Hematopoietic stem cells (HSCs) are unique in their capacity to maintain blood formation following transplantation into immunocompromised hosts. Expansion of HSCs in vitro is therefore important for many clinical applications but has met with limited success because the mechanisms regulating the self-renewal process are poorly defined. We have previously shown that expression of the LIM-homeobox gene Lhx2 in hematopoietic progenitor cells derived from embryonic stem cells differentiated in vitro generates immortalized multipotent hematopoietic progenitor cell lines. However, HSCs of early embryonic origin, including those derived from differentiated embryonic stem cells, are inefficient in engrafting adult recipients upon transplantation. To address whetherLhx2 can immortalize hematopoietic progenitor/stem cells that can engraft adult recipients, we expressed Lhx2 in hematopoietic progenitor/stem cells derived from adult bone marrow. This approach allowed for the generation of immortalized growth factor–dependent hematopoietic progenitor/stem cell lines that can generate erythroid, myeloid, and lymphoid cells upon transplantation into lethally irradiated mice. When transplanted into stem cell–deficient mice, these cell lines can generate a significant proportion of circulating erythrocytes in primary, secondary, and tertiary recipients for at least 18 months. Thus, Lhx2immortalizes multipotent hematopoietic progenitor/stem cells that can generate functional progeny following transplantation into lethally irradiated hosts and can long-term repopulate stem cell–deficient hosts.
Introduction
Hematopoietic stem cells (HSCs) can be isolated on the basis of cell surface markers and have the unique capacity to regenerate and maintain a functional hematopoietic system following transplantation into immunocompromised mice.1 The ability to isolate HSCs and their importance in clinical applications have led to the development of a variety of different culture systems for expanding HSCs in vitro.2-5 Expansion of HSCs using these different culture systems has met with modest success because the molecular and cellular mechanisms regulating the self-renewal process in HSCs are largely unknown.6
An alternative approach for expanding HSCs in vitro is to immortalize them by genetic manipulation, and stem cell–like cell lines have been established using such methods. Most of these cell lines either show a limited ability to generate functional hematopoietic cells after transplantation or have thus far not been thoroughly analyzed in this respect.7-10 One cell line was reported to generate functional cells in vivo, but the genetic event that led to the immortalization was unknown.11 Hence, generation of this cell line cannot be easily reproduced. We have previously generated HSC-like cell lines by expressing the LIM-homeobox gene Lhx2in hematopoietic progenitor cells (HPCs) derived from embryonic stem (ES) cells differentiated in vitro.12 However, HSCs of early fetal origin, including those derived from ES cells differentiated in vitro, are for unknown reasons inefficient in engrafting in an adult environment.13-17
To address whether Lhx2 can reproducibly immortalize hematopoietic progenitor/stem cells that have the capacity to engraft adult recipients, we expressed the Lhx2 gene in hematopoietic progenitor/stem cells derived from adult bone marrow (BM). This approach allowed for the generation of clonal and cytokine-dependent stem cell–like cell lines. These cell lines can generate different functional hematopoietic cells in vivo, including a high proportion of circulating erythrocytes in stem cell–deficient hosts for an extended time.
Materials and methods
Mice
Mice used in these studies were obtained from Jackson Lab and maintained at the animal facility at Umeå University under pathogen-free conditions. Cell lines were generated from the C57BL/6-cast (B6-cast) mouse strain (genotypeGpi1a/Gpi1a, Ly5.2/Ly5.2). The C57BL/6-SJL (B6-SJL) mouse strain (Gpi1b/Gpi1b, Ly5.1/Ly5.1) and the c-kit kinase–deficient C57BL/6-W41 /W41 (B6W) mouse strain (Gpi1b/Gpi1b, Ly5.2/Ly5.2) were used as recipient mouse strains in the transplantation experiments. All animal experiments carried out in this report were approved by the ethical committee at the Umeå University.
Retrovirus production and infection of BM cells
The complementary DNA of mouse Lhx2 was inserted into the EcoRI site upstream of the pgk-neo cassette in the MND-X-SN retroviral vector.18 The GP+E-86 cell line was transfected with the respective construct, and stable subclones producing the highest virus titers were selected for transduction of BM cells. B6-cast mice 10 to 12 weeks old were treated with 150 mg/kg 5-fluorouracil (5-FU) (SIGMA, St Louis, MO) 3 days prior to BM harvest. Harvested BM cells were cultured for 48 hours in Iscoves modified Dulbecco medium (IMDM) (Gibco-BRL, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS) (Integro, the Netherlands), 1.5 × 10−4 M monothiolglycerol (MTG) (SIGMA), 100 ng/mL Steel factor (SF) (R&D Systems, Abingdon, United Kingdom), 10 ng/mL interleukin-6 (IL-6) (R&D Systems), and IL-3 used at 1% final concentration. IL-3 was obtained from media conditioned by X63Ag8-653 myeloma cells transfected with a vector containing murine IL-3.19 The cells were subsequently transferred to the virus producing GP+E-86 cells in the same media supplemented with 4 μg/mL Polybrene (hexadimethrine bromide) (SIGMA). After 48 hours of coculture with virus-producing cells, the BM cells were harvested by vigorous pipetting and transferred to IMDM media supplemented with 5% FCS, 1.5 × 10−4 M MTG, 0.75 mg/mL G418 (Gibco-BRL), 100 ng/mL SF, and with or without 10 ng/mL IL-6. G418 was kept in the media for 4 to 6 weeks, and nonadherent cells were continuously passaged. Because Lhx2 immortalizes HPCs by a cell nonautonomous mechanism,20 the transduced cells were continuously kept at a density of at least 2 × 106/mL until a homogenous population of blastlike cells was obtained. Established cell lines were maintained in IMDM media supplemented with 5% FCS, 1.5 × 10−4 M MTG, SF, and with or without IL-6 and were continuously kept at a cell density between 5 × 105/mL and 2 × 106/mL as previously described for SF-dependent HPCs immortalized byLhx2.12 20
Clonal assays in vitro and spleen colony-forming unit assays
Methylcellulose-based clonal assays were carried out in IMDM containing 1% methyl cellulose (Fluka, Neu-Ulm, Switzerland) supplemented with l-glutamine, 300 μg/mL iron-saturated transferrin (Boehringer, Ingelheim, Germany), 5% protein-free hybridoma medium II (Gibco-BRL), 1.5 × 10−4 M MTG, and 10% plasma-derived serum (Antech, Tyler, TX). The cells were plated in final volume of 1.25 mL in 35-mm Petri dishes (Falcon 3008) in triplicates. The following factors were used at a predetermined optimal concentration: erythropoietin (Epo) (Eprex Cilag, Sollentuna, Sweden) 4 U/mL, macrophage colony-stimulating factor (M-CSF) 20 ng/mL, granulocyte-CSF (G-CSF) 20 ng/mL, GM-CSF 10 ng/mL, thrombopoietin (Tpo) 20 ng/mL, and Flt3L 10 ng/mL. SF, IL-6, and IL-3 were used as previously described. All growth factors, except where indicated, were obtained from R&D Systems. In clonal assays of BM cells G418 was used at 1 mg/mL. For spleen colony-forming unit (CFU-S) assays different numbers of BM-HPCs, ranging between 300 to 106, were intravenously injected into lethally irradiated (10 Gy in a single dose from a 60Co source) B6-SJL mice. Ten days later the spleens of the animals receiving transplants were removed and fixed in Bouin solution (SIGMA), and the number of visible colonies on the spleen were counted to determine the frequency of CFU-Ss for each cell line.
Northern and Southern blot analysis
Total RNA was prepared using the RNAgents system (Promega, Madison, WI). Ten micrograms of total RNA was separated on a 1% formaldehyde agarose gel, blotted onto a Zeta-Probe GT blotting membrane (Bio-Rad, Hercules, CA), and hybridized to radioactively labeled probes according to standard procedures.21 Genomic DNA was prepared by standard procedures and subsequently digested withBamHI, separated on 1% agarose gel, blotted onto Zeta-Probe GT blotting membrane, and hybridized to radioactively labeled Neo-probe. All membranes were analyzed in a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Transplantation of BM-HPC lines and Gpi assays
Different numbers of BM-HPCs were transplanted by intravenous injection into lethally irradiated B6-SJL mice or sublethally irradiated (4-5 Gy) B6W mice. Mice were bled at various times after transplantation, and the blood was collected in microhematocrit tubes (Drummond Scientific, Broomall, PA). Erythrocytes were isolated from the erythrocyte fraction after centrifugation, lysed in a 7 mg/mL ethylenediaminetetraacetic acid solution, and subjected to glucose phosphate isomera (Gpi) analysis. Similarly, hemato/lymphoid organs were removed, single-cell suspensions were prepared, and following osmotic shock to eliminate erythrocytes, nucleated cells were lysed in ethylenediaminetetraacetic acid solution and subjected to Gpi analysis. B6-SJL and B6W mice have the Gpi1b allele at theGpi locus. The Gpi1b type forms a more rapidly migrating band from positive to negative on electrophoresis than does the Gpi1a type. Electrophoresis and detection of the Gpi types was adapted from Eppig et al and Harrison et al.22 23 Gpi assays were scanned in a flatbed scanner (ScanMaker III, MICROTEK, Redondo Beach, CA) and analyzed using the image analyzing program Scion Image (Scion, Frederick, MD). Donor contribution (percent donor) was calculated as % donor = 100 × (intensity Gpi1a band/[intensity Gpi1aband + intensity Gpi1b band]).
Serial transplantation
Total BM cells were harvested from primary B6W recipients at 4, 7, 9, and 12 months after transplantation, and erythrocytes were eliminated by osmotic shock. One million or 2 million unfractionated nucleated cells were injected intravenously into sublethally irradiated B6W mice or lethally irradiated B6-SJL mice. For transplantation into tertiary recipients, total BM cells were harvested from a secondary B6W recipient 6 months after transplantation. Two million unfractionated nucleated cells from the secondary recipient were injected intravenously into sublethally irradiated B6W mice. The secondary recipient used as a source of cells for serial transplantation into the tertiary recipients had received BM cells harvested from a primary recipient 9 months after transplantation. Gpi analyses of erythrocytes and hematopoietic organs were carried out as described above.
Flow cytometry
The monoclonal antibodies used in this study were direct conjugates with phycoerythrin (PE), fluorescein isothiocyanate, or biotin. The following antibodies were purchased from Pharmingen (San Diego, CA): anti-CD45.1 (A20), anti-CD45.2 (104), anti–Sca-1 (E13-161.7), anti–Gr-1 (RB6-8C5), anti–c-kit (2B6), anti–Mac-1 (M1/70), PE-TER119, F4/80, anti-CD3 (145-2C11), anti-CD4 (GK1.5), anti-CD8 (1044D), anti-CD19 (1D3), anti-Thy1.2 (53-2.1), anti–H-2Kb (AF-88.5), and anti-CD34 (RAM34). Unspecific antibody binding was prevented by incubating cells on ice in supernatant from the 2.4G2 hybridoma 15 minutes prior to all antibody labeling. The cells were incubated with specific antibodies on ice for 20 minutes, washed twice, and subsequently incubated with PE-conjugated streptavidin (Southern Biotechnology, Birmingham, AL). Labeled cells were washed twice before being analyzed in a FACScan (Becton Dickinson, Silicon Valley, CA).
Results
Generation of cytokine-dependent cell lines from adult BM
We previously showed that Lhx2 could immortalize HPCs derived from ES cells differentiated in vitro.12 To elucidate whether Lhx2 also can immortalize hematopoietic progenitor/stem cells derived from adult mice, BM cells from 5-FU–treated B6-cast mice were transduced with virus containing theLhx2 gene or control vector. Transduced cells were subsequently cultured in the presence of SF or SF/IL-6. After 4 to 5 weeks in culture, a high proportion (> 99%) of the cells transduced with Lhx2 showed blastlike morphology with a large nuclei and a small rim of cytoplasm (Figure 1A). These cultures of expanded blastlike cells are referred to as BM-derived HPC (BM-HPC) lines. The cells transduced with control vector differentiated into neutrophils and mast cells after 3 to 4 weeks of culture, and no viable cells were present after an additional 2 to 3 weeks. The doubling time of BM-HPC lines established in SF or in SF/IL-6 was 40 hours and 22 hours, respectively. However, the lines established in SF/IL-6 did not proliferate in IL-6 alone because after 24 hours in culture in the presence of IL-6 alone, more than 80% of the cells were not viable based on trypan exclusion. Thus, BM-HPC lines are strictly dependent on SF for growth, whereas IL-6 only promotes proliferation in combination with SF. Northern blot analysis revealed that the BM-HPC lines express Lhx2 from the retroviral vector at a similar level as a cell line generated from ES cells differentiated in vitro (Figure 1B). The 2 BM-HPC lines we chose to study further were clonal because they both harbor one unique insertion site of the retroviral vector in the genome (Figure 1C). Cell surface marker analysis of the BM-HPC lines showed that those generated in SF/IL-6 expressed high levels of the mouse HSC marker Sca-1 (Figure 1D, top), whereas the cell line generated in SF alone displayed lower expression of this marker (Figure 1D, bottom). Other cell surface markers analyzed did not reveal any major differences between these cell lines because both were c-kit+, H-2Kb+ (major histocompatibility complex class I), CD45.2+, CD34−, Gr-1− (marker for neutrophils), Mac-1low/− (monocyte/macrophage), F4/80− (macrophage), TER119− (erythroid), CD19− (B cell), CD3− (T cell) (Figure 1E). Both the doubling time and cell surface marker phenotype for the BM-HPC lines have been stable for at least 10 weeks in vitro. Thus, expression of Lhx2 in hematopoietic progenitor/stem cells derived from BM of adult mice generates immortalized and cytokine-dependent cell lines.
Molecular and cellular analyses of the BM-HPC lines.
(A) May-Grünwald Giemsa staining of the BM-HPCs. Magnification × 100. (B) Northern blot analysis showing Lhx2 expression in 3 independently generated BM-HPC lines compared with a cell line generated from ES cells (HPC line no. 7). (C) Southern blot analysis of genomic DNA derived from the 2 BM-HPC lines used in this study showing a single proviral integration site in both cell lines. Cell surface marker analyses showing the difference in expression of the stem cell marker Sca-1 on the BM-HPC lines generated in SF/IL-6 (BM-HPC no. 5) (D, top) and in SF alone (BM-HPC no. 9) (D, bottom). (E) Flow cytometry analyses of cell surface markers showing similar expression pattern on BM-HPC line nos. 5 and 9.
Molecular and cellular analyses of the BM-HPC lines.
(A) May-Grünwald Giemsa staining of the BM-HPCs. Magnification × 100. (B) Northern blot analysis showing Lhx2 expression in 3 independently generated BM-HPC lines compared with a cell line generated from ES cells (HPC line no. 7). (C) Southern blot analysis of genomic DNA derived from the 2 BM-HPC lines used in this study showing a single proviral integration site in both cell lines. Cell surface marker analyses showing the difference in expression of the stem cell marker Sca-1 on the BM-HPC lines generated in SF/IL-6 (BM-HPC no. 5) (D, top) and in SF alone (BM-HPC no. 9) (D, bottom). (E) Flow cytometry analyses of cell surface markers showing similar expression pattern on BM-HPC line nos. 5 and 9.
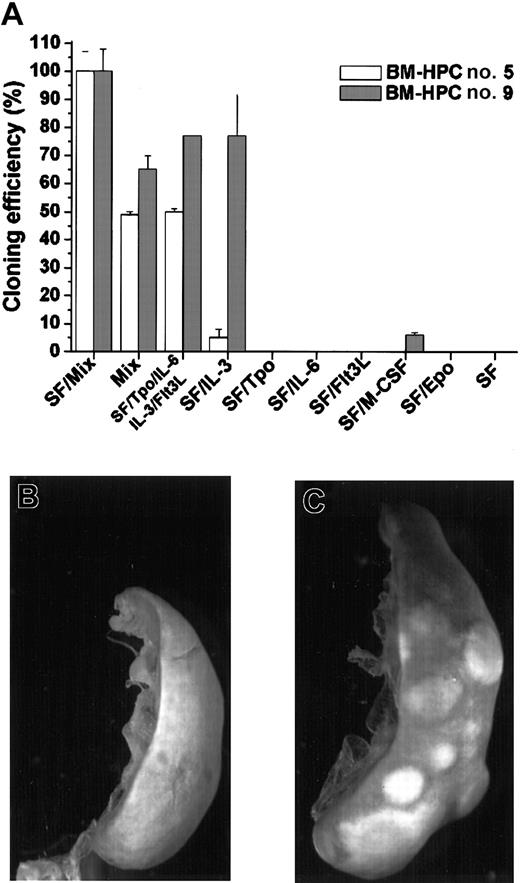
A broad combination of growth factors efficiently promotes proliferation of the BM-HPC lines at low cell density, whereas no significant proliferation was observed in SF or SF/IL-6
Normal hematopoietic progenitor/stem cells only proliferate when cultured in a broad combination of early-acting growth factors.24-26 We have also previously shown that SF-dependent HPCs immortalized by Lhx2 do not respond to SF at low cell density because the SF-dependent proliferation is due to an additional and cell nonautonomous mechanism.20 This prompted us to analyze the response of the BM-HPC lines to different growth factors/growth factor combinations in clonal assays. The total number of colonies generated by the BM-HPCs in clonal assays in the presence SF/Mix (SF/Tpo/IL-3/IL-6/GM-CSF/G-CSF/M-CSF/Epo) was arbitrarily set as 100% and served as reference for all other combinations (Figure 2A). The frequency of colony-forming cells (CFCs) using this factor combination did not differ between the 2 cell lines and ranged between 30 and 40 CFCs per 100 cells plated. The main difference between line nos. 5 and 9 is that the latter is highly responsive to IL-3 because the plating efficiency in SF/IL-3 was 77% for line no. 9 and 5% for line no. 5 (Figure 2A). Thus, line no. 5 required a more complex mix of growth factors for efficient proliferation. None of the cell lines responded to Tpo, Flt3L, or Epo in combination with SF, and M-CSF in combination with SF promoted a low response of line no. 9, where the plating efficiency was low (6%) and the colonies generated were small (< 100 cells). The BM-HPCs reveal a limited potential in vitro because the major cell type in the colonies, independent of factor combination, was large macrophagelike cells expressing Mac-1 (data not shown). Neither line no. 5 nor line no. 9 showed significant proliferation in response to SF or SF/IL-6 at these cell densities (< 104/mL) (Figure2A). Thus, the BM-HPC lines respond efficiently to a broad combination of growth factors, whereas their response to SF and SF/IL-6 appear to be cell density–dependent similar to the cell lines we generated from Lhx2-transduced ES cells differentiated in vitro.20
BM-HPCs efficiently generate colonies in vitro in response to a broad spectrum of growth factors and have CFU-S activity.
(A) Colony formation in vitro in the indicated factor/factor combinations of the BM-HPC line generated in SF/IL-6 (no. 5) and the BM-HPC line generated in SF (no. 9). Cloning efficiency is measured as the total number of colonies generated in the different factor/factor combinations compared with the total number of colonies generated in SF/Mix (SF/Tpo/IL-3/IL-6/GM-CSF/G-CSF/M-CSF/Epo), which is arbitrarily set as 100%. Spleen of a lethally irradiated B6-SJL control mouse (B) and a spleen of a mouse transplanted with BM-HPCs (C) 10 days after transplantation and fixed in Bouin solution. Original magnification B-C, × 9.6.
BM-HPCs efficiently generate colonies in vitro in response to a broad spectrum of growth factors and have CFU-S activity.
(A) Colony formation in vitro in the indicated factor/factor combinations of the BM-HPC line generated in SF/IL-6 (no. 5) and the BM-HPC line generated in SF (no. 9). Cloning efficiency is measured as the total number of colonies generated in the different factor/factor combinations compared with the total number of colonies generated in SF/Mix (SF/Tpo/IL-3/IL-6/GM-CSF/G-CSF/M-CSF/Epo), which is arbitrarily set as 100%. Spleen of a lethally irradiated B6-SJL control mouse (B) and a spleen of a mouse transplanted with BM-HPCs (C) 10 days after transplantation and fixed in Bouin solution. Original magnification B-C, × 9.6.
The BM-HPC lines have CFU-S activity
Immature hematopoietic progenitor/stem cells can generate colonies on the spleen upon transplantation into lethally irradiated recipients.1 27 To test whether the BM-HPC lines have this potential, we transplanted different numbers of the respective cell line into lethally irradiated B6-SJL. Control mice did not show any visible colonies on the spleen after 10 days (Figure 2B), whereas all mice injected with BM-HPCs showed numerous colonies on the spleen (Figure 2C). The estimated frequency of CFU-Ss in the respective cell line was 2 ± 1 per 300 cells for line no. 5 and 4 ± 2 per 103 cells for line no 9.
BM-HPC lines are multipotent and generate functional cells in vivo
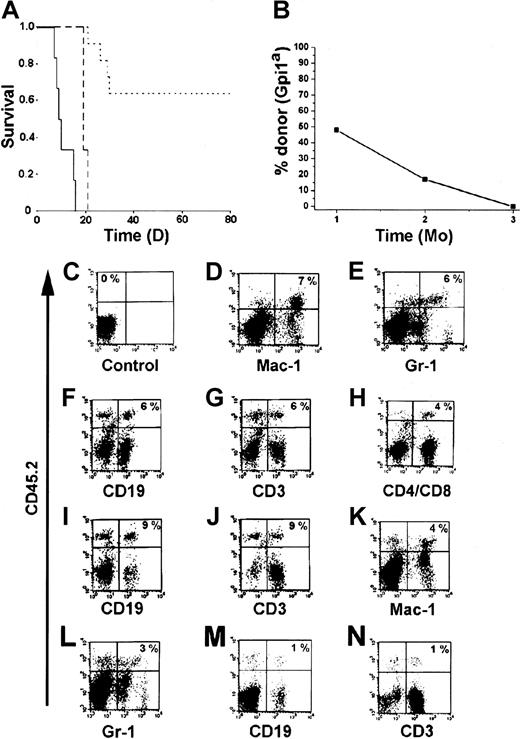
An important characteristic of HSCs is that they should be able to generate mature and functional hematopoietic cells in vivo. One of the most stringent assays to test for function in vivo is to analyze whether the cells can protect mice from radiation-induced death.1 28 Therefore, we transplanted the BM-HPC lines (Gpi1a, CD45.2) into lethally irradiated B6-SJL mice (Gpi1b, CD45.1). Sixty-four percent of the mice that received 3 × 106 BM-HPCs survived the acute effects of irradiation (Figure 3A). All recipients transplanted with less than 3 × 106 cells died within 21 days, and the control mice receiving no cells died within 16 days (Figure 3A). Gpi analysis of erythrocytes in peripheral blood of the surviving mice 1 month after transplantation confirmed the presence of significant numbers of donor erythrocytes in the circulation (Figure3B). Donor contribution to circulating erythrocytes decreased over time and was undetectable 3 months after transplantation (Figure 3B), at which time endogenous erythropoiesis was completely restored. Flow cytometry analysis of different hematopoietic organs from animals receiving transplants revealed the presence of donor-derived (CD45.2+) leukocytes. Leukocytes generated by the BM-HPC lines were myeloid cells (Mac-1+ and Gr-1+) (Figure 3D,E,K,L), B cells (CD19+) (Figure 3F,I,M), and T cells (CD3+, CD4+/CD8+) (Figure3G,H,J,N). The Sca-1low line appears to be less efficient in generating lymphoid cells as compared with the Sca-1+line (compare Figure 3M,N with Figure 3F,G). These findings clearly demonstrate that the BM-HPC lines are multipotent—able to generate myeloid cells and lymphoid cells in addition to erythrocytes. The decrease in donor cells in peripheral blood was followed by a similar decrease of donor cells in all hematopoietic organs analyzed (BM, spleen, thymus, lymph nodes), and donor cells were undetectable 3 to 4 months after transplantation (data not shown). Together, these observations indicate that the BM-HPC lines are multipotent, can provide some radioprotection, and can short-term repopulate lethally irradiated wild-type B6 hosts.
The BM-HPC lines are multipotent and generate functional cells upon transplantation into lethally irradiated mice.
(A) Kaplan-Meier plot of mice receiving a lethal dose (10 Gy) of radiation and transplanted with 3 × 106 BM-HPCs (11 mice), 106 (3 mice) BM-HPCs, or mock-transplanted controls (6 mice). Data for line nos. 5 and 9 are pooled. Solid line indicates control mice; broken line, mice receiving 106cells; dotted line, mice receiving 3 × 106cells. (B) Average donor contribution to circulating erythrocytes based on Gpi assays in surviving mice at different time points after transplantation. SE is included in each time point. Cell surface marker analyses for the presence of donor cells (CD45.2+ vertical axes) of different lineages (horizontal axes) in control B6-SJL mice (C) and in representative B6-SJL mice receiving either BM-HPC line no. 5 (D-J) or no. 9 (K-N) analyzed 6 to 8 weeks after transplantation. Percentage donor cells of different lineages is indicated in the upper right quadrant for the mice receiving transplants (D-N) and in the upper left quadrant in the control (C) (eg, no lineage marker is included). Organs analyzed were spleen (D-G,K-N) and lymph nodes (C,H-J).
The BM-HPC lines are multipotent and generate functional cells upon transplantation into lethally irradiated mice.
(A) Kaplan-Meier plot of mice receiving a lethal dose (10 Gy) of radiation and transplanted with 3 × 106 BM-HPCs (11 mice), 106 (3 mice) BM-HPCs, or mock-transplanted controls (6 mice). Data for line nos. 5 and 9 are pooled. Solid line indicates control mice; broken line, mice receiving 106cells; dotted line, mice receiving 3 × 106cells. (B) Average donor contribution to circulating erythrocytes based on Gpi assays in surviving mice at different time points after transplantation. SE is included in each time point. Cell surface marker analyses for the presence of donor cells (CD45.2+ vertical axes) of different lineages (horizontal axes) in control B6-SJL mice (C) and in representative B6-SJL mice receiving either BM-HPC line no. 5 (D-J) or no. 9 (K-N) analyzed 6 to 8 weeks after transplantation. Percentage donor cells of different lineages is indicated in the upper right quadrant for the mice receiving transplants (D-N) and in the upper left quadrant in the control (C) (eg, no lineage marker is included). Organs analyzed were spleen (D-G,K-N) and lymph nodes (C,H-J).
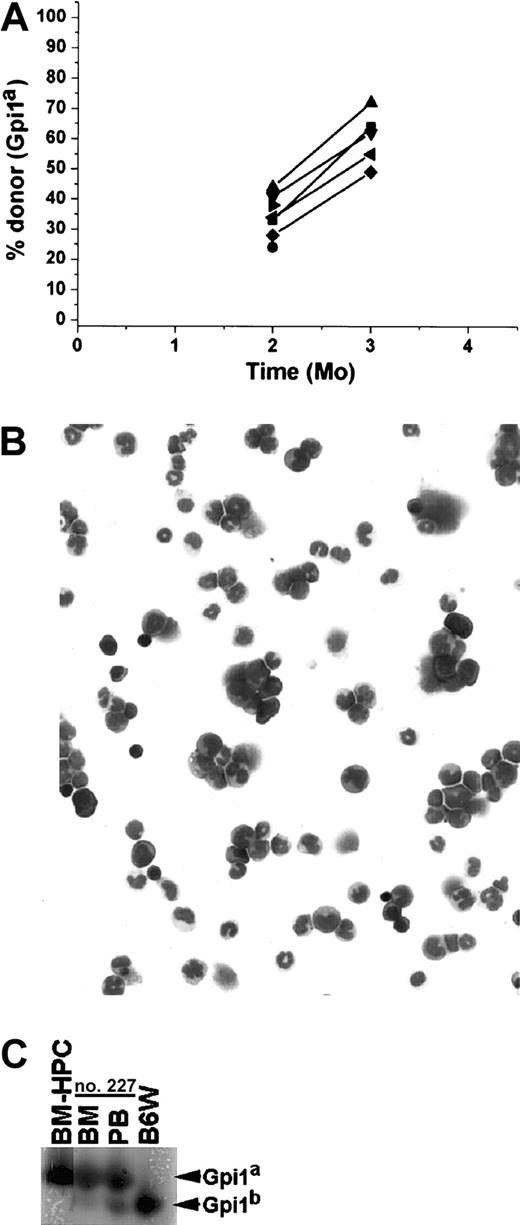
BM-HPC lines long-term repopulate stem cell–deficient mice
To further investigate the long-term repopulating potential of the BM-HPC lines, they were transplanted into the stem cell–deficient B6W mouse strain, which provides a less competitive environment for engraftment.29 BM-HPC lines were transplanted at different cell doses (3 × 104-3 × 106 cells) into sublethally irradiated B6W recipients, and donor contribution to circulating erythrocytes was monitored regularly. All recipients showed significant donor contribution to erythrocytes in peripheral blood 1 month after transplantation. The proportion of donor-derived erythrocytes ranged from 9% to 85%, roughly correlating to the number of cells transplanted (Figure 4A). All mice receiving 2 × 106 or fewer BM-HPCs lost donor contribution in all hematopoietic organs analyzed within 4 to 5 months after transplantation (data not shown). However, most of the animals receiving 3 × 106 cells had a significant proportion of donor-derived cells in the erythrocyte fraction in peripheral blood for an extended time (Figure 4B). Donor contribution decreased in all recipient mice 2 to 3 months after transplantation, and thereafter it stabilized, increased, or fluctuated. Six of 13 recipients had a donor contribution above 20% during the whole time period, and 7 of 13 recipients had undetectable levels of donor erythrocytes at one or more time points. Six mice analyzed 1 year after transplantation showed significant donor contribution, ranging from 23% to 72% (Figure 4B). Mice killed at various time points, including those showing no donor contribution to peripheral blood (Figure 4C), revealed no significant difference in the donor contribution to nucleated cells in the BM (average 54% ± 8%). These data reveal that all mice receiving 3 × 106 BM-HPCs showed sustained engraftment, suggesting that lack of donor contribution to circulating erythrocytes reflects fluctuations in erythropoiesis that are not due to progenitor/stem cell exhaustion.
The BM-HPC lines engraft stem cell–deficient mice for an extended time.
Data for BM-HPC line nos. 5 and 9 are pooled because no difference in reconstitution ability could be observed. (A) Gpi analyses 1 month after transplantation of circulating erythrocytes in B6W mice receiving different doses of BM-HPCs. Displayed are the Gpi assays together with the calculated value for the fraction (in percent) of donor Gpi1a isoform for each individual mice receiving the indicated cell dose. (B) Percentage donor-derived erythrocytes in peripheral blood at different time points of 13 individual recipients receiving 3 × 106 BM-HPCs. Each line indicates an individual mouse. (C) Three examples of primary recipient animals (nos. 51, 64, 150) receiving 3 × 106 BM-HPCs showing no donor contribution to peripheral blood (PB) but significant donor contribution (≥ 50%) to nucleated cells in the BM. Bands corresponding to the respective Gpi isoforms are indicated.
The BM-HPC lines engraft stem cell–deficient mice for an extended time.
Data for BM-HPC line nos. 5 and 9 are pooled because no difference in reconstitution ability could be observed. (A) Gpi analyses 1 month after transplantation of circulating erythrocytes in B6W mice receiving different doses of BM-HPCs. Displayed are the Gpi assays together with the calculated value for the fraction (in percent) of donor Gpi1a isoform for each individual mice receiving the indicated cell dose. (B) Percentage donor-derived erythrocytes in peripheral blood at different time points of 13 individual recipients receiving 3 × 106 BM-HPCs. Each line indicates an individual mouse. (C) Three examples of primary recipient animals (nos. 51, 64, 150) receiving 3 × 106 BM-HPCs showing no donor contribution to peripheral blood (PB) but significant donor contribution (≥ 50%) to nucleated cells in the BM. Bands corresponding to the respective Gpi isoforms are indicated.
BM-HPCs efficiently engraft secondary recipients
A unique characteristic of HSCs is their capacity to generate repopulating cells that can be detected following transplantation into secondary recipients.30 31 To test if the BM-HPC lines display this potential, we transplanted 1 × 106 to 2 × 106 BM cells from primary B6W recipients into sublethally irradiated secondary B6W recipients and lethally irradiated B6-SJL recipients. Eight secondary B6W recipients showed a significant fraction of donor-derived erythrocytes in peripheral blood at 1 month after transplantation, and all mice in this group maintained a high donor contribution thereafter (> 70%) throughout the analysis period (Figure 5A). One mouse in this group had 100% donor contribution to circulating erythrocytes 3 months after transplantation, and 3 mice in this group had 100% donor-derived erythrocytes in peripheral blood 6 months after transplantation (Figure5A). Six secondary B6W recipients did not generate detectable levels of donor erythrocytes in peripheral blood until 4 months after transplantation, and mice in this group analyzed at 7 months after transplantation maintained a significant donor contribution in peripheral blood. In one mouse we did not detect donor-derived erythrocytes until 6 months after transplantation, and this mouse maintained a significant donor contribution at 8 months after transplantation (Figure 5A). Six of 13 secondary wild-type B6-SJL recipient animals showed a significant fraction of donor-derived erythrocytes in peripheral blood 3 months after transplantation, ranging from 36% to 58% (Figure 5B). Four of 5 secondary wild-type B6-SJL mice analyzed 4 months after transplantation showed significant donor contribution to circulating erythrocytes, ranging from 30% to 51% (Figure 5B). This is in contrast to the primary B6-SJL recipient animals where no donor-derived erythrocytes could be detected beyond 2 months after transplantation (compare Figure 5B with Figure 3B). Secondary B6W and B6-SJL recipients lacking donor-derived erythrocytes in peripheral blood had at least 50% donor contribution to nucleated cells in BM (Figure 5C). This observation supports the idea that lack of donor contribution to erythrocytes reflects fluctuations in erythropoiesis and is not due to progenitor/stem cell exhaustion.
The BM-HPCs engraft after serial transplantation into secondary recipients.
(A) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 22 individual secondary stem cell[–deficient B6W recipients. Fifty-five percent of the animals received 106 and 45% of the animals received 2 × 106BM cells from primary B6W recipients. (B) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 13 individual secondary B6-SJL wild-type recipients. All mice received 2 × 106 BM cells from the primary B6W recipients. Each line indicates an individual mouse. (C) Three examples of secondary recipient animals (nos. 130, 165, 132) showing no donor contribution to peripheral blood (PB) but significant donor contribution (≥ 50%) to nucleated cells in the BM. Bands corresponding to the respective Gpi isoforms are indicated.
The BM-HPCs engraft after serial transplantation into secondary recipients.
(A) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 22 individual secondary stem cell[–deficient B6W recipients. Fifty-five percent of the animals received 106 and 45% of the animals received 2 × 106BM cells from primary B6W recipients. (B) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 13 individual secondary B6-SJL wild-type recipients. All mice received 2 × 106 BM cells from the primary B6W recipients. Each line indicates an individual mouse. (C) Three examples of secondary recipient animals (nos. 130, 165, 132) showing no donor contribution to peripheral blood (PB) but significant donor contribution (≥ 50%) to nucleated cells in the BM. Bands corresponding to the respective Gpi isoforms are indicated.
BM-HPCs engraft tertiary recipients
An additional property that has been used to characterize HSCs is their ability to generate repopulating cells in the secondary recipient that can be detected following transplantation into tertiary recipient.32 33 To test whether the BM-HPCs also have this property, 2 million BM cells from a secondary B6W recipient were serially transplanted into sublethally irradiated B6W tertiary recipients. Analysis of the tertiary recipients revealed that all 7 mice analyzed had significant donor contribution to circulating erythrocytes at 2 months after transplantation, ranging from 24% to 44% (Figure 6A). Five of the tertiary recipients analyzed at 3 months after transplantation had donor-derived erythrocytes in peripheral blood, ranging from 49% to 72% (Figure6A). Morphologic analysis of the nucleated cells in the BM of tertiary recipients showed numerous myeloid cells (Figure 6B). Gpi assays of the nucleated cells in the BM of the tertiary recipients revealed a high proportion (> 90%) of donor cells (Figure 6C), indicating that other hematopoietic lineages in addition to the erythroid lineage were generated in the tertiary recipients. Thus, similar to normal HSCs, the BM-HPC lines can generate repopulating cells in the secondary recipient that can be detected by serial transplantation into tertiary recipients.
The BM-HPCs engraft after serial transplantation into tertiary recipients.
(A) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 7 individual tertiary stem cell–deficient B6W recipients. All mice received 2 × 106 BM cells from the secondary B6W recipient. The secondary recipient used was killed 6 months after transplantation. The secondary recipient received BM cells from a primary recipient killed 9 months after transplantation. (B) May-Grünwald Giemsa staining of nucleated cells from BM isolated from a tertiary recipient killed 4 months after transplantation. Original magnification B, × 40. (C) Gpi assay of the nucleated cells in the BM and of erythrocytes in peripheral blood (PB) of the same individual analyzed in panel B.
The BM-HPCs engraft after serial transplantation into tertiary recipients.
(A) Fraction of donor-derived erythrocytes in peripheral blood at different time points of 7 individual tertiary stem cell–deficient B6W recipients. All mice received 2 × 106 BM cells from the secondary B6W recipient. The secondary recipient used was killed 6 months after transplantation. The secondary recipient received BM cells from a primary recipient killed 9 months after transplantation. (B) May-Grünwald Giemsa staining of nucleated cells from BM isolated from a tertiary recipient killed 4 months after transplantation. Original magnification B, × 40. (C) Gpi assay of the nucleated cells in the BM and of erythrocytes in peripheral blood (PB) of the same individual analyzed in panel B.
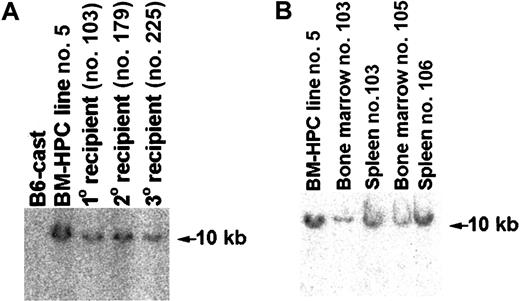
Transplanted mice are engrafted with transduced cells
To confirm that the cells of donor Gpi type in engrafted mice were transduced with the retroviral vector, genomic DNA prepared from hemato/lymphoid organs of primary, secondary, and tertiary recipient animals was analyzed for the presence of proviral sequence. A proviral insertion could be detected in genomic DNA derived from spleen and BM of all mice receiving transplants showing donor cells based on the presence of Gpi1a marker in these organs (Figure7 and data not shown). Mice with donor cells in the BM as determined Gpi assays also had a significant fraction of G418-resistant (G418R) CFCs in the BM (Table 1), further supporting that the donor cells contained retroviral DNA. Furthermore, the integration site of the provirus in the genome of cells in different hemato/lymphoid organs of engrafted primary, secondary, and tertiary recipients appears to be identical to that of the original cell line (Figure 7A-B). These results show that cells engrafting primary, secondary, and tertiary recipients are derived from the original hematopoietic progenitor/stem cell transduced with Lhx2 and expanded in vitro.
Mice transplanted with BM-HPCs are engrafted with cells showing the same proviral integration site as the original BM-HPC line.
(A) Southern blot analysis of genomic DNA using Neo as a probe comparing the proviral integration site in the original BM-HPC lines (no. 5) with that of a spleen of a primary recipient (no. 103), a spleen of a secondary recipient (no. 179), and a spleen of a tertiary recipient (no. 225). All organs analyzed showed at least 50% donor contribution based on Gpi assays. Normal BM from the donor mouse strain (B6-cast) is used as negative control. (B) Similar to panel A, but analysis of the BM cells of the primary recipient analyzed in panel A (no. 103) and the BM and spleen of 2 additional primary recipients (nos. 105, 106) were also included.
Mice transplanted with BM-HPCs are engrafted with cells showing the same proviral integration site as the original BM-HPC line.
(A) Southern blot analysis of genomic DNA using Neo as a probe comparing the proviral integration site in the original BM-HPC lines (no. 5) with that of a spleen of a primary recipient (no. 103), a spleen of a secondary recipient (no. 179), and a spleen of a tertiary recipient (no. 225). All organs analyzed showed at least 50% donor contribution based on Gpi assays. Normal BM from the donor mouse strain (B6-cast) is used as negative control. (B) Similar to panel A, but analysis of the BM cells of the primary recipient analyzed in panel A (no. 103) and the BM and spleen of 2 additional primary recipients (nos. 105, 106) were also included.
Discussion
Expression of the LIM-homeobox gene Lhx2 in adult hematopoietic progenitor/stem cells reproducibly generates multipotent hematopoietic progenitor/stem cell lines immortalized by a similar mechanism as previously described for hematopoietic progenitor/stem cells derived from ES cells differentiated in vitro.12,20The BM-HPC lines have some radioprotective properties and can generate erythroid, myeloid, and T and B lymphoid cells upon transplantation. The BM-HPC lines also demonstrate robust contribution to the mature erythrocyte population in stem cell–deficient primary, secondary, and tertiary recipients mice for an aggregate time of at least 18 months, revealing a remarkable potential for self-renewal and differentiation in vivo. Our results also indicate that recipients can survive when the erythrocytes are exclusively derived from the cell line, because in some instances 100% of the circulating erythrocytes in the stem cell–deficient animals receiving transplants were of donor origin. This strongly suggests that the BM-HPC lines produce functional erythrocytes in vivo. Collectively, these characteristics make the BM-HPC lines unique in comparison with the previously described immortalized stem cell–like cell lines.7-11
Although the data presented suggest that the BM-HPC lines are HSC-like, the BM-HPC lines are less efficient in engrafting immunocompromised mice as compared with normal HSCs. Because Lhx2 is most likely not expressed in HSCs,34 the ectopic expression ofLhx2 in HSCs may alter these cells to reduce their fitness in vivo. This idea is supported by the observations that they can long-term repopulate in a less competitive environment provided by the BM of a stem cell–deficient host. The dependence on a certain host environment for long-term engraftment is unexpected because the difference between so-called long-term and short-term repopulating stem cells has been suggested to be due to intrinsic differences in their ability to self-renew.35 Whether properties distinct from intrinsic capacity to self-renew between different subpopulations of HSCs can contribute to the difference between long-term and short-term repopulating stem cells remains to be elucidated.
Normal HSCs show decreased efficiency in engrafting upon serial transplantation that is suggested to be caused by intrinsic changes and/or to dilution of HSCs.36-39 However, engraftment of BM-HPCs in the secondary and tertiary recipients appears to be more efficient than in the primary recipients because reproducible engraftment was observed in all secondary and tertiary recipients with fewer BM-HPCs as compared with the number injected into primary recipients. Furthermore, the BM-HPCs could also contribute to a significant proportion of circulating erythrocytes in secondary wild-type recipients 4 months after transplantation, whereas primary wild-type recipients never showed donor contribution in peripheral blood beyond 2 months after transplantation. These observations suggest that the BM-HPC lines acquire characteristics in the BM environment that increase their ability to compete with host cells. The nature of this change of phenotype is at present unknown, but it is further supported by the observation that we have thus far been unable to reestablish any type of cell line from animals receiving transplants, suggesting that the change is intrinsic to the BM-HPCs.
Another striking observation is the homogeneity of the BM-HPC lines despite being generated from such a heterogeneous cell population as BM from 5-FU–treated mice. Also, the third independently generated BM-HPC line (line no. 28, Figure 1A) shows the identical pattern of cell surface marker expression as BM-HPC line no. 5 (eg, Sca-1+/c-kit+/CD34−/Lin−/low; data not shown). This suggests that the mechanism wherebyLhx2 immortalizes cells is specific for stem cells and that a highly purified population of HSCs is not required for generating HSC-like cell lines. Furthermore, Lhx2-deficient mice die in utero due to severe anemia, and both the anemia in these mutant mice and the self-renewal of the stem cell–like cells immortalized byLhx2 is based on a cell nonautonomous mechanism.20,40 The specific mediator(s) responsible for the cell nonautonomous phenotype has not yet been identified. A wide range of cytokines/growth factors cannot substitute for the cell density–dependent self-renewal of cell lines immortalized byLhx2, indicating that a novel growth factor/growth factor combination is responsible for Lhx2-induced immortalization. Because Lhx2 is a putative transcription factor,41 identification of genes regulated byLhx2 could therefore shed light on the molecular and cellular mechanisms responsible for expansion of HSCs both in vivo and in vitro. These aspects of HSC biology are critical for improving different clinical applications of stem cells.
We thank Dr Sara Wilson for critical reading of the manuscript.
Supported by the Swedish Cancer Society and the Tobias Foundation. K.R. was supported by the Foundation for Strategic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leif Carlsson, Dept of Molecular Biology, Umeå University, 901 87 Umeå, Sweden; e-mail: leif.carlsson@micro.umu.se.