHD prophylaxis was used.
Definitions of response
Responses were classified by using standard criteria. Immunoelectrophoresis and immunofixation were used to evaluate for the presence of serum and/or urine monoclonal immunoglobulin. Marrow examination included staining to demonstrate immunoglobulin light chain restriction. A CR was defined by the presence of less than 5% polyclonal plasma cells in the BM and the absence of a monoclonal protein in serum or urine by immunofixation. A partial response (PR) was the presence of a decrease of 50% or more in the serum and/or urine monoclonal protein and less than 10% monoclonal plasma cells on BM biopsy. Stable disease was a less than 50% reduction in paraprotein. Progressive disease was defined as the reappearance of serum and/or urine monoclonal protein for patients in CR, or more than 25% increase in serum and/or urine monoclonal protein from the lowest value observed for patients in PR.
Statistical analysis
Patient characteristics are summarized using proportions and medians. OS and PFS curves were constructed using the method of Kaplan and Meier.12 Current PFS is the time from BMT to the first relapse, progression, or death among those patients who did not receive DLI and is the time from BMT to the first relapse, progression, or death after DLI among those patients who received DLI.13Cox proportional hazards model was used in multivariate modeling to determine predictors of OS and PFS for all patients. Comparisons between the patients receiving CD6-depleted BMT and DLI and patients receiving CD6-depleted BMT alone were done using the Fisher exact test, Wilcoxon test, and the log rank test where appropriate.14A landmark analysis was performed to compare OS and PFS in the patients receiving CD6-depleted BMT plus DLI compared with patients receiving CD6-depleted BMT alone. In this analysis patients who received DLI after BMT were compared with a cohort of patients treated with CD6-depleted BMT alone who otherwise would have been candidates to receive DLI. This cohort was defined as those who were alive at the median time of DLI (6.4 months) and had not developed GVHD after BMT. This analysis was done because those groups provide a better comparison to assess the effect of DLI.
Results
Patients treated
A total of 24 patients underwent allogeneic BMT (Table1). The median age of all patients was 46 years (range, 36-54). There were 12 men and 12 women. The majority (54%) of patients had Durie-Salmon Stage III disease at diagnosis. Sixty-six percent of patients had immunoglobulin (Ig) G myeloma, 17% IgA, and 17% light chain only. All patients had evidence of chemotherapy-sensitive disease at the time of transplantation. The median time from diagnosis to BMT was 10 months. Patients had received a median of 3 regimens (range, 1-7) before BMT. Four (17%) patients were in CR at the time of BMT. Twenty patients had evidence of persistent disease at the time of BMT. Ablative therapy included cyclophosphamide and TBI in 21 patients (88%); cyclophosphamide and busulfan were used in 3 patients (12%).
Engraftment
All patients engrafted after infusion of CD6 T-cell–depleted BM. With the use of G-CSF, the median time to reach an absolute neutrophil count more than 500/μL was 12 days (range, 10-17) and platelets more than 20 000/μL was 19 days (range, 16-28). The median duration of hospitalization was 21 days (range, 18-33). The median number of nucleated cells infused was 3.98 × 107 cells/kg (range, 1.51-8.89). The median number of CD3+ cells/kg infused was 4.0 × 105 cells/kg (range, 0.80-24.71). The median number of CD34+ cells infused was 1.4 × 106CD34+ cells/kg (range, 0.49-3.89).
Response to BMT
Of the 4 patients who were in CR at the time of BMT, 2 patients remained in CR at 6 months after BMT, 1 patient developed progressive disease, and 1 patient died early after BMT of a treatment-related complication (Table 2). Twenty patients had evidence of persistent disease at the time of BMT. Six months after BMT, 1 of these patients achieved a CR, 8 patients a PR, and 10 evaluable patients (50%) had either stable or progressive disease. A single patient died less than 100 days after BMT.
Response to CD4+ DLI
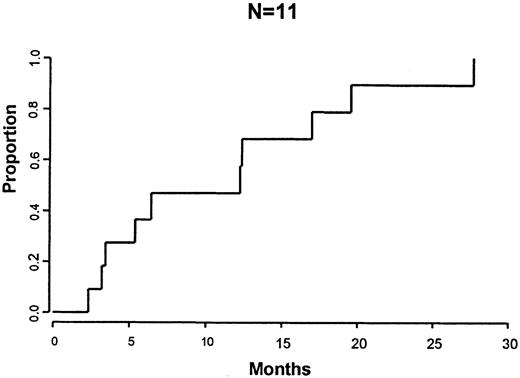
Patients were eligible to receive CD4+ DLI 6 to 9 months after BMT as prophylaxis if they had no evidence of GVHD and were not receiving immune-suppressive medications. Fourteen of 24 (58%) patients received DLI at 6 to 9 months after DLI. Three patients were in CR and 11 patients had persistent disease at the time of DLI. CD8 depletion of the DLI product resulted in a 98.8% (range, 94%-100%) depletion of CD8+ cells. Two of 14 patients received DLI that contained no detectable CD8+ cells by flow cytometry. Twelve patients received DLI that contained a median of 1.7 × 105 CD8+ cells/kg (range, 1.2-2.3). Of the 11 patients with persistent disease receiving DLI, 6 patients developed a CR and 4 patients a PR after DLI. The remaining patient has stable disease 6 months after DLI with a stable monoclonal protein The median time to best response was 6.4 months (range, 2-28) after DLI (Figure 1). The median time to CR after DLI was 11.7 months.
Time from DLI to response.
Time from DLI to maximal response following CD4+ DLI for the 10 patients with persistent disease after BMT.
Time from DLI to response.
Time from DLI to maximal response following CD4+ DLI for the 10 patients with persistent disease after BMT.
Overall, 5 (36%) patients remain in a CR after DLI with a median follow-up of 28 months (range, 15-43) after DLI. Of the 3 patients who received DLI while in CR, 1 patient has relapsed. One patient who achieved a CR after DLI died of a myocardial infarction 4 years after DLI while in remission. Of the 11 patients with persistent disease at the time of BMT, 1 patient who obtained a CR after DLI relapsed 12 months after DLI, and 2 patients who achieved a PR after DLI developed progressive disease 6 and 12 months after DLI.
Ten (42%) patients were not able to receive DLI at 6 to 9 months after DLI. Of these 10 patients, 3 patients had developed either complications of GVHD or had ongoing GVHD that precluded DLI. Three patients developed other transplantation-related complications after BMT including veno-occlusive disease, interstitial pneumonitis, and Epstein-Barr virus (EBV) posttransplant lymphoma that prevented planned CD4+ DLI. Two patients had early relapse and died of progressive disease. One patient had an active infection that prevented DLI, and a single patient refused.
Treatment-related toxicity associated with BMT and DLI
Five of 24 (21%) patients developed evidence of acute grade II-IV GVHD after T-cell–depleted BMT with 4 patients developing grade II GVHD and 1 (4%) patient with grade III GVHD. Five (21%) patients died of transplantation-related complications, including complications related to GVHD in 2 cases and veno-occlusive disease, EBV non-Hodgkin lymphoma–lymphoproliferative disease, and noninfectious interstitial pneumonitis in the remaining 3 cases.
The development of GVHD was the most significant complication of DLI. Overall 7 of 14 (50%) patients receiving DLI developed grade II-IV acute GVHD or extensive chronic GVHD. One patient died of complications of GVHD following DLI. With long-term follow-up, 3 patients who received DLI remain in CR without evidence of active GVHD. Five patients developed evidence of extensive chronic GVHD. No patients developed evidence of pancytopenia following DLI.
The development of GVHD in 5 patients after BMT was not associated with significant disease responses. Two of these 5 patients subsequently developed evidence of progressive disease despite the presence of GVHD. Significant acute or chronic GVHD occurred in 7 patients (50%) after DLI. Six of 7 patients demonstrated initial evidence of a GVM response at the time of the development of GVHD and before the initiation of steroid therapy for GHVD. The development of GVHD after DLI was not protective of relapse, however, because 2 patients with GVHD subsequently developed evidence of progressive disease. Six patients had evidence of a GVM response in the absence of GVHD with 3 patients achieving CR. One patient with stable disease at 6 months after DLI has no evidence of GVHD.
Overall response to BMT and DLI
With a median follow-up of 2.3 years among those patients alive, the estimated 1- and 2-year OS for all 24 patients enrolled in the study are 71% (95% confidence interval [CI], 53%-89%) and 55% (95% CI, 34%-76%), respectively. The estimated 1- and 2-year PFS is 54% (95% CI, 34%-74%) and 30% (95% CI, 10%-50%), respectively. The 100-day mortality was 4%.
Comparison with the outcome of patients treated with T-cell–depleted BMT alone
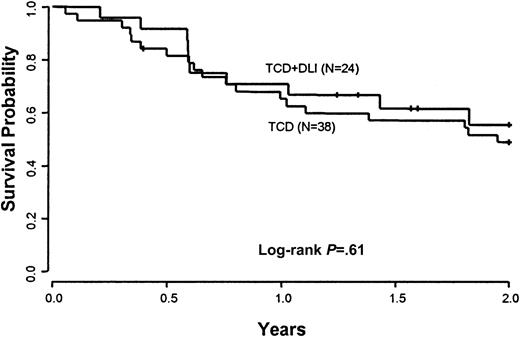
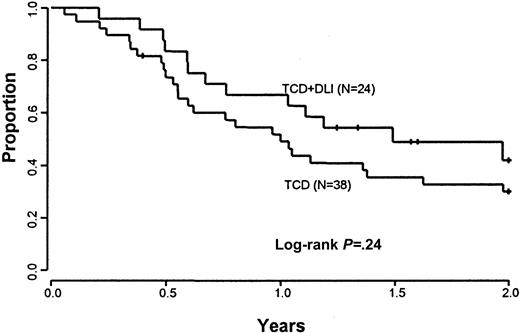
A comparison was performed between patients treated with CD6-depleted BMT and DLI versus a cohort of 38 patients previously treated with CD6-depleted BMT alone at the Dana-Farber Cancer Institute. This control population was similar to the current treatment group in terms of demographic characteristics (Table 1). The conditioning regimens and GVHD prophylaxis with CD6 T-cell depletion were identical. The estimated 2-year OS in the current study of CD6-depleted BMT and DLI of 57% compares with the 2-year OS of 49% (95% CI, 33%-65%) in the group who received CD6-depleted BMT alone (P = .88) (Figure 2). The estimated 2-year current PFS in the current study of CD6-depleted BMT plus DLI is 42% (95% CI, 20%-64%) as compared with 30% (95% CI, 15%-45%) in the control population (P = .24) (Figure3).
Overall survival.
Comparison of overall survival between patients eligible to receive CD6 T-cell–depleted BMT and CD4+ DLI and a cohort of patients treated previously with CD6 BMT alone.
Overall survival.
Comparison of overall survival between patients eligible to receive CD6 T-cell–depleted BMT and CD4+ DLI and a cohort of patients treated previously with CD6 BMT alone.
Current progression-free survival.
Comparison of current progression-free survival between patients eligible to receive CD6 T-cell–depleted BMT and CD4+ DLI and a cohort of patients treated previously with CD6 BMT alone.
Current progression-free survival.
Comparison of current progression-free survival between patients eligible to receive CD6 T-cell–depleted BMT and CD4+ DLI and a cohort of patients treated previously with CD6 BMT alone.
In an attempt to assess the effect of DLI, we performed a landmark analysis comparing OS and PFS within the first 2 years for patients treated with CD6-depleted BMT and DLI compared with control patients treated with CD6-depleted BMT alone. In this analysis, only patients receiving BMT alone who did not develop GVHD and survived 6 months after transplantation were included (n = 22). This comparison was done because these patients would have been eligible to receive DLI and therefore would serve as a better comparison group. The OS at 2 years in this subset of 14 patients who received both CD 6-depleted BMT and DLI, 83% (95% CI, 60%-100%), was similar to the subset of patients who received CD6-depleted BMT alone, 68% (95% CI, 49%-89%) (P = .40). However, in this subset analysis the current PFS at 1 and 2 years after transplantation for the CD6-depleted BMT and DLI group is 93% (95% CI, 80%-100%) and 65% (95% CI, 37%-93%), respectively, compared with 68% (95% CI, 49%-87%) and 41% (95% CI, 20%-62%) in the BMT only group (P = .13) (Figure4). Longer follow-up will be needed to assess the full effect of DLI on PFS.
Current progression-free survival for patients eligible to receive DLI; landmark analysis.
Comparison of current progression-free survival for patients who received CD4+ DLI after BMT compared with a cohort of patients treated previously with CD6 BMT alone who would have been eligible to receive DLI.
Current progression-free survival for patients eligible to receive DLI; landmark analysis.
Comparison of current progression-free survival for patients who received CD4+ DLI after BMT compared with a cohort of patients treated previously with CD6 BMT alone who would have been eligible to receive DLI.
Prognostic features associated with OS and PFS
All 62 patients, including the 24 from the current study and 38 patients who received CD6 T-cell–depleted BMT alone, were analyzed for prognostic features that predicted OS and PFS. Features analyzed included age, stage of disease at diagnosis, MM immunoglobulin class, donor gender–patient gender, and interval from diagnosis to BMT (Table3). Univariate analysis demonstrated that both advanced stage at diagnosis and IgA predicted for worse OS (P = .024 and P = .04, respectively) and worse PFS (P < .001 and P = 0.07, respectively). Response at BMT was also predictive of PFS (P = .01) in univariate analyses. After adjusting for other factors in a multivariate model, stage of disease was determined to be significantly associated with OS and PFS (P = .01 and P < .001, respectively). A 2.5-increased risk of mortality was noted in patients with stage III disease compared with earlier stages of disease at diagnosis. For PFS, the estimated relative risk for stage III patients compared with earlier stages was 3.3. Similar results were found for current PFS, with stage III patients at a 2.7-fold increased risk versus earlier stage disease (P = .004).
Discussion
In the present study, CD4+ DLI induced a significant GVM effect when administered 6 to 9 months after BMT. Thirteen of 14 (93%) patients with persistent disease after transplantation responded to DLI, with 6 patients obtaining a CR. With the addition of DLI to CD6-depleted BMT there appears to be a trend toward improved PFS after transplantation because recipients of CD6-depleted BMT plus DLI have a 2-year current PFS of 65%, compared with 41% in a cohort of patients treated with CD6-depleted BMT alone who would have been eligible to receive DLI. Although improvement in PFS may translate into improved OS with additional follow-up, only 58% of patients in the current study were able to receive DLI after BMT because of BMT-related complications. Therefore, despite the encouraging results of CD6-depleted BMT and CD4+ DLI, not all patients are able to benefit from this treatment strategy because of the toxicities noted after BMT. This experience suggests that new transplantation conditioning approaches are necessary to allow the majority of patients to receive DLI and to benefit from the GVM effect.
Previous reports of allogeneic BMT in patients with MM have documented a high TRM of 25% to 56%.1,2 The high TRM is related to an increased incidence of GVHD in these patients, as well as transplantation-related complications such as veno-occlusive disease, interstitial pneumonitis, and infection. The increased risk of GVHD may be due to the advanced age of patients with MM undergoing transplantation and to the increased risk of infection after transplantation attributable to the prior use of high doses of corticosteroid therapy. Importantly, despite the high TRM, allogeneic BMT results in long-term disease-free survival for some patients. Bensinger et al2 reported a 36% CR rate after allogeneic BMT in patients with highly refractory MM, with an OS and PFS of 20% and 16%, respectively, at 5 years after transplantation. In a more heterogeneous population of patients receiving a variety of transplantation regimens and GVHD prophylaxis, the EBMTR reported a lower (25%) overall TRM.1 In the EBMTR experience, the CR rate was 44%, with an actuarial OS at 4 and 9 years after transplantation of 32% and 18%, respectively. In the current study, the goal was to reduce the TRM using a CD6-depleted BMT and to reduce relapse by administering CD4+ DLI to induce a GVM effect after transplantation. Although significant transplantation-related toxicity was decreased and a potent GVM effect was demonstrated after DLI, complications of BMT still precluded DLI in a significant number of patients.
The significant GVM effect induced by DLI confirms prior observations of anti-MM activity mediated by donor lymphocytes in patients who have relapsed after allogeneic BMT.5,7-9 In a summary of DLI in patients with relapsed MM after allogeneic BMT, 62% of patients demonstrated a response after DLI.10 In the current study, all patients with persistent disease demonstrated a response to DLI, suggesting that early treatment after BMT may improve response rates. This finding mirrors the experience in CML in which DLI is more effective in patients with molecular and cytogenetic evidence of disease after transplantation compared with patients treated in hematologic relapse.
Infusion of a lower number of cells may be able to achieve a significant GVM effect when administered early after BMT. Infusions of more than 1 × 108 cells/kg appear to be needed to treat patients with relapsed MM.9 Unfortunately, toxicity of DLI is increased in patients who receive this cell dose, with 100% of responding patients developing GVHD in that recent study. Infusion of lower cell doses appears capable of inducing CRs in other diseases, such as CML. A cell dose of 1 × 107 CD3+cells/kg is associated with a complete cytogenetic response rate in 42% of patients with relapsed stable-phase CML in the absence of significant GVHD.15 The high response rate to DLI observed in the current study suggests that a lower cell dose may be effective in treating patients with persistent disease early after BMT, as compared with the higher doses needed to treat patients in overt relapse.
As with other diseases responsive to DLI, the median time to maximal response after DLI in patients with MM is prolonged. Our experience demonstrates that patients' paraprotein levels may rise in the first month after DLI even in those patients destined to develop significant responses. The median time to maximal response in our study after a single DLI is 6 months, with a single patient achieving CR only at 35 months after DLI. Because time to response after DLI may be prolonged and the infusion of additional donor cells associated with increased toxicity, patients should be monitored for response for a significant period of time after DLI.
The optimal timing of DLI to maximize efficacy and to minimize toxicity after BMT remains to be defined. DLI very early after BMT is associated with an increased risk of GVHD compared with later infusions: DLI at day 30 after BMT led to an increased risk of GVHD compared with DLI after day 45.16 However, timing of DLI may not be such an important factor at prolonged intervals after transplantation. For example, in a previous study we observed no difference in the incidence of GVHD when patients received DLI at less than one year versus more than a year after BMT.8 In addition, no relationship between time from transplantation to DLI was noted in 2 large registry reports.5 6 The toxicity and efficacy of DLI prior to 6 months after BMT should be further explored because early DLI may be more efficacious.
As with other diseases, there appears to be a strong association between GVM and GVHD. In a review of multiple studies of DLI in patients with relapsed MM, a statistically significant correlation between GVHD and response was noted in MM patients treated with DLI: 18 of 22 patients who responded to DLI developed GVHD, whereas only 2 of 7 patients who did not respond developed GVHD.10 Although depletion of CD8+ cells has been shown to reduce the incidence of GVHD after DLI,8,17 GVHD remains a significant complication of DLI in the current study. A comparative trial of CD8-depleted DLI and unmanipulated DLI is under way at our institute to clarify whether CD8 depletion leads to a reduction in GVHD after DLI. Ideally, the infusion of antigen-specific T cells would be the most effective way to induce a GVM effect in the absence of GVHD, because some experimental evidence demonstrates that distinct populations of T cells may mediate GVHD versus GVM. In a detailed analysis of patients with relapsed MM treated with CD4+DLI, we have identified distinct clonal populations of T cells that correlated with GVM and GVHD.18 Moreover, in the current study, 2 patients developed a CR in the absence of clinical GHVD, further suggesting that GVM and GVHD may, at least in some cases, be distinct. Ultimately, it may be possible to generate antigen-specific T cells in vivo by vaccination or ex vivo for adoptive immunotherapy and thereby achieve GVM without GVHD. Already, idiotype-specific donor T cells have also been generated from donor vaccination.19
Although this study demonstrates that DLI is capable of inducing a significant GVM effect when administered 6 to 9 months after transplantation, only 58% of patients were able to receive the DLI. Despite T-cell–depleted transplantation, nearly half of the patients still developed complications that prevented them from receiving DLI. For allogeneic transplantation and DLI to be an effective treatment strategy in MM, a transplantation regimen associated with less toxicity is therefore needed. Nonmyeloablative transplantation strategies that use lower doses of chemotherapy or radiotherapy may provide this approach and allow more patients to benefit from DLI administration. Giralt et al20 have reported results on 13 patients with MM treated with melphalan and fludarabine as nonmyeloablative therapy, followed by CD34+-selected allogeneic peripheral blood stem cell transplantation. All evaluable patients achieved 100% donor cell engraftment by day 30; however, 5 patients developed significant GVHD after BMT despite GVHD prophylaxis. Ongoing studies will define the optimal nonmyeloablative transplantation regimen to both avoid the toxicity of transplantation and provide a platform for the administration of DLI.
Supported by grants AI29530 and CA78378 from the National Institutes of Health.
E.A. is a Special Fellow of the Leukemia and Lymphoma Society of America, R.S. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society of America, and K.A. Anderson is a Doris Duke Distinguished Clinical Research Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edwin Alyea, Dana-Farber Cancer Institute, Boston, MA 02115; e-mail: edwin_alyea@dfci.harvard.edu.