Abstract
This study reports a lentiviral gene transfer protocol for efficient transduction of adult human peripheral blood (PB)–derived CD34+ NOD/SCID-repopulating cells (SRCs) using vesicular stomatitis virus–G protein (VSV-G)–pseudotyped lentiviruses encoding for enhanced green fluorescence protein (eGFP). Lentiviral stocks were concentrated by anion exchange chromatography, and transduction was performed under serum-free conditions at a multiplicity of infection (MOI) between 3 and 50. Similar transduction efficiencies were achieved in the presence and absence of cytokines. Transduction of PB-derived CD34+cells at a MOI of 3 resulted in gene transfer efficiencies into SRCs of 9.2% and 12.0% in the absence and presence of cytokines, respectively. Using improved lentiviral vectors, transduction frequency varied between 42.0% (MOI 10) and 36.0% (MOI 50) with multilineage transgene expression within SRC-derived myeloid and lymphoid cells. The protocol described can be adapted for clinical application of lentiviral gene transfer into PB-derived CD34+ cells from adult patients.
Introduction
Efficient ex vivo gene transfer into autologous hematopoietic stem cells (HSCs) and their subsequent transplantation could offer new therapeutic approaches for a variety of diseases such as inherited immunodeficiencies,1hemoglobinopathies,2 and metabolic defects. Successful correction of severe combined immunodeficiency (SCID-X1) has recently been achieved in infants by retroviral transduction of autologous bone marrow (BM) cells.1 However, the use of conventional oncoretroviruses requires cytokine stimulation and multiple cycles of infection, which interfere with the engraftment capacity of HSCs.3 In contrast, lentivirus-based vectors allow transduction of nondividing cells like HSCs or terminally differentiated cells.4-14 Protocols for effective lentiviral transduction of CD34+, CD34+/CD38−, and primary and secondary NOD/SCID-repopulating cells (SRCs) have been developed using brief transduction protocols, high multiplicities of infection (MOIs), and cytokine-free conditions in some cases.5,7-14 In these studies, CD34+ SRCs were derived from cord blood (CB)9,11-14 or BM,12 and stable long-term transgene expression was observed in SRC-derived lymphoid, myeloid, and colony-forming unit (CFU) progenitor cells. Because SRCs are enriched in CB compared to adult BM or peripheral blood (PB),15 the use of CB-derived CD34+ SRCs does not allow for prediction of lentiviral transduction in adult HSCs. Furthermore, adult BM-derived CD34+ cells were found more refractory to retroviral gene transfer as compared to cells derived from CB.16 17Whereas transplantation with autologous CB-derived CD34+cells is impossible for most adult patients, the harvest and purification of CD34+ cells from granulocyte colony-stimulating factor (G-CSF)–primed PB blood by leukapheresis is presently a routine clinical procedure. We therefore studied lentiviral transduction of PB-derived adult SRCs and report a protocol allowing highly efficient lentiviral gene transfer under serum- and cytokine-free conditions at low MOIs.
Study design
Preparation of lentiviral stocks
Self-inactivating lentiviral vectors18 containing the enhanced green fluorescent protein(eGFP) gene and a woodchuck posttranscriptional regulatory element (WPRE)19 either with (improved vectors) or without the central polypurine tract/central termination sequence (PPT/CTS element)20 were generated by transient transfection in 293T cells using pMD.G for vesicular stomatitis virus–G protein (VSV-G) pseudotyping as described.21 22After 8 hours, the medium was replaced by Dulbecco modified Eagle medium (DMEM)/10 mM sodium butyrate for 20 hours. Lentiviral particles were collected in X-VIVO10 (Biowhitthaker, Wokingham, United Kingdom) every 24 hours for 2 days, pooled (200-250 mL), cleared by low-speed centrifugation, and filtered through 0.45-μm filters. The absence of replication-competent virus was confirmed by monitoring p24 antigen expression in 293T culture medium for 3 weeks.
Concentration and titration of lentiviral supernatants
Lentiviral supernatants were concentrated by anion exchange chromatography on a Fractoflow 80-6 column (Merck, Darmstadt, Germany). Columns were washed with phosphate-buffered saline (PBS), and viral particles were eluted at 2 M NaCl in PBS (flow rate 4 mL/min), desalted, and concentrated by centrifugation in 100 000 MW cut-off Vivaspin filters (Vivascience, Lincoln, United Kingdom). Finally, the viral supernatant was equilibrated in X-VIVO10/1% human serum albumin (HSA). Real-time polymerase chain reaction (PCR)–based estimation of viral particle number and the biologic titration of viral supernatants have been described.22
Isolation and lentiviral transduction of PB-derived CD34+cells
The G-CSF–primed CD34+ cells were harvested by leukapheresis from a healthy volunteer, purified to at least 98% CD34+ content by magnetic cell sorting (Clini MACS, Miltenyi Biotech, Bergisch-Gladbach, Germany), and cryopreserved in liquid nitrogen. For lentiviral transduction, 5 × 105 cells/mL were cultured in 24-well plates coated with CH-296 recombinant fibronectin fragment in serum-free X-VIVO10/1% HSA with or without recombinant human stem cell factor (SCF; 100 ng/mL), Flt-3 ligand (100 ng/mL), TPO (20 ng/mL) (all from R&D Systems, Abingdon, United Kingdom), and interleukin 6 (IL-6; 20 ng/mL, Boehringer Mannheim, Mannheim, Germany). Transduction was carried out by spinoculation (1000g) for 90 minutes at 32°C in the presence of protamine sulfate (4 μg/mL) and 100 μM deoxynucleoside triphosphates, followed by 16 hours of incubation. After fresh viral supernatant was added for another 5 hours, CD34+ cells were injected intravenously into NOD/SCID mice (24-32 hours after cell culture initiation) or incubated for 6 to 8 days with SCF plus Flt-3 ligand plus TPO plus IL-6. Methylcellulose colony assays were performed as described.23
NOD/SCID mice
The NOD/SCID mice underwent transplantation with transduced and mock-transduced human CD34+ cells (2 × 106cells in 300 μL Iscoves modified Dulbecco medium per mouse) as described.23 Mice were killed 6 to 7 weeks after transplantation, and engraftment and multilineage eGFP expression were analyzed by FACS.23
Results and discussion
Generation and concentration of high-titer lentiviral vector stocks
The VSV-G–pseudotyped lentiviral supernatants were applied to a Fractoflow column and vector particles bound to the weak anion exchanger were eluted, desalted, and further concentrated by ultrafiltration. In 12 independent experiments, the recovery of virus particles ranged between 33% and 68% as assessed by quantitative real-time PCR (data not shown). Viral stocks were concentrated between 100- and 300-fold, and the biologic titers of concentrated vector preparations ranged between 1 and 5 × 108 IU/mL. This procedure allows for concentration of large volumes of viral supernatants with at least similar efficacy and recovery as standard ultracentrifugation.
Lentiviral transduction of PB-derived CD34+cells and SRCs
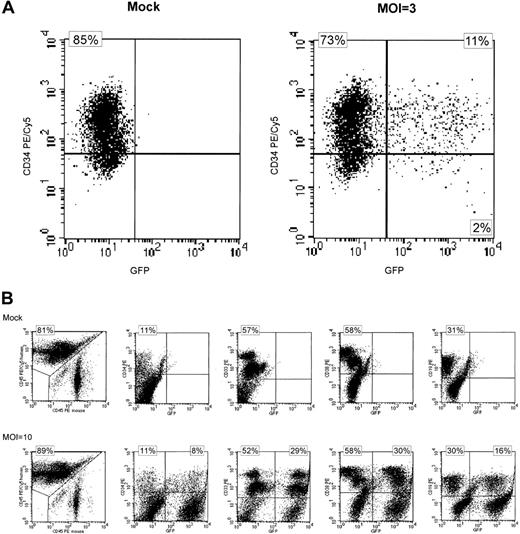
In initial experiments, purified PB-derived CD34+cells were transduced at MOI 3 in the presence (+) or absence (−) of SCF, TPO, Flt-3 ligand, and IL-6. Total transduction efficiency was 13% under both conditions as determined by FACS analysis of eGFP-expressing cells after short-term suspension culture with 11% of the cells found to be double positive for CD34 and eGFP (Table1 and Figure1A). In addition, transplantation of transduced cells into NOD/SCID mice resulted in similar engraftment levels and eGFP expression in human CD34+, CD33+, CD38+, CD19+, and CD14+ cells independent of cytokine stimulation during gene transfer (Table 1).
Expression of eGFP in transduced CD34+ and SRC-derived cells.
(A) FACS analysis of transgenic eGFP (GFP) and CD34+expression 6 days after lentiviral transduction. Cells were stained with an anti–CD34-PE/Cy5 monoclonal antibody. Mock-transduced CD34+ cells are shown on the left and cells transduced with eGFP in the absence of cytokine stimulation at an MOI of 3 are shown at the right. Almost identical results were seen after lentiviral transduction in the presence of cytokine stimulation (data not shown). Quadrants were set according to isotype-matched negative controls. (B) Lymphohematopoietic engraftment of SRC-derived cells in NOD/SCID mice 6 to 7 weeks after transplantation. Expression of eGFP in subpopulations of cells is shown. Engraftment was quantitated using anti–human CD45-PE/Cy5 and anti–murine CD45 PE antibodies (left 2 panels). Expression of eGFP (x-axis) was analyzed in CD34+, CD33+, CD38+, and CD19+ human cells (from left to right, y-axis). Quadrants were set according to isotype-matched negative controls. These data are representative of 28 mice (mock n = 9, eGFP-transduction n = 19). The numbers refer to the upper left and upper right quadrants, respectively. PE indicates phycoerythrin; PE/Cy5, phycoerythrin-cyanin 5.1.
Expression of eGFP in transduced CD34+ and SRC-derived cells.
(A) FACS analysis of transgenic eGFP (GFP) and CD34+expression 6 days after lentiviral transduction. Cells were stained with an anti–CD34-PE/Cy5 monoclonal antibody. Mock-transduced CD34+ cells are shown on the left and cells transduced with eGFP in the absence of cytokine stimulation at an MOI of 3 are shown at the right. Almost identical results were seen after lentiviral transduction in the presence of cytokine stimulation (data not shown). Quadrants were set according to isotype-matched negative controls. (B) Lymphohematopoietic engraftment of SRC-derived cells in NOD/SCID mice 6 to 7 weeks after transplantation. Expression of eGFP in subpopulations of cells is shown. Engraftment was quantitated using anti–human CD45-PE/Cy5 and anti–murine CD45 PE antibodies (left 2 panels). Expression of eGFP (x-axis) was analyzed in CD34+, CD33+, CD38+, and CD19+ human cells (from left to right, y-axis). Quadrants were set according to isotype-matched negative controls. These data are representative of 28 mice (mock n = 9, eGFP-transduction n = 19). The numbers refer to the upper left and upper right quadrants, respectively. PE indicates phycoerythrin; PE/Cy5, phycoerythrin-cyanin 5.1.
Transduction of PB-derived CD34+ cells and SRCs using improved lentiviral vectors
Because cytokine stimulation of PB-derived CD34+cells was not required for efficient gene transfer, PB-derived CD34+ cells were transduced in parallel at MOIs of 10 and 50, using improved lentiviral vectors containing the central polypurine tract (cPPT/CTS) in the absence of cytokine stimulation. FACS analysis after transduction at MOI 10 revealed eGFP expression in 42% of CD34+ cells after short-term suspension culture (mean of 2 experiments), whereas 36% of the CD34+ cells expressed eGFP on transduction at an MOI of 50 (single experiment, Table 2). Transplantation of transduced CD34+ cells into NOD/SCID mice revealed similar engraftment and transgene expression in subsets of hematopoietic cells independent of the MOI used during transduction (Table 2 and Figure1B). However, engraftment of CD19+ cells was higher and that of CD33+ cells lower at MOI 10 compared to MOI 50 (and MOI 3, Table 1) in these experiments.
We demonstrate efficient lentiviral transduction of adult PB-derived SRCs in the absence of cytokines at low MOIs (between 3 and 50). Similar to a recent study using MOI 5,24 we achieved efficient gene transfer at MOIs more suitable for clinical application than those previously reported (ranging from 12 to 600).
We found transduction and engraftment of PB-derived SRCs comparable to that reported for CB- and BM-derived SRCs.9,12-14Consistent with published data,12 lentiviral transduction seems to be independent of cytokine stimulation during short-term lentiviral transduction. Additionally, the concentration of VSV-G–pseudotyped lentiviruses by anion exchange chromatography may improve the efficacy of lentiviral transduction, for example, by reducing toxic contaminants. In summary, we have established a simple and efficient protocol for the purification of lentiviral vectors and for the transduction of adult PB-derived SRCs that can be adapted for future clinical applications.
We thank Drs R. Zufferey and D. Trono, University of Geneva, Switzerland for providing us with lentiviral plasmids, and Dietmar Klose for excellent secretarial help. NOD/SCID mouse experiments were performed in part at the German Cancer Research Center. The technical assistance of B. Berkus, J. Engel, and S. Heil are gratefully acknowledged.
Supported in part by a grant of the H. W. and J. Hector Stiftung and the Hermann J. Abs Program of the Deutsche Bank AG. The Georg-Speyer-Haus is supported by the Bundesministerium für Gesundheit and the Hessisches Ministerium für Wissenschaft und Kunst.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michaela Scherr, Medizinische Hochschule Hannover, Zentrum Innere Medizin, Abteilung Hämatologie und Onkologie, Carl-Neuberg Str 1, D-30623 Hannover, Germany; e-mail:m.scherr@t-online.de.