Abstract
DT388–GM-CSF, a targeted fusion toxin constructed by conjugation of human granulocyte-macrophage colony-stimulating factor (GM-CSF) with the catalytic and translocation domains of diphtheria toxin, is presently in phase I trials for patients with resistant acute myeloid leukemia. HL-60/VCR, a multidrug-resistant human myeloid leukemia cell line, and wild-type HL-60 cells were used to study the impact of DT388–GM-CSF on metabolism of ceramide, a modulator of apoptosis. After 48 hours with DT388–GM-CSF (10 nM), ceramide levels in HL-60/VCR cells rose 6-fold and viability fell to 10%, whereas GM-CSF alone was without influence. Similar results were obtained in HL-60 cells. Examination of the time course revealed that protein synthesis decreased by about 50% and cellular ceramide levels increased by about 80% between 4 and 6 hours after addition of DT388–GM-CSF. By 6 hours this was accompanied by activation of caspase-9, followed by activation of caspase-3, cleavage of caspase substrates, and chromatin fragmentation. Hygromycin B and emetine failed to elevate ceramide levels or induce apoptosis at concentrations that inhibited protein synthesis by 50%. Exposure to C6-ceramide inhibited protein synthesis (EC50∼5 μM) and decreased viability (EC50 ∼6 μM). Sphingomyelinase treatment depleted sphingomyelin by about 10%, while increasing ceramide levels and inhibiting protein synthesis. Diphtheria toxin increased ceramide and decreased sphingomyelin in U-937 cells, a cell line extremely sensitive to diphtheria toxin; exposure to DT388–GM-CSF showed sensitivity at less than 1.0 pM. Diphtheria toxin and conjugate trigger ceramide formation that contributes to apoptosis in human leukemia cells through caspase activation and inhibition of protein synthesis.
Introduction
Despite recent advances in chemotherapy and use of allogeneic bone marrow transplantation, a large number of patients with acute myelogenous leukemia (AML) eventually die of this disease. Complete remissions can be induced in up to 60% to 80% of new cases with current treatment modalities, but development of resistance precludes long-term cure.1 2
A number of drug-resistance mechanisms have been described in AML. These include overexpression of drug efflux pumps such as P-glycoprotein (P-gp) and lung resistance protein, increased activity of the drug metabolizing enzyme, glutathione-S-transferase (GSTπ), and alterations in apoptotic regulators such as Bcl-2 and p53.1-5 At least 3 of these proteins, P-gp, GSTπ, and Bcl-2, have been shown to have rapid turnover.6-8Moreover, their concentration in leukemia blasts correlates with response to therapy and remission duration.9-11 It has been proposed that cell-specific inhibitors of protein synthesis, such as targeted toxins, might exert their effects by down-regulating these drug-resistance proteins.12-15
Targeted toxins or fusion toxins are recombinant polypeptides that consist of the catalytic and translocation domains of a toxin fused with a tumor-selective ligand such as an antibody or a growth factor. The latter is able to bind target cells and trigger receptor-mediated endocytosis, enabling the toxin to gain access to cytosol. Genetic engineering of new growth factors opens the door for synthesis of new fused toxins targeting different cells, tissues, and tumors. The combination of high specificity and toxicity makes fused toxins a potentially important treatment modality in oncology. A number of targeted cytotoxic conjugates have been developed over the last decade using diphtheria toxin, which is among the most potent toxins known.16 The catalytic domain of diphtheria toxin catalyzes the adenosine diphosphate (ADP)–ribosylation and inactivation of elongation factor-2 (EF-2, also known as translocase), thereby inhibiting protein synthesis.17 It has also been suggested, however, that diphtheria toxin triggers other events that contribute to the induction of apoptosis.18 19
Granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors are present on the cell surface of leukemia blasts in a majority of patients with AML.13 In an effort to target multidrug- resistant AML blasts expressing GM-CSF receptors, a recombinant diphtheria fusion toxin has been synthesized by linking a truncated form of diphtheria toxin (DT388) to human GM-CSF (DT388–GM-CSF).20,21 In vincristine-resistant HL-60 cells, modulation of doxorubicin resistance by DT388–GM-CSF correlated with reductions in membrane P-gp levels and increased uptake of doxorubicin,13 raising the possibility that DT388–GM-CSF acts by decreasing synthesis of drug-resistance proteins.
In the present study, we have tested an alternative hypothesis for the action of DT388–GM-CSF. Over the last decade, it has become clear that the sphingomyelin cycle (reviewed in Rizzieri and Hannun22 and Kolesnick and Kronke23) plays an important role in cell maturation, senscence, and apoptosis. In particular, a number of investigations have shown that ceramide and sphingolipids are involved in chemotherapy-induced apoptotic events in cancer cells.24-29 For example, synthesis of ceramide has been shown to mediate daunorubicin-induced apoptosis in leukemia cells.26,27 Likewise, etoposide has been reported to induce apoptosis via generation of ceramide.30 Further genetic targeting through cellular transfection with glucosylceramide synthase, which enhances removal of ceramide by way of glycosylation, confers doxorubicin and tumor necrosis factor-α (TNF-α) resistance in human breast cancer cells.28,31 The association of ceramide with mechanisms of cytotoxic insult prompted us to examine whether the DT388–GM-CSF death signal in leukemia cells involved alterations in sphingolipid metabolism. Here we show that GM-CSF–conjugated diphtheria toxin and diphtheria toxin alone elicit cellular sphingomyelin degradation and ceramide generation in human leukemia cells. This is followed by activation of caspase-9 and caspase-3 as well as additional apoptotic biochemical changes. Ceramide also inhibited protein synthesis in the cells. These results have important implications for current understanding of the biochemical basis for killing by targeted toxin molecules and are of utility when downstream modulators of ceramide metabolism are used to heighten cytotoxic response to chemotherapy.29
Materials and methods
Materials
DT388–GM-CSF was prepared and purified as previously described.32 A stock solution (30 μM) was made in DMSO, stored at −70°C in 5-μL aliquots, and diluted into medium before use. All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). [9,10-3H] Palmitic acid (56.5 Ci/mmol) was from DuPont/NEN (Boston, MA) and [4,5-3H(N)]l-leucine was from American Radiolabeled Chemicals (St Louis, MO). RPMI-1640 and leucine-free RPMI-1640 medium were from Gibco BRL (Gaithersburg, MD). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Thin-layer chromatography (TLC) plates, Silica Gel G, 0.25 mm thick, were purchased from Analtech (Newark, DE). Sodium azide, 2-deoxyglucose, nicotinamide, sphingomyelinase (human placenta) 100 U/mg protein, and N-acetyl-5-farnesyl-L-cysteine (AFC) were purchased from Sigma (St Louis, MO). Plastic tissue cultureware was from Corning-Costar (Cambridge, MA). Etoposide and N-acetyl-Asp-Glu-Val-Asp-AFC (7-amino-4-trifluoromethyl coumarin (DEVD-AFC) were from Biomol (Plymouth Meeting, PA). Enhanced chemiluminescence (ECL) reagents were from Amersham (Newark, NJ). Murine monoclonal anti–caspase-2 and anti–caspase-3 were obtained from Transduction Laboratories (Lexington, KY). Rabbit anti–protein kinase Cδ was from Santa Cruz Biotechnology (Santa Cruz, CA), and peroxidase-labeled secondary antibodies were from KPL (Gaithersburg, MD). Reagents that recognize poly(ADP-ribose) polymerase (PARP), procaspase-9, and procaspase-8 were kindly provided by Guy Poirier (Laval University, Ste-Foy, Quebec, Canada), Yuri Lazebnik (Cold Spring Harbor Laboratory, NY), and John Reed and Stan Krajewski (Burnham Institute, La Jolla, CA), respectively. Chicken serum that reacts with lamin B1 and rabbit sera that recognize the large subunit of caspase-3, the PEPD epitope at the C-terminus of the caspase-9 large subunit, and the VETD epitope at the C-terminus of the caspase-8 large subunit were raised as previously described.33 34
Cells
The HL-60 cells were obtained form the American Type Culture Collection (ATCC; Rockville, MD) and from Robert Abraham (Duke University, Durham, NC). HL-60/VCR, a vincristine-resistant line, was developed by stepwise exposure of HL-60 to increasing concentrations of vincristine.35 Cells were grown in RPMI-1640 containing 10% FBS and additives as previously described.36 To maintain HL-60/VCR cells, 1.0 μg/mL vincristine was added to the medium. U-937, a human myeloid leukemia cell line, was obtained from the ATCC and maintained in high-glucose RPMI-1640 medium containing 10% FBS, 2 mM l-glutamine, 10 mM HEPES (pH 7.2), and 1.0 mM sodium pyruvate.
Lipid metabolism and analysis
To determine the influence of DT388–GM-CSF, GM-CSF, or diphtheria toxin on ceramide and sphingomyelin metabolism, stock cell cultures were washed twice with room-temperature phosphate-buffered saline (PBS), suspended in medium at 2.5 × 105 cells/mL, and 2.0 mL seeded into 6-well plates. Vincristine was removed from HL-60/VCR medium during the course of each experiment. Cellular lipids were radiolabeled by the addition of [3H]palmitic acid, 1.0 μCi/mL medium; the agent under study was added, and incubations were continued for the indicated times at 37°C in a humidified, 5% CO2 atmosphere tissue culture incubator.
Total cellular lipids were extracted by the method of Bligh and Dyer,37 modified to contain 2% acetic acid in methanol, in a manner previously described.38 After evaporation of the lipid-rich organic lower phase using a stream of nitrogen, total cellular radiolabeled lipids were resuspended in 50 μL chloroform/methanol (1:1, v/v), and equal aliquots from each sample were spotted onto the origin of TLC plates. Commercial lipid standards were spotted and cochromatographed. Ceramide was resolved from other lipids in a solvent system containing chloroform/acetic acid (90:10, v/v). Sphingomyelin was resolved using a solvent system containing chloroform/methanol/acetic acid/water (50:30:7:4, v/v). After drying, the appropriate areas on the plate were identified by iodine vapor staining of the lipids; identification was made by comparison of Rf values, and spots were scraped into plastic scintillation vials containing 0.5 mL water. After 4.5 mL EcoLume was added, radioactivity was analyzed by liquid scintillation spectroscopy.36 38
Sphingomyelinase treatment
The HL-60/VCR cells (250 000/mL 5% FBS medium) were labeled with [3H]palmitic acid (1.0 μCi/mL) for 24 hours, washed by centrifugation, incubated in fresh tritium-free medium for 1 hour, and rewashed for distribution in 6-well plates. Cells (250 000/mL 5% FBS medium) were exposed to sphingomyelinase (1-5 U/mL) at 37°C for 4 hours. After harvest and lipid extraction, [3H]sphingomyelin and [3H]ceramide were analyzed by TLC as described above.
Cytotoxicity assays
Assays were performed as described previously.28Briefly, cells from stock cultures were washed twice with PBS and seeded into 96-well plates at 10 000 cells/well in 0.1 mL medium containing 10% FBS. After a 2-hour acclimation period, 0.1 mL serum-free medium containing the agent under study was added and the incubation continued. Vincristine was eliminated from HL-60/VCR medium during cytotoxicity studies. Cytotoxicity was determined using the Promega 96 aqueous cell proliferation kit (Promega, Madison, WI), a tetrazolium-based colorimetric assay. Absorbance at 490 nm was recorded using a microplate reader, model FL600 (Bio-Tek, Winooski, VT).
Apoptosis
Apoptosis, in response to drug exposure, was quantitated by the Cell Death Detection enzyme-linked immunosorbent assay (ELISA; Boehringer Mannheim, Indianapolis, IN). After cell harvest and lysis (3000 cells/tube), mononucleosomes and oligonucleosomes in the soluble fraction were recognized by DNA-histone antibody and detected by peroxidase-coupled anti-DNA antibody according to instructions from the manufacturer. Absorbance was measured at 405 nm.
Protein synthesis assay
Cells from stock cultures were washed twice with PBS and resuspended at 5 × 105 cells/mL in leucine-free RPMI-1640 medium containing 2.5% FBS. Aliquots of 0.5 mL were added to wells of a 24-well plate. After 2 hours, the agents under investigation were coadministered along with 1.0 μCi [3H]leucine in a final well volume of 1.0 mL. Controls received vehicle and [3H]leucine only. At the indicated times, cells were transferred along with a PBS wash to Eppendorf spin tubes and pelleted by centrifugation at 10 000 rpm, 10 minutes, in an IEC Micromax centrifuge (International Equipment, Needham Heights, MA). Cell pellets were resuspended at 4°C in 10% trichloroacetic acid to precipitate cell protein. Pellets were washed in methanol, dried, dissolved in 0.5 mL 1% sodium dodecylsulfate/0.3 M sodium hydroxide, and analyzed by liquid scintillation spectroscopy using EcoLume. Data represent percent protein synthesis in treated cells compared to protein synthesis at each time point in untreated controls. Each increment with time in control synthesis is set at 100%.
Caspase activity and cleavage of caspase substrates
At the start of each experiment, nonviable HL-60 cells were removed by sedimentation at 200g for 20 minutes on Ficoll-Hypaque step gradients (density = 1.119 g/cm3). Cells harvested from the interface were diluted with complete RPMI 1640 medium, sedimented at 200g for 10 minutes, and resuspended in fresh medium. Cells were treated with 40 nM DT388–GM-CSF for 3 to 30 hours. Alternatively, cells were treated with etoposide (prepared as a 1000-fold concentrated stock in DMSO) at a concentration of 68 μM, a high but clinically sustainable concentration39,40 that has previously been shown to induce apoptosis in more than 85% of HL-60 cells within 6 hours41 through a caspase-9–dependent pathway.34 42
After treatment, aliquots of cells were sedimented at 200gfor 10 minutes and washed once with ice cold RPMI 1640 containing 10 mM HEPES (pH 7.4). Replicate aliquots were either fixed for morphologic examination after Hoechst staining,41 solubilized in 6 M guanidine hydrochloride under reducing conditions in preparation for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting,43 or subjected to subcellular fractionation followed by fluorogenic measurement of caspase-3–like activity.44 In brief, cells for subcellular fractionation were sedimented, washed with calcium-, magnesium-free PBS, and resuspended in buffer A (25 mM HEPES, pH 7.5 at 4°C, 5 mM MgCl2, 1 mM EGTA supplemented immediately before use with 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL pepstatin A, and 10 μg/mL leupeptin). After a 20-minute incubation on ice, cells were lysed with 20 to 30 strokes in a tight-fitting Dounce homogenizer and sedimented at 800g for 10 minutes (to remove nuclei) followed by 280 000g for 60 minutes to sediment other membranous cellular components. The supernatant (cytosol) was frozen in 50-μL aliquots at −70°C after addition of EDTA to a final concentration of 0.5 mM and dithiothreitol (DTT) to a final concentration of 2 mM.
Aliquots containing 50 μg cytosolic protein, estimated by the bicinchonic acid method,45 in 50 μL buffer A were diluted with 225 μL of freshly prepared buffer B (25 mM HEPES, pH 7.5, 0.1% [w/v] CHAPS, 10 mM DTT, 100 U/mL aprotinin, 1 mM PMSF) containing 100 μM DEVD-AFC and incubated for 2 hours at 37°C. Reactions were terminated by addition of 1.225 mL ice-cold buffer B, and fluorescence was measured in a Sequoia-Turner fluorometer using an excitation wavelength of 360 nm and emission wavelength of 475 nm. Reagent blanks containing 50 μL buffer A and 225 μL buffer B were incubated at 37 °C for 2 hours, then diluted with 1.225 mL ice-cold buffer B. Standards containing 0 to 1500 pmol of AFC were used to determine the amount of fluorochrome released. Preliminary studies demonstrated that (1) the Kd for the substrate was 20 μM,46 and (2) the release of product under these conditions was linear for at least 4 hours. These controls rule out the possibility that changes in the amount of AFC liberated reflect alterations in enzyme affinity or stability as opposed to increases in amount of active enzyme.
Immunoblotting
Whole cell lysates containing protein from 3 × 105 cells were solubilized in sample buffer consisting of 4 M urea, 2% SDS, 62.5 mM Tris-HCL (pH 6.8 at 4°C), and 1 mM EDTA, heated to 65°C and loaded on SDS-polyacrylamide gels containing a 5% to 15% (w/v) acrylamide gradient. Subsequent transfer to nitrocellulose and immunoblotting were performed as described.44 46
Results
Previous studies have shown that DT388–GM-CSF induces apoptosis in human acute myeloid leukemia blasts and that sensitivity is dependent on GM-CSF receptors.20 Work with HL-60/VCR cells also demonstrated the utility of DT388–GM-CSF in modulating resistance to anthracyclines.13 In light of work showing that ceramide mediates anthracycline-induced26,27 and etoposide-induced30 apoptosis in leukemia cell models, we have now investigated the influence of DT388–GM-CSF on ceramide metabolism.
Initial experiments, conducted with HL-60/VCR cells, showed that DT388–GM-CSF had a profound impact on ceramide production. Figure 1 shows that a 48-hour exposure to DT388–GM-CSF elicits ceramide generation in HL-60/VCR cells in a dose-dependent manner. With DT388–GM-CSF concentrations as low as 0.1 nM, ceramide increased to 3.5 times the level in untreated cells, and at 10 nM, ceramide increased 6-fold. The steep increase in ceramide in HL-60/VCR cells reached a plateau at concentrations greater than 10 nM. DT388–GM-GSF also promoted ceramide formation in HL-60 cells in a similar manner (data not shown). In contrast, GM-CSF alone had little influence on cellular ceramide metabolism (Figure 1).
Influence of DT388–GM-CSF and GM-CSF on ceramide metabolism in HL-60/VCR cells.
Cells, seeded in 6-well plates, were exposed to the agents at the indicated doses for 48 hours in medium containing [3H]palmitic acid. Radiolabeled ceramide was resolved from the total cell lipid extract by TLC and quantitated by liquid scintillation counting.
Influence of DT388–GM-CSF and GM-CSF on ceramide metabolism in HL-60/VCR cells.
Cells, seeded in 6-well plates, were exposed to the agents at the indicated doses for 48 hours in medium containing [3H]palmitic acid. Radiolabeled ceramide was resolved from the total cell lipid extract by TLC and quantitated by liquid scintillation counting.
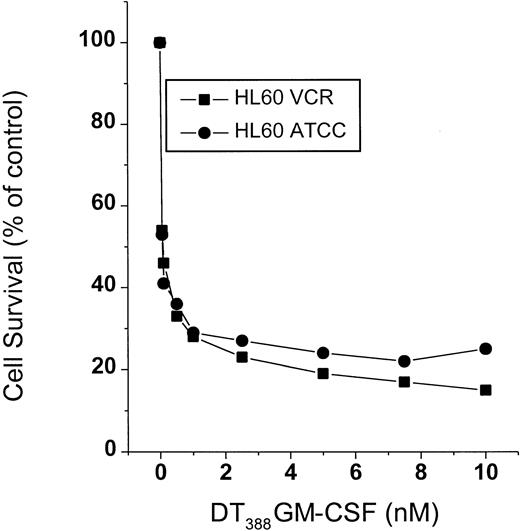
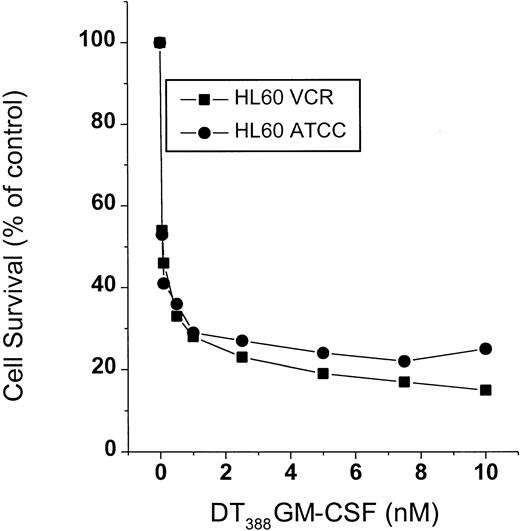
Concomitant with ceramide formation induced by DT388–GM-CSF, survival of HL-60/VCR cells fell sharply in response to treatment (Figure 2). HL-60 cells were similarly susceptible to treatment (Figure 2). The decline in cell survival (Figure 2) mirrored the increase in levels of intracellular ceramide (Figure 1) in response to treatment.
Cell survival in response to DT388–GM-CSF treatment.
HL-60 and HL-60/VCR cells, seeded in 96-well plates, were treated with increasing concentrations of DT388–GM-CSF for a 3-day period. Cell viability was assessed spectrophotometrically using Promega reagents as described in “Materials and methods.” Data represent the mean ± SD of 6 replicates. SD bars were coincident with symbols.
Cell survival in response to DT388–GM-CSF treatment.
HL-60 and HL-60/VCR cells, seeded in 96-well plates, were treated with increasing concentrations of DT388–GM-CSF for a 3-day period. Cell viability was assessed spectrophotometrically using Promega reagents as described in “Materials and methods.” Data represent the mean ± SD of 6 replicates. SD bars were coincident with symbols.
To more clearly define the relationship of ceramide to the cytotoxic action of DT388–GM-CSF, the time courses for inhibition of cellular protein synthesis, ceramide production, and initiation of apoptosis were compared. Protein synthesis was inhibited by 20% at 2.5 hours after addition of DT388–GM-GSF and 50% at 6 hours (Figure 3A). Despite this modest reduction in protein synthesis (see below), ceramide production in response to DT388–GM-GSF was initiated between 4 and 6 hours after addition, attaining a level about 75% above control at 6 hours. Increases in chromatin fragmentation were apparent within the same time frame (4-6 hours) and were 230% of control values at 8 hours (Figure 3B). A reduction in the level of cellular sphingomyelin was evident by 8 hours, with depletion of 60% of the total cellular sphingomyelin by 24 hours (Figure 3A).
Influence of DT388–GM-CSF on sphingomyelin and ceramide metabolism, protein synthesis, and apoptosis in HL-60/VCR cells.
(A) Sphingomyelin and ceramide metabolism, and protein synthesis. Cells were exposed to 1.0 nM DT388–GM-CSF in medium containing [3H]palmitic acid for the times indicated. Radiolabeled sphingomyelin and ceramide were analyzed as detailed in “Materials and methods.” Protein synthesis was followed by [3H]leucine utilization as described in “Materials and methods,” and is represented as percent decrease in protein synthesis compared with rate in untreated control cells. (B) Apoptosis. HL-60/VCR cells were treated with 1.0 nM DT388–GM-CSF for the times shown and chromatin fragmentation was measured by the Cell Death Detection ELISA as described.
Influence of DT388–GM-CSF on sphingomyelin and ceramide metabolism, protein synthesis, and apoptosis in HL-60/VCR cells.
(A) Sphingomyelin and ceramide metabolism, and protein synthesis. Cells were exposed to 1.0 nM DT388–GM-CSF in medium containing [3H]palmitic acid for the times indicated. Radiolabeled sphingomyelin and ceramide were analyzed as detailed in “Materials and methods.” Protein synthesis was followed by [3H]leucine utilization as described in “Materials and methods,” and is represented as percent decrease in protein synthesis compared with rate in untreated control cells. (B) Apoptosis. HL-60/VCR cells were treated with 1.0 nM DT388–GM-CSF for the times shown and chromatin fragmentation was measured by the Cell Death Detection ELISA as described.
To determine if increased ceramide formation is a response common to the action of protein synthesis inhibitors, the influence of emetine and hygromycin B were investigated. As shown in Figure4, these compounds, at concentrations that inhibit protein synthesis by 50%, did not significantly alter ceramide metabolism in HL-60/VCR cells. After 6 hours of exposure to either emetine or hygromycin B, cellular ceramide levels were only 5% below and 7% above untreated control levels, respectively. GM-CSF in combination with protein synthesis inhibitors also failed to increase ceramide, whereas the diphtheria toxin–GM-CSF conjugate, DT388–GM-CSF, at 6 hours, elicited a 75% increase in ceramide levels (Figure 4).
Effect of protein synthesis inhibitors on ceramide metabolism in HL-60/VCR cells.
Cells (500 000/well) were seeded in 6-well plates in complete medium containing 1.0 μCi [3H]palmitic acid, and incubated for 6 hours with the agents indicated: emetine (0.5 μg/mL); hygromycin B (80 μg/mL); GM-CSF (1.0 nM); DT388–GM-CSF (1.0 nM), or combinations. Total cellular lipids were extracted, and [3H]ceramide was quantitated. The concentrations of emetine and hygromycin B are those that promote a 50% suppression of protein synthesis in HL-60/VCR cells at approximately 6 hours. Each experimental group represents the average of 3 cultures with variation less than 10%.
Effect of protein synthesis inhibitors on ceramide metabolism in HL-60/VCR cells.
Cells (500 000/well) were seeded in 6-well plates in complete medium containing 1.0 μCi [3H]palmitic acid, and incubated for 6 hours with the agents indicated: emetine (0.5 μg/mL); hygromycin B (80 μg/mL); GM-CSF (1.0 nM); DT388–GM-CSF (1.0 nM), or combinations. Total cellular lipids were extracted, and [3H]ceramide was quantitated. The concentrations of emetine and hygromycin B are those that promote a 50% suppression of protein synthesis in HL-60/VCR cells at approximately 6 hours. Each experimental group represents the average of 3 cultures with variation less than 10%.
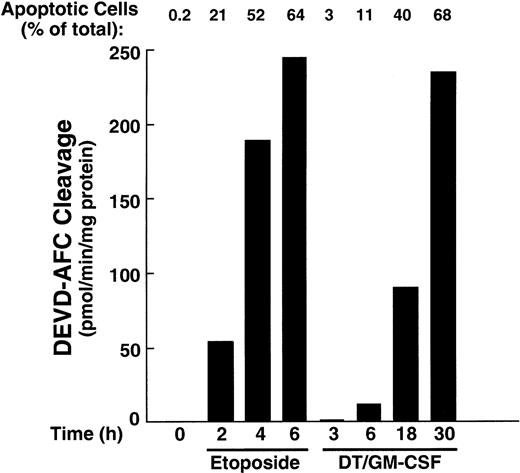
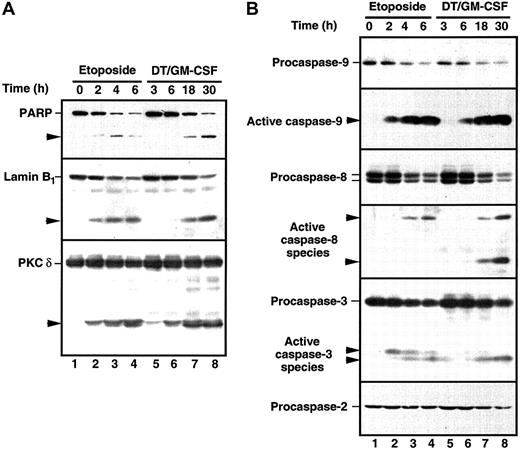
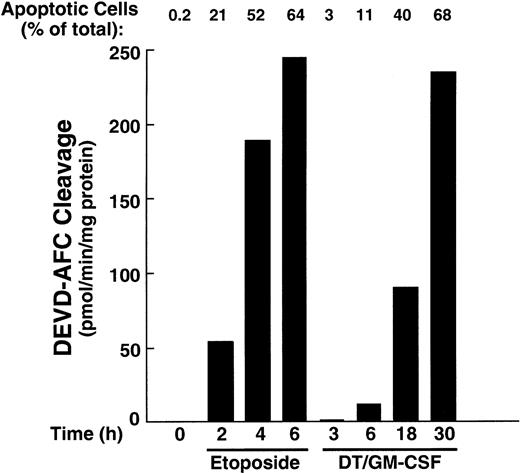
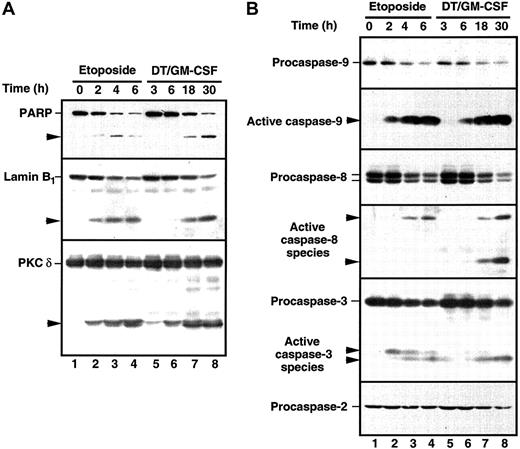
The HL-60 wild-type cells demonstrate ceramide production and are extremely sensitive when challenged with DT388–GM-CSF (Figure 2). Because recent studies have documented the participation of ceramide in etoposide-induced apoptosis30 47 and because caspase activation is a hallmark of the cell death cascade, we studied the effects of DT388–GM-CSF on cellular caspase activity. Etoposide was used in these studies as a positive control. Treatment with DT388–GM-CSF caused time-dependent caspase activation, as evidenced by DEVD-AFC cleavage (Figure5), which was readily detectable at 6 hours. Consistent with these results, active species of caspase-9 and caspase-3 were also detectable at this time point (Figure6, right), with proteolytic fragments of caspase-8 becoming detectable later. Cleavage of the caspase substrates PARP, lamin B1, and protein kinase Cδ (PKCδ; Figure 6, left) was observed concomitant with caspase activation.
Caspase-3–like enzymatic activity in HL-60 cells treated with etoposide or DT388–GM-CSF.
Cells were incubated with etoposide (68 μM) or DT388–GM-CSF (40 nM) for the indicated times, and cytosol was prepared and assayed for activity that cleaves the fluorogenic substrate DEVD-AFC.
Caspase-3–like enzymatic activity in HL-60 cells treated with etoposide or DT388–GM-CSF.
Cells were incubated with etoposide (68 μM) or DT388–GM-CSF (40 nM) for the indicated times, and cytosol was prepared and assayed for activity that cleaves the fluorogenic substrate DEVD-AFC.
Cleavage of caspase substrates and zymogens in HL-60 cells treated with etoposide or DT388–GM-CSF.
(A) Whole cell lysates were prepared from HL-60 cells treated with 68 μM etoposide or 40 nM DT388–GM-CSF for the indicated times. After SDS-PAGE, samples were probed with reagents that recognize PARP, lamin B1, or PKCδ. Arrowheads indicate cleavage products as a result of caspase action. (B) Duplicate blots were probed with reagents that recognize procaspase-9, the epitope PEPD that becomes accessible on cleavage between the large and small subunits of caspase-9, the procaspase-8 splice variants expressed in HL-60 cells, the epitope VETD that becomes accessible on cleavage between the large and small subunits of caspase-8, the large subunit of caspase-3, or procaspase-2. Arrowheads indicate products that represent active caspase species in HL-60 cells.
Cleavage of caspase substrates and zymogens in HL-60 cells treated with etoposide or DT388–GM-CSF.
(A) Whole cell lysates were prepared from HL-60 cells treated with 68 μM etoposide or 40 nM DT388–GM-CSF for the indicated times. After SDS-PAGE, samples were probed with reagents that recognize PARP, lamin B1, or PKCδ. Arrowheads indicate cleavage products as a result of caspase action. (B) Duplicate blots were probed with reagents that recognize procaspase-9, the epitope PEPD that becomes accessible on cleavage between the large and small subunits of caspase-9, the procaspase-8 splice variants expressed in HL-60 cells, the epitope VETD that becomes accessible on cleavage between the large and small subunits of caspase-8, the large subunit of caspase-3, or procaspase-2. Arrowheads indicate products that represent active caspase species in HL-60 cells.
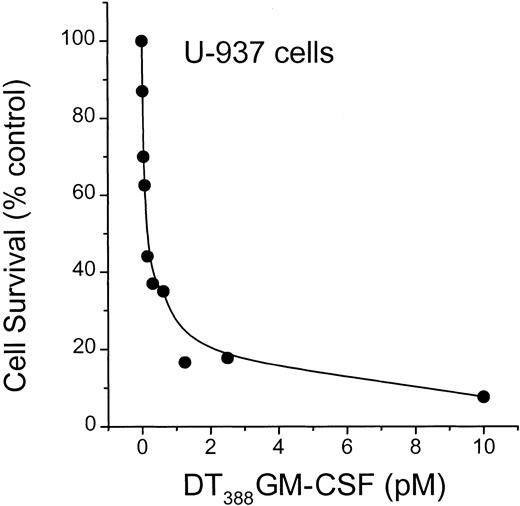
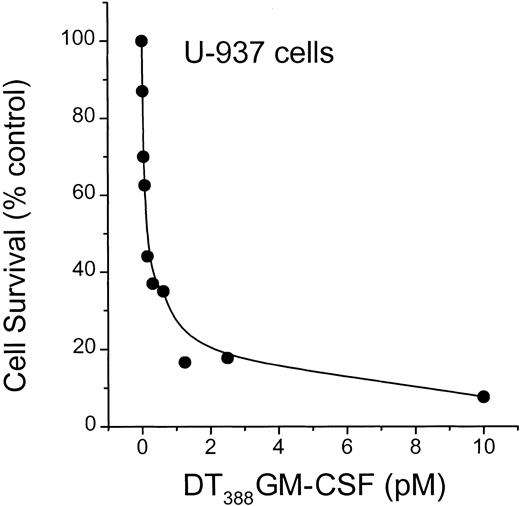
To determine whether diphtheria toxin alone would activate ceramide generation, we used U-937 myeloid leukemia cells, which are extremely sensitive to diphtheria toxin.18 As illustrated in Figure7, diphtheria toxin promoted formation of ceramide with a simultaneous decline in sphingomyelin content in U-937 cells. Thus, the pattern of lipid changes is very similar to the impact of DT388–GM-CSF on lipid metabolism in HL-60/VCR cells (Figure 3A). Because U-937 cells express high levels of GM-CSF receptor, it was of interest to determine the sensitivity of cells to conjugated toxin. The data of Figure 8clearly show that U-937 cells are exceptionally responsive to DT388–GM-CSF with an EC50 (amount of drug eliciting 50% kill) of approximately 0.2 pM. This represents a 3-log increase in sensitivity, compared to HL-60 cells (Figure 2).
Influence of diphtheria toxin on ceramide metabolism in U-937 cells.
Cells were seeded in 6-well plates and treated with toxin (37.5 ng/mL) for the times indicated, in medium containing [3H]palmitic acid. Total cellular lipids were extracted and analyzed for [3H]sphingomyelin and [3H]ceramide by TLC. Minus toxin controls were run at each time point. Data, shown as percent of control, were calculated from disintegrations per minute (dpm) tritium in sphingomyelin or ceramide per 500 000 dpm total lipid. n = 3 ± SD for each experimental point.
Influence of diphtheria toxin on ceramide metabolism in U-937 cells.
Cells were seeded in 6-well plates and treated with toxin (37.5 ng/mL) for the times indicated, in medium containing [3H]palmitic acid. Total cellular lipids were extracted and analyzed for [3H]sphingomyelin and [3H]ceramide by TLC. Minus toxin controls were run at each time point. Data, shown as percent of control, were calculated from disintegrations per minute (dpm) tritium in sphingomyelin or ceramide per 500 000 dpm total lipid. n = 3 ± SD for each experimental point.
Influence of DT388–GM-CSF on U-937 cell survival.
U-937 cells were seeded in 96-well plates and treated with increasing concentrations (0.1-10 pM) DT388–GM-CSF over a 3-day period. Cell viability was determined spectrophotometrically, and data represent the mean ± SD (n = 6). The experiment was repeated 3 times. SD bars are coincident with symbols.
Influence of DT388–GM-CSF on U-937 cell survival.
U-937 cells were seeded in 96-well plates and treated with increasing concentrations (0.1-10 pM) DT388–GM-CSF over a 3-day period. Cell viability was determined spectrophotometrically, and data represent the mean ± SD (n = 6). The experiment was repeated 3 times. SD bars are coincident with symbols.
To more firmly support the premise that ceramide generation by diphtheria toxin contributes to cytotoxicity, we tested the impact of exogenous ceramide exposure and sphingomyelinase treatment on cell response. HL-60 and HL-60/VCR cells were sensitive to supplements of cell-permeable C6-ceramide (EC50 ∼6.5 μM, data not shown). C6-Ceramide also strongly inhibited protein synthesis after only 4 hours of exposure (EC50 ∼5 μM, data not shown). Another route to increase ceramide levels, sphingomyelinase treatment of cells, also resulted in inhibition of protein synthesis. As shown in Figure 9, enzyme treatment of HL-60/VCR cells caused an approximate 10% depletion in sphingomyelin, a 2.5-fold increase in endogenous ceramide, and inhibition of protein synthesis. After 4 hours in the presence of 2 U enzyme/mL culture medium, cellular ceramide levels doubled and protein synthesis was inhibited 50% (Figure 9B,C).
Influence of sphingomyelinase treatment on ceramide metabolism and protein synthesis.
(A,B) HL-60/VCR cells, prelabeled with [3H]palmitic acid, were incubated with sphingomyelinase for 4 hours, and lipids were extracted and analyzed. (C) To measure protein synthesis, cells were incubated with sphingomyelinase for 1 hour, before addition of [3H]leucine (1.0 μCI/well) for 3 hours. Data points represent the mean ± SD (n = 3). SD bars are not shown (error < 5%). SM indicates sphingomyelin.
Influence of sphingomyelinase treatment on ceramide metabolism and protein synthesis.
(A,B) HL-60/VCR cells, prelabeled with [3H]palmitic acid, were incubated with sphingomyelinase for 4 hours, and lipids were extracted and analyzed. (C) To measure protein synthesis, cells were incubated with sphingomyelinase for 1 hour, before addition of [3H]leucine (1.0 μCI/well) for 3 hours. Data points represent the mean ± SD (n = 3). SD bars are not shown (error < 5%). SM indicates sphingomyelin.
Discussion
A number of fusion toxins have been engineered with use of the diphtheria toxin enzymatic domain. Diphtheria toxin conjugates constructed with interleukin (IL)-2 are being investigated for treatment of lymphoma48-50 and human immunodeficiency virus.51,52 Other diphtheria fusion toxins targeting brain tumors have been described.53 Diphtheria toxin fused to IL-3 is toxic to blasts from patients with myeloid leukemias,54 and DT388–GM-CSF, the object of the present study, is being evaluated in a clinical trial for patients with relapsed AML.55
Despite this widespread interest in toxin molecules, there is relatively limited information about how they actually kill target cells. Here for the first time we have identified changes in sphingomyelin metabolism leading to ceramide generation in response to diphtheria toxin and the targeted conjugate DT388–GM-CSF. Moreover, we have placed these changes in ceramide metabolism upstream of caspase-9 activation. These results have potentially important implications for current understanding of how toxin molecules kill cells.
In both U-937 and K-562 cells, diphtheria toxin induces internucleosomal DNA fragmentation, a characteristic of apoptosis, whereas other protein synthesis inhibitors were without influence at similar levels of protein synthesis inhibition.18,56 Thus, cytolysis initiated by diphtheria toxin does not appear to be a simple consequence of translation inhibition, but also has been hypothesized to involve a second pathway of cytotoxicity.56 Consistent with this hypothesis, when we exposed HL-60/VCR cells to either hygromycin B or emetine at concentrations that inhibited protein synthesis by 50%, neither agent caused a remarkable change in cellular ceramide levels, and further, both agents were relatively nontoxic. This strengthens the argument that ceramide generation contributes to diphtheria toxin toxicity. Because diphtheria toxin also potentiates the cytotoxic effect of TNF-α, which signals via ceramide, in renal cell carcinoma,57 ovarian carcinoma,58 and prostate cancer cells,59 it has been suggested that the agents share the same pathway of cytotoxicity.60
We propose that ceramide constitutes an element in the death-signaling cascade initiated by diphtheria toxin and DT388–GM-CSF. Several observations support this view. First, the coincidence of the dose-response curves for ceramide elevation (Figure 1) and decreased cell survival (Figure 2) suggest that these are linked processes after treatment with DT388–GM-CSF. Second, the time course experiments show that ceramide elevation becomes detectable 4 to 6 hours after DT388–GM-CSF addition (Figure 3), with detectable activation of caspase-9 and caspase-3 (Figure 6, right) as well as internucleosomal cleavage (Figure 3B) by 6 hours. These results not only place ceramide elevation temporally upstream of other apoptotic changes, but also suggest that ceramide might be triggering the mitochondrial pathway of caspase activation. Third, in the absence of diphtheria toxin, we have shown that ceramide potently inhibits cellular protein synthesis. Moreover, either exogenous C6-ceramide, or endogenous ceramide, generated by sphingomyelinase, inhibits cell growth. These data provide direct evidence that ceramide is cytotoxic and corroborate recent studies showing that ceramide inhibits protein synthesis.62 The data also indicate that inhibition of protein synthesis by DT388–GM-CSF is not responsible for the elevation in ceramide. Thus, diphtheria toxin appears to signal not only caspase activation but also inhibition of protein synthesis through ceramide. We have not determined if ceramide inactivates EF-2. This is obviously a complex situation warranting further evaluation.
Our observations raise the possibility that diphtheria toxin–induced ADP-ribosylation of another polypeptide lies upstream of ceramide elevation. This event then appears to lead to ceramide generation through sphingomyelin hydrolysis (Figures 3 and 7). Consistent with this hypothesis, 2 agents that block de novo ceramide formation (fumonisin B1 and l-cycloserine), failed to alter the apoptotic index of HL-60/VCR cells treated with DT388–GM-CSF. The influence of diphtheria toxin on sphingomyelinase activity has yet to be determined. In addition, diphtheria toxin activated sphingomyelinase activity in the human myeloid leukemia cell line, U-937. The extreme sensitivity of U-937 cells to the conjugate, DT388–GM-CSF, approximately 1000-fold greater than HL-60 cells, may be related to GM-CSF receptor number; however, the literature is not clear on this point.
Current understanding suggests that there are at least 2 major pathways for drug-induced apoptosis, one triggered by ligation of the death receptor Fas, and another triggered by mitochondrial release of cytotochrome c (reviewed in Kaufmann and Earnshaw61). Several observations suggest that DT388–GM-CSF is activating the mitochondrial pathway rather than the Fas pathway. First, the HL-60 subline used in Figures 5 and 6 fails to express detectable levels of the Fas receptor and fails to die in response to agonistic anti-Fas antibodies.63 Second, active caspase-8 is detectable in cells treated with DT388–GM-CSF only at late time points (Figure 6), a result consistent with the activation of caspase-8 downstream of caspase-364 rather than upstream.
A body of work supports a role for ceramide metabolism in cancer chemotherapy. For example, the accumulation of ceramide in cancer cells is involved in the induction of apoptosis,26,27,38,65,66whereas glycosylation of ceramide is associated with resistance to anthracyclines and vinblastine28,29,67-69 as well as TNF-α.31 Resistance to anthracyclines, which induce ceramide generation,26,27,65 can be conferred by cell transfection with the complementary DNA encoding for glucosylceramide synthase.28 Importantly, DT388–GM-CSF and anthracyclines show supra-additive toxicity in leukemia blasts.13 The conspicuous impact of DT388–GM-CSF on ceramide metabolism suggests that sphingomyelin hydrolysis may be critical in mediating the synergy of these agents in leukemia blasts. Because it has been shown that DT388–GM-CSF, although highly cytotoxic to AML blasts70,71 is not toxic for normal hematopoietic progenitors,71 clinical results obtained with the DT388–GM-CSF/daunorubicin combination are awaited with interest.
Supported by grants from the National Institutes of Health (CA77632 to M.C.C.; CA 69008 to S.H.K.; CA76178 to A.E.F.), Strauss Foundation, Los Angeles (Sandra Krause, Trustee), Associates for Breast and Prostate Cancer Studies, Los Angeles, and the Leukemia Lymphoma Society (6114 to A.E.F.).
This work was presented at the 92nd Annual Meeting, American Association for Cancer Research, March 24-28, 2001, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Myles C. Cabot, John Wayne Cancer Institute, 2200 Santa Monica Blvd, Santa Monica, CA 90404; e-mail: cabot@jwci.org.

![Fig. 1. Influence of DT388–GM-CSF and GM-CSF on ceramide metabolism in HL-60/VCR cells. / Cells, seeded in 6-well plates, were exposed to the agents at the indicated doses for 48 hours in medium containing [3H]palmitic acid. Radiolabeled ceramide was resolved from the total cell lipid extract by TLC and quantitated by liquid scintillation counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534001.jpeg?Expires=1763684851&Signature=P4YsTnK5AaUuByHrour8yHeV2FTEZIL~meLGuhCHZnW6cnrtM5TL5bTHh-33KofecT4w36FBwWxeSEtJ62jjONBUCBllx8Xl68FyVe7ZbG1-SHWxJ5TZYPNZnyuOOX1gOEzHBFXYB1MYkRTS7NR-G1nv94Ztx5rEgDU7IPe7KYgRgeaAANjQSSBDrtPX4LqUtMy2axv1n~s0o-qnSxn0-z3k3S6AVliTokJM91uKT0oKtMK5H7oAVMXUGRLd22LZYWAQO0fX5HbWyytMR4qeZe3ETSSfOrEWw89RshkVNKC7IOgr5hWu1KrrjuxEZeW99Afqr8CqK9vSI5hKNW4FJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Influence of DT388–GM-CSF on sphingomyelin and ceramide metabolism, protein synthesis, and apoptosis in HL-60/VCR cells. / (A) Sphingomyelin and ceramide metabolism, and protein synthesis. Cells were exposed to 1.0 nM DT388–GM-CSF in medium containing [3H]palmitic acid for the times indicated. Radiolabeled sphingomyelin and ceramide were analyzed as detailed in “Materials and methods.” Protein synthesis was followed by [3H]leucine utilization as described in “Materials and methods,” and is represented as percent decrease in protein synthesis compared with rate in untreated control cells. (B) Apoptosis. HL-60/VCR cells were treated with 1.0 nM DT388–GM-CSF for the times shown and chromatin fragmentation was measured by the Cell Death Detection ELISA as described.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534003.jpeg?Expires=1763684851&Signature=HpHBYICUcjvxmLCSsh8ZHxqQTaGy9tPFWAcCV0ESWBg3KR2pNKq304WsIsNV2sl3eiJRngrMeuAPoQZccHUU3InqJg72jup~Gm78M6TlTz2bPE1fpPukGJixZCQZKWwXGCbfFGzbq9ooIcKpB3rm5vJ9myqfbYHiClp9W6bOKjgop69NblTr7bZYvnhH8fCM08Ng83YYdxJ9Wru7lNvuav1RUvJFJRaA4UxJ5KpI98e~zjBnFUaynEG2BcKKMi90luxPQfV5Z7Ge924GAbxUhm~lSgEvAubQ02sRa38n6QJ7sRlViEQEf7opRFzMf2aoKm0FCiWAyIZU1gdL3zycPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of protein synthesis inhibitors on ceramide metabolism in HL-60/VCR cells. / Cells (500 000/well) were seeded in 6-well plates in complete medium containing 1.0 μCi [3H]palmitic acid, and incubated for 6 hours with the agents indicated: emetine (0.5 μg/mL); hygromycin B (80 μg/mL); GM-CSF (1.0 nM); DT388–GM-CSF (1.0 nM), or combinations. Total cellular lipids were extracted, and [3H]ceramide was quantitated. The concentrations of emetine and hygromycin B are those that promote a 50% suppression of protein synthesis in HL-60/VCR cells at approximately 6 hours. Each experimental group represents the average of 3 cultures with variation less than 10%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534004.jpeg?Expires=1763684851&Signature=Zvk25XjI9f0SvpEtI06oYT5oxiCc26gRELhsxinbcYbIQOFBHlG2uG81F5UfQpTmrZn9qWBTwFl65aFteKC6DcuP0UM~nu4A3zAWOop8nE~RHiPG2u1U3SRc~LmOXOf4vU0mleg~vNbcgjaVKYhgpEvjX9-KxlsLTc1T2HYSTRO0pFBTNcOp79wELjJa8KJr3~bxVJlEAfraM0thqLrW5ROj1B4RsVkCN39l2JhEXV-wYZXeuLSUedV6~qb8t94s90z3xea6y-Bf292EYWl7FEdM19FzV873Bxy3fyBRNx~CQvl2-wycFaVpjUusonRQFQVC1DQW7rbcw2P6k4G1LQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Influence of diphtheria toxin on ceramide metabolism in U-937 cells. / Cells were seeded in 6-well plates and treated with toxin (37.5 ng/mL) for the times indicated, in medium containing [3H]palmitic acid. Total cellular lipids were extracted and analyzed for [3H]sphingomyelin and [3H]ceramide by TLC. Minus toxin controls were run at each time point. Data, shown as percent of control, were calculated from disintegrations per minute (dpm) tritium in sphingomyelin or ceramide per 500 000 dpm total lipid. n = 3 ± SD for each experimental point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534007.jpeg?Expires=1763684851&Signature=OOD6M-y-mkoQBK1kKwrnsL8r9dpELoLbQi3QQHW~SlZEKoBhwOhimWDtSAU4UPwMhQ5UTISQC9OipQge07~IWApTW0Sgl-MXbFDj35--CgYNHaJsdqYy0ffcZ9Vy8iXy8gNyGeWsUy-T5e-Q04ZPh7yQbEL4Sm9wW2z6k44uKpFCsFXd1Jgcd1fTdpWWy1M1pRMaZ0aaKyaekNacaWUr0BJu7kteBv62rx~tqa8aIM13cU5h0Tz0xhhXJsGQU393VWz4GvhYuRqHaEejAlAeybq0o96qzOKwezQxqwW3A9HJ7JfiTeRCLdQLpGEXtcmxK1opz7aPkdyvKMvz2Su~xQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Influence of sphingomyelinase treatment on ceramide metabolism and protein synthesis. / (A,B) HL-60/VCR cells, prelabeled with [3H]palmitic acid, were incubated with sphingomyelinase for 4 hours, and lipids were extracted and analyzed. (C) To measure protein synthesis, cells were incubated with sphingomyelinase for 1 hour, before addition of [3H]leucine (1.0 μCI/well) for 3 hours. Data points represent the mean ± SD (n = 3). SD bars are not shown (error < 5%). SM indicates sphingomyelin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534009.jpeg?Expires=1763684851&Signature=Y1v0ccHBbNF~S1OBHAEyFDf1r7Ut3LawOs4J~reFnC0-PqUbKdZ6R62hYfrcU6CmMjPE5ACKx9VrdwoMmmTU3CgRDrGeKwvSZE7NQXhVabknjV0zdK2NHvTbu3fukb~R2QlSR19f0siA3duNgGyLPOSyFffOccGndypNwMwwRQt8USrGnvvcWkZTtpBXA-urNWbjt-yeS2Uekj6K90~4REQApYibO3BpspC3DMkradcb8MH2OnV4FQDT3RAPYLQ6yqeqn5HjxeDL~xezEGZIuDmj0HmNA53OoWWdjSCZjk2kwt-c24GnFGqJ6KeDpwRTT7-Ste4H225rFLJ9aJZmIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Influence of DT388–GM-CSF and GM-CSF on ceramide metabolism in HL-60/VCR cells. / Cells, seeded in 6-well plates, were exposed to the agents at the indicated doses for 48 hours in medium containing [3H]palmitic acid. Radiolabeled ceramide was resolved from the total cell lipid extract by TLC and quantitated by liquid scintillation counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534001.jpeg?Expires=1763684852&Signature=Ev~VIe~Rxab0Q-k83WQhaZ8DfjTNxL9SVeWyhD9Ica4iIe2YDpzH5cAg61eIYfxeXGisEYm6cHofRwX8xSk48EAUgzRiqge7KEuvhOZsHvVKkqXxjCHeGXubjidV~5q2JCecufasJiz~nEccEKHK5Sj9S8l5KDHiQe0fWDop8LHfQL7W-N2i4Bi-Cscw1yQ7Y0oiAcbUSPIRVLM8~U1kYc4td9YS0487d1NRN7JqsapumUv-dXrUb63QxSIJcw2e-PpbC9QA1Hn38uYp4qfg1Ict6YxY4WJOeJMXOTmKx~uFL-9TqFtdF3PFsRWo-h9M5Vrkvq-ZLGBnY~VbU4v2kA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Influence of DT388–GM-CSF on sphingomyelin and ceramide metabolism, protein synthesis, and apoptosis in HL-60/VCR cells. / (A) Sphingomyelin and ceramide metabolism, and protein synthesis. Cells were exposed to 1.0 nM DT388–GM-CSF in medium containing [3H]palmitic acid for the times indicated. Radiolabeled sphingomyelin and ceramide were analyzed as detailed in “Materials and methods.” Protein synthesis was followed by [3H]leucine utilization as described in “Materials and methods,” and is represented as percent decrease in protein synthesis compared with rate in untreated control cells. (B) Apoptosis. HL-60/VCR cells were treated with 1.0 nM DT388–GM-CSF for the times shown and chromatin fragmentation was measured by the Cell Death Detection ELISA as described.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534003.jpeg?Expires=1763684852&Signature=SJ857Vz5QilayvHBQRb90eDoZ-jvYBHfjBcpMIZbrkWAOgWCIxB4w7yys-TWp--1RLNAWhIE45RSzcK~m-T-bgBVCGM9JH5wcaPmu-6XYB0DzJn9Z0alGlVheUO1vXuijQQCV7Uy7g-JZwc2GZOwty33DUJaeCOdiEf6~H2CTS4jzxC9AgPLbxi25IS6Iy~UnD6oT~XBTF98F0lS65Pjb36OWuguviiAHUXoKXeZLLJuLRnQqId44Y~tvPR8NP1nsbXFqLI~YIkkLWPjLB9PYHll~VJw~sq9oz6VwqvYIV9wjoAL~JOvq6E4tl2NXksSHKSb2KINZI1GWgAab05Mrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of protein synthesis inhibitors on ceramide metabolism in HL-60/VCR cells. / Cells (500 000/well) were seeded in 6-well plates in complete medium containing 1.0 μCi [3H]palmitic acid, and incubated for 6 hours with the agents indicated: emetine (0.5 μg/mL); hygromycin B (80 μg/mL); GM-CSF (1.0 nM); DT388–GM-CSF (1.0 nM), or combinations. Total cellular lipids were extracted, and [3H]ceramide was quantitated. The concentrations of emetine and hygromycin B are those that promote a 50% suppression of protein synthesis in HL-60/VCR cells at approximately 6 hours. Each experimental group represents the average of 3 cultures with variation less than 10%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534004.jpeg?Expires=1763684852&Signature=wsyuZt2crAic1wOg-QKiToGRrOzQLfrByuF9j6DoCVtor8ATZkyRxusvZonXjrv0wwGBIHzokHBMkbH4FCvuY6fDhZNJ~HPEl8iOyeMvK7uws4eukqbXM4k8zm~VGl0Wez~fUyiwbkSiwnYxxpHrxuXbPV9k13jQcdJ2aMV2HT1k0e3nWhhAfKJ9KSwUHDArYrZQmTBMfiEQSlMHQGR7fU7tipxusxchOKX0fB4jonHj5ZTcSKIWpviu68HL3gdj3POZx8s7-9v9M2oxyjBb3lOwgMLf9sFJioyxsDb~xHitjLrf4ieZg69T~v8K~7Asvtk7XzM0gx41034M35JaWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Influence of diphtheria toxin on ceramide metabolism in U-937 cells. / Cells were seeded in 6-well plates and treated with toxin (37.5 ng/mL) for the times indicated, in medium containing [3H]palmitic acid. Total cellular lipids were extracted and analyzed for [3H]sphingomyelin and [3H]ceramide by TLC. Minus toxin controls were run at each time point. Data, shown as percent of control, were calculated from disintegrations per minute (dpm) tritium in sphingomyelin or ceramide per 500 000 dpm total lipid. n = 3 ± SD for each experimental point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534007.jpeg?Expires=1763684852&Signature=eBL9QxJelHOCLyMHvO6B~ld4A4GvKD8FLZY5gWfZIDwPAPFWKkT4K0fWTKa1Vqda4SFLskTxHsTa5rYlRrkjgXiWJVxwqnn2XKACpyjL7G5yf2PgCJOummy9BsIaTFJBXht7GhlqbldD09fg2xKsS-bzC~1wTdY1PfybYm1eor7mRPEAKWC5XCpA1P5hScmg8K1iAjIucnGhkNVUuRJ2KuuKUC3RUsykv3yQTKZch2pb~xHfBsbBFZtxO6Skk1VcaejykHcfR9Z1ikK4WKPLFiFKFeAh4SFtjAYHlvXBx5pKbgSlV5l4ONvodYbNhQo-iGmLAJFBfc4W6su6h1telw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Influence of sphingomyelinase treatment on ceramide metabolism and protein synthesis. / (A,B) HL-60/VCR cells, prelabeled with [3H]palmitic acid, were incubated with sphingomyelinase for 4 hours, and lipids were extracted and analyzed. (C) To measure protein synthesis, cells were incubated with sphingomyelinase for 1 hour, before addition of [3H]leucine (1.0 μCI/well) for 3 hours. Data points represent the mean ± SD (n = 3). SD bars are not shown (error < 5%). SM indicates sphingomyelin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1927/7/m_h81811534009.jpeg?Expires=1763684852&Signature=vWmLgN0h6nTH1RJU9B9hjaFj7uj4Tdo4YUzCVFIt2JpAVJS2ydJEz8pxOooAeb1gcTbJl3hg0OlEooKC8M1HV2pyo-uIfv3jdGKcyklWFNqfGsn~xtBOD4Bav8r87hexIHkPBK3aKKfskTJN3XzP0BRp7PDHhvZj1m8eep320JGOcQVWPWYj4fGrr72lVzOpQC0B8bHyJC0XHsH4wq9Nu~t5Z6ju4oDHJW2gXw1rDeMFSiqhu4taPnefL5fn4vdi4CSx~DhA6AC94H2ySOQcyvtSXE203lSIbSm4vshmITWOG-KwFjARDe2JFPYK2YBd-cWW9mL6ILL9tQyKeaDj9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)