Homeobox genes are well known for their crucial role during embryogenesis but have also been found to be critically involved in normal and leukemic hematopoiesis. Because most previous studies focused on the role of aberrant HOX gene expression in leukemogenesis and because HOX-A10 is expressed in human CD34+ precursor cells, this study investigated whetherHOX-A10 also plays a pivotal role in normal hematopoietic-lineage determination. The effect of enforced expression of this transcription factor on hematopoietic differentiation of highly purified human cord-blood progenitors was examined by using in vitro assays. In fetal thymic organ cultures, a dramatic reduction in cells expressing high levels of HOX-A10 was observed, along with absence of thymocytes positive for CD3+ T-cell receptor αβ. Furthermore, in MS-5 stromal cell cultures, there was a 7-fold reduction in the number of natural killer cells and a 9-fold reduction in the number of B cells, thus showing a profound defect in differentiation toward the lymphoid lineage inHOX-A10–transduced progenitors. In contrast, the number of CD14+ monocytic cells in the stromal cell culture was 6-fold higher, suggesting an enhanced differentiation toward the myeloid differentiation pathway of HOX-A10–transduced progenitors. However, there was a slight reduction in the number of CD15+ granulocytic cells, which were blocked in their final maturation. These data show that HOX-A10 can act as an important key regulator of lineage determination in human hematopoietic progenitor cells.
Introduction
Hematopoietic stem cells can differentiate into all types of blood cells, although how this lineage determination occurs is poorly understood. Several transcription factors that control this process at the molecular level have been identified.1-3These factors enhance or direct expression of lineage-specific genes. Most of these identified regulators of transcription are critical for one specific hematopoietic lineage. Thus, Pax-5,4 early B-cell factor,5 and Sox-46 are essential for B-cell development; GATA-37 and T-cell factor 18 for T-cell differentiation; c-myb for early myeloid differentiation9; and GATA-1 for red blood cell formation.10 Other factors control more than one blood cell lineage. Low levels of expression of PU.1 drive hematopoietic progenitor cells toward the B-cell lineage, whereas a high concentration of PU.1 results in myeloid differentiation.11 On the other hand, Ikaros plays a crucial role in lymphoid but not myeloid development.12
Several studies have suggested that HOX genes, originally identified as key regulators of embryonic development,13,14 also play a crucial role in both normal and leukemic hematopoiesis.15,16 Of the 39 members of theHOX family identified in humans, most HOX-A andHOX-B cluster genes were preferentially expressed in CD34+ bone marrow precursor cells in gene-expression studies, and these genes were activated from 3′ to 5′ during hematopoiesis,17 similar to the situation during embryonic development.18 This spatial and temporal colinear expression pattern was also observed during activation of natural killer (NK) cells19 and T cells.20
Functional studies of HOX genes in hematopoiesis done by generating knockout mice are lacking, except for investigations ofHOX-A9. Mice deficient in HOX-A9 have serious hematopoietic defects in the lymphoid, erythroid, and myeloid compartments.21 Most information on the role ofHOX genes in hematopoiesis is derived from studies in which one HOX gene was constitutively expressed in normal murine bone marrow. Overexpression of HOX-B4 was found to produce an increased number of transplantable hematopoietic stem cells without changing their differentiation potential; thus, no malignant disease resulted.22 On the other hand, enforced expression ofHOX-B3 increased the number of mature granulocyte-macrophage colony-forming cells, perturbed B-cell differentiation, and skewed T-cell progenitors toward the γδ-lineage instead of the αβ-lineage.23 Finally, overexpression ofHOX-B8 led to an expansion of progenitor cells and, eventually, a leukemic phenotype.24 The possible role ofHOX-A5 in hematopoiesis was elucidated by using antisense oligodeoxynucleotides in human bone marrow cells.25 Use of these oligodeoxynucleotides inhibited the granulocytic/monocytic pathway and increased generation of erythroid progenitors.
Expression of HOX-A10 was shown to be restricted to CD34+ precursor cells and early stages of myeloid differentiation.26 In one study, overexpression of this gene in human progenitor cells resulted in severely perturbed hematopoiesis, a dramatic reduction in B-cell differentiation, and a myeloproliferative effect.27 No data on the effect of continuous expression of HOX-A10 on the development of T and NK cells, monocytes, and dendritic cells were reported.
In this study, we analyzed the role of HOX-A10 in hematopoietic-lineage determination by producing overexpression of this gene in CD34+ cord-blood (CB) progenitor cells with use of retrovirus-mediated gene transfer. Such overexpression resulted in a severe disruption of the T-, B-, and NK-cell differentiation pathways and granulocytic precursor cells were unable to mature fully. In contrast, the monocytic differentiation pathway was enhanced. These findings suggest that HOX-A10 is an important regulator of lineage determination in human stem cells and provides a novel marker for monocytic differentiation.
Materials and methods
Monoclonal antibodies
Mouse anti–human monoclonal antibodies (MoAbs) used were CD1a (fluorescein isothiocyanate–conjugated [FITC] OKT6; American Type Culture Collection [ATCC], Rockville, MD), CD3 (FITC Leu-4 or allophycocyanin [APC]; Becton Dickinson Immunocytometry Systems [BDIS], Mountain View, CA), CD4 (FITC Leu-3a, phycoerythrin [PE], or APC [BDIS];), CD7 (FITC clone 3A1; ATCC), CD8α (FITC OKT8; ATCC), CD8β (2ST8.5H7 PE; Coulter, Miami, FL), CD14 (MP9 PE; BDIS), CD15 (HI98 PE; PharMingen, San Diego, CA), CD19 (Leu-12; ATCC or Leu-12 PE; BDIS), CD34 (HPCA-2 PE; BDIS), CD41 (clone P2; Coulter), CD45 (2D1 PE; PharMingen), CD56 (N901-NKH1 APC; Coulter), CD80 (L307.4 PE; BDIS), glycophorin-A (10F7MN; Dr L. Lanier, DNAX, Palo Alto, CA), human leukocyte antigen (HLA) DR (L243 APC; BDIS), T-cell receptor (TCR) panαβ (BMA031 PE; Coulter), and TCR-γδ (PE; BDIS).
Rat anti–mouse MoAb CD45-Cychrome (30F1 1.1; PharMingen) was used to gate out mouse cells during flow cytometry, and anti-FcγRII/III MoAb (clone 2.4.G2; Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY) was used to block murine Fc receptors.
Mice
Nonobese diabetic/LtSz-severe combined immunodeficient (scid)/scid (NOD-SCID) mice originally purchased from Jackson Laboratory (Bar Harbor, ME) were obtained from our pathogen-free breeding facility. For timed pregnancies, females were housed separately from males until mating. The appearance of vaginal plugs after overnight mating was considered to indicate day 0 of pregnancy. Mice that were 14 or 15 days pregnant were killed by cervical dislocation to obtain embryos for isolation of fetal thymic lobes. Animals were cared for and used in accordance with the guidelines of the Laboratory Animal Ethical Commission of Ghent University Hospital.
Purification of CD34+ CB stem cells
Cord blood was obtained and used in accordance with the guidelines of the Medical Ethical Commission of Ghent University Hospital. Umbilical CB was obtained from full-term, healthy newborns. Within 24 hours after CB collection, mononuclear cells were isolated by using a Lymphoprep density gradient (Nyegaard, Oslo, Norway), resuspended in 9 vol fetal-calf serum (FCS; Invitrogen, Carlsbad, CA) and 1 vol dimethyl sulfoxide (Serva, Heidelberg, Germany), and cryopreserved in liquid nitrogen until use. After the CB cells were thawed and washed, they were labeled with glycophorin A, CD19, CD41, and FITC CD7. For immunomagnetic depletion, the cells were resuspended in 1 mL cold phosphate-buffered saline (PBS) and 2% FCS and mixed with 1 mL prewashed (to remove the preservative) sheep anti–mouse immunoglobulin-coated Dynabeads (Dynal AS, Oslo, Norway) to obtain a 1:5 ratio of cells to Dynabeads. After 30 minutes at 4°C, the suspension was subjected to a magnetic field in a magnetic particle concentrator (Dynal AS). The supernatant containing the unlabeled cells was recovered. The remaining cells were resuspended in 0.2 mL PBS and 2% FCS and labeled with CD34-PE and FITC-labeled CD1, CD3, CD4, and CD8. Finally, cells that were positive for CD34-PE and negative for FITC (called CD34+Lin− cells) were sorted on a fluorescence-activated cell-sorter scanner (FACS; Vantage; BDIS). Sorted cells were checked for purity, which was always at least 99.0%.
Cell cultures
All cultures were maintained at 37°C in a humidified atmosphere containing 7.5% (vol/vol) carbon dioxide in air. Cells were cultured in Iscoves modified Dulbecco medium (IMDM) supplemented with penicillin (100 IU/mL), streptomycin (100 μg/mL), and 10% heat-inactivated FCS (complete IMDM; Invitrogen). The CD34+Lin− CB stem cell coculture on MS-5 cells28 was done in complete IMDM containing 10% heat-inactivated human AB serum (BioWhittaker, Walkersville, MD) and 5% FCS instead of 10% FCS. Fetal thymic organ cultures (FTOCs) were done in complete IMDM containing 10% heat-inactivated human AB serum (BioWhittaker) instead of 10% FCS.
Cloning of human HOX-A10 cDNA
We previously used bicistronic vectors with a gene of interest linked to a downstream internal ribosome entry site (IRES) and a marker gene that allow independent translation of the products of both genes in the transduced target cells.29 TheHOX-A10 cDNA was isolated from the Bluescript SK–positive–HA10 plasmid30 (Dr C. Largman, University of California VA Medical Center, San Francisco, CA) by using the restriction enzymes BbsI (New England Biolabs, Beverly, MA) and EcoRI (Roche Diagnostics, Mannheim, Germany). TheBbsI site was made to have a blunt end by using T4 DNA polymerase (Roche Diagnostics). This DNA fragment was then cloned into the LZRS-IRES–enhanced green fluorescent protein (EGFP) retroviral vector,29 cut with SnaBI (Roche Diagnostics) and EcoRI, to generate the LZRS-HOX-A10-IRES-EGFP vector. The LZRS-IRES-EGFP retroviral vector was used as a control. Direct sequencing (ABI; Perkin Elmer, Foster City, CA) confirmed the integrity of the construct and showed that the clone used contained the published coding region of the human HOX-A10 gene (GenBank accession number AF040714).
Generation of HOX-A10 encoding retrovirus
The Phoenix-A–based amphotropic packaging cell line (Dr P. Achacoso and Dr G.P. Nolan, Stanford University School of Medicine, Stanford, CA) was transfected with the LZRS-IRES-EGFP and LZRS-HOX-A10-IRES-EGFP plasmids by using calcium phosphate precipitation (Invitrogen) to generate both retroviruses. The viral supernatant was stored in aliquots at −70°C until use. The IRES-EGFP and HOX-A10-IRES-EGFP batches used in this study contained approximately 9 × 105 and 1 × 106transducing units/mL, respectively, titrated on Jurkat cells (ATCC).
Retroviral gene transfer
Sorted CD34+ Lin− CB cells were cultured in complete IMDM supplemented with stem cell factor (SCF; 100 ng/mL), ftl3/flk-2 ligand (FL; 100 ng/mL), and thrombopoietin (TPO, 20 ng/mL) for 2 days (all cytokines from R&D Systems Europe, Abingdon, United Kingdom). Afterward, the cells were transduced once in 24 hours. For transduction, 2 to 15 × 104 cells were seeded on 96-well culture plates coated with RetroNectin (Takara Biomedicals, Otsu Shiga, Japan) in a final volume of 150 μL containing 75 μL retroviral supernatant and 75 μL complete IMDM supplemented with 200 ng/mL SCF, 200 ng/mL FL, and 40 ng/mL TPO to keep the final cytokine concentration constant. After transduction, cells were harvested and washed to remove the virus particles, transduction efficiency was determined, and the cells were used in subsequent assays.
FTOCs
Thymic lobes were isolated from fetal NOD-SCID mice on day 14 or 15 of pregnancy.31 Hanging drops were prepared in Terasaki plates by adding to each well 25 μL complete medium containing the progeny of 10 × 103 CD34+Lin−CB cells transduced as described above. One fetal thymic lobe was added to each well, and the plates were inverted to form hanging drops and incubated for 48 to 72 hours. After incubation, on day 0 of FTOC, the lobes were removed, washed in complete medium, put on a Nuclepore filter (Costar, Cambridge, MA) resting on a Gelfoam sponge (Upjohn, Kalamazoo, MI) soaked in complete medium in a 6-well tissue-culture plate (BDIS), and cultured for 10 to 25 days. Subsequently, the lobes were mechanically disrupted with a tissue grinder to obtain a single-cell suspension that was used in flow cytometry.
MS-5 stromal cell cultures
Transduced CD34+ Lin− CB stem cells were incubated in the presence of various human cytokines in 24-well plates precoated with confluent MS-5 cells derived from murine marrow28 (Dr Coulombel, Institut Gustave Roussy, Villejuif, France, with the permission of Kirin Brewery, Tokyo, Japan). For NK- or B-cell development, 2 × 103 CD34+Lin− CB cells were cultured for 3 weeks in the presence of SCF (50 ng/mL), interleukin-2 (IL-2) (5 ng/mL), and IL-15 (10 ng/mL) (for NK cells) or SCF (50 ng/mL) and IL-7 (20 ng/mL) (for B cells). Monocytic and granulocytic differentiation was assessed by culturing 1 × 103 CD34+ Lin− CB cells for 2 weeks in the presence of SCF (50 ng/mL), FL (50 ng/mL), TPO (20 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL) (monocytic) or SCF (50 ng/mL), FL (50 ng/mL), TPO (20 ng/mL), and granulocyte colony-stimulating factor (G-CSF; 10 ng/mL) (granulocytic).
Generation of dendritic cells
EGFP-positive (EGFP+) CD14+ monocytes generated from IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB stem cells after 9 days of MS-5 stromal cell culture were sorted to a purity of at least 98.5%. Between 10 × 103 and 25 × 103 of these monocytes were cultured for 4 days in the presence of GM-CSF (10 ng/mL), tumor necrosis factor α (TNF-α; 10 ng/mL) and IL-4 (20 ng/mL) in a flat-bottomed, 96-well plate to generate mature dendritic cells.32
Calculation of absolute cell numbers and statistical analysis
Because of the difference in virus concentration between the IRES-EGFP and HOX-A10-IRES-EGFP retroviral batches used and the differences in transduction efficiency among experiments, the number of transduced cells at the beginning of culture was not the same in the 2 cultures and in all experiments. To compare the yields of cells obtained from EGFP+-transduced CD34+Lin− CB progenitors and EGFP+-transduced CD34+Lin− CB progenitors positive for HOX-A10, we multiplied the absolute number of EGFP+ cells (obtained by multiplying the total number of cells counted under a microscope by the fraction of EGFP+ human cells determined by FACS analysis) by a factor so that the number of EGFP+ cells at initiation of the cultures was 1 × 103 for both EGFP+– andHOX-A10-positive EGFP+-transduced CD34+Lin− CB cells. Statistical analysis was done with the nonparametric paired Wilcoxon test (SPSS, version 9.0; SPSS, Chicago, IL).
Flow cytometry
Before labeling, cells were suspended in PBS, 1% bovine serum albumin, and 0.1% sodium azide. In all experiments in which human cells were stained in the presence of mouse cells, the mixture of cells was preincubated for 15 minutes with saturating amounts of anti-FcγRII/III MoAb to avoid nonspecific binding of MoAbs by the murine cells. Subsequently, the cells were stained with a panel of MoAbs. Dead cells were gated out by propidium iodide; murine thymocytes in FTOCs were gated out by using mouse CD45-Cychrome. For each analysis of MS-5 stromal cell cultures, at least one staining was done with the human CD45 antibody to show that gates were set on human cells. Negative controls included isotype MoAbs conjugated with the corresponding fluorochrome. The cells were analyzed on a FACScalibur instrument (BDIS) with an argon-ion laser tuned at 488 nm and a red-diode laser at 635 nm. Forward light scattering, orthogonal scattering, and 4 fluorescence signals were determined and stored in list-mode data files. Data acquisition and analysis were done with use of CellQuest software (BDIS).
Results
Continuous expression of HOX-A10 in CB progenitors severely hampers their lymphoid differentiation potential
T-cell development.
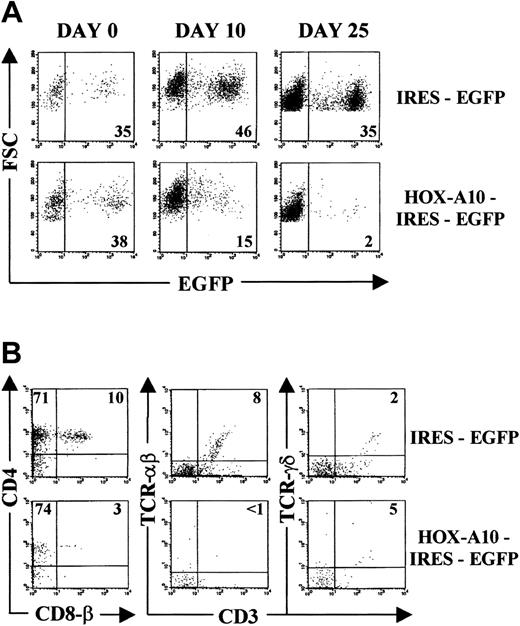
To analyze the effect of HOX-A10 overexpression on human T-cell development, CD34+ Lin− CB cells were sorted and transduced with IRES-EGFP and HOX-A10-IRES-EGFP encoding retrovirus. T-cell development from these transduced progenitor cells was assayed in vitro by using FTOCs.31,33 On day 0 of FTOC, the percentage of EGFP+ cells among IRES-EGFP–transduced cells was comparable to that among HOX-A10-IRES-EGFP–transduced cells (Figure1A). Among the IRES-EGFP–transduced cells, the percentage of EGFP+ cells observed at that time was sustained during the culture period, a finding in agreement with previously reported data.33 In contrast, there was a dramatic decrease in EGFP+ cells in HOX-A10-IRES-EGFP–transduced cultures. A similar decrease in EGFP+ cells was observed in FTOCs initiated with HOX-A10-IRES-EGFP–transduced CD34+ thymocytes (data not shown).
HOX-A10 –transduced CB progenitors have a strongly reduced T-cell differentiation potential in FTOC.
(A) Kinetic analysis of the percentage of EGFP-expressing cells in FTOC initiated with IRES-EGFP–transduced and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells. Dot plots gated on human thymocytes show forward scatter (FSC) compared with EGFP for IRES-EGFP– (top) and HOX-A10-IRES-EGFP–transduced CD34+Lin− CB cells (bottom) at the indicated time points of FTOC. Figures in the corresponding dot plots indicate the percentage of EGFP+ cells. (B) Bivariate dot plots (gated on human EGFP+ cells on day 25 of FTOC) of flow cytometric results with IRES-EGFP– (top) and HOX-A10-IRES-EGFP–transduced cells (bottom) showing CD4-APC compared with CD8β-PE expression and TCRαβ-PE or TCRγδ-PE compared with CD3-APC. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values indicate the percentage of cells in the corresponding quadrants.
HOX-A10 –transduced CB progenitors have a strongly reduced T-cell differentiation potential in FTOC.
(A) Kinetic analysis of the percentage of EGFP-expressing cells in FTOC initiated with IRES-EGFP–transduced and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells. Dot plots gated on human thymocytes show forward scatter (FSC) compared with EGFP for IRES-EGFP– (top) and HOX-A10-IRES-EGFP–transduced CD34+Lin− CB cells (bottom) at the indicated time points of FTOC. Figures in the corresponding dot plots indicate the percentage of EGFP+ cells. (B) Bivariate dot plots (gated on human EGFP+ cells on day 25 of FTOC) of flow cytometric results with IRES-EGFP– (top) and HOX-A10-IRES-EGFP–transduced cells (bottom) showing CD4-APC compared with CD8β-PE expression and TCRαβ-PE or TCRγδ-PE compared with CD3-APC. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values indicate the percentage of cells in the corresponding quadrants.
Cell-surface staining with T-cell–specific markers after 25 days of FTOC showed that within the few remainingHOX-A10–transduced thymocytes, the fraction of CD4+ CD8β double-positive cells was reduced in comparison with that in control transduced cells (Figure 1B) or untransduced cells (data not shown). Moreover, virtually no TCR-αβ–positive CD3+ thymocytes were detected among the HOX-A10-EGFP–transduced cells, and despite a slight increase in the fraction of TCR-γδ–positive CD3+ T cells, there was no obvious skewing of T-cell progenitors toward the γδ T-cell lineage (Figure 1B).
NK-cell and B-cell development.
To study the effect of HOX-A10 overexpression on development of NK and B cells, the progeny cells of 2000 transduced CD34+ Lin− CB cells were cocultured on an MS-5 stromal cell layer in the presence of human SCF, IL-2, and IL-15 (for NK cells) or SCF and IL-7 (for B cells). The fraction of EGFP+ cells at initiation of cultures (day 0) is shown in Figure 2.
Reduced NK-cell and B-cell differentiation potential of
HOX-A10–transduced CB stem cells.EGFP-expression profile of IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells at initiation (day 0) and harvesting (day 21) of MS-5 stromal cell cultures in the presence of either SCF, IL-2, and IL-15 or SCF and IL-7. Upper dot plots show FSC compared with EGFP. Values in the corresponding dot plots indicate the percentage of EGFP+cells. Lower dot plots show 4-color flow cytometric analysis of the MS-5 stromal cell cultures on day 21, gated on EGFP+ cells. Dot plots show CD56-APC compared with CD45-PE and HLA-DR-APC compared with CD19-PE. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values in the corresponding dot plots indicate the percentage of cells in the upper right quadrant.
Reduced NK-cell and B-cell differentiation potential of
HOX-A10–transduced CB stem cells.EGFP-expression profile of IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells at initiation (day 0) and harvesting (day 21) of MS-5 stromal cell cultures in the presence of either SCF, IL-2, and IL-15 or SCF and IL-7. Upper dot plots show FSC compared with EGFP. Values in the corresponding dot plots indicate the percentage of EGFP+cells. Lower dot plots show 4-color flow cytometric analysis of the MS-5 stromal cell cultures on day 21, gated on EGFP+ cells. Dot plots show CD56-APC compared with CD45-PE and HLA-DR-APC compared with CD19-PE. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values in the corresponding dot plots indicate the percentage of cells in the upper right quadrant.
After 3 weeks of culture, a significant decline in the percentage of HOX-A10-IRES-EGFP–transduced cells compared with that of control IRES-EGFP–transduced cells had occurred in the NK-cell cultures (Figure 2). In addition, the percentage of human CD45+CD56+ cells was dramatically reduced in the EGFP+ gated cells of the HOX-A10–transduced cultures compared with control transduced cultures (Figure 2) or untransduced cells (data not shown). As a result, the absolute number of NK cells in cultures with HOX-A10 overexpression had decreased an average of 7-fold compared with those in control cultures (Table 1).
In the B-cell cultures, a similar decrease in EGFP+ cells was observed in HOX-A10-EGFP–transduced cultures compared with control cultures (Figure 2). This was accompanied by a lower percentage of HLA-DR–positive CD19+ human B cells inHOX-A10–transduced cells (Figure 2), resulting in an average 9-fold reduction in the absolute number of B cells generated from HOX-A10-IRES-EGFP–transduced CB progenitors compared with controls (Table 1). Together, these data show that the development of lymphoid cells from CB progenitor cells that overexpressHOX-A10 is severely impaired.
Overexpression of HOX-A10 has different effects on the myeloid differentiation potential of CB progenitors
Monocyte differentiation.
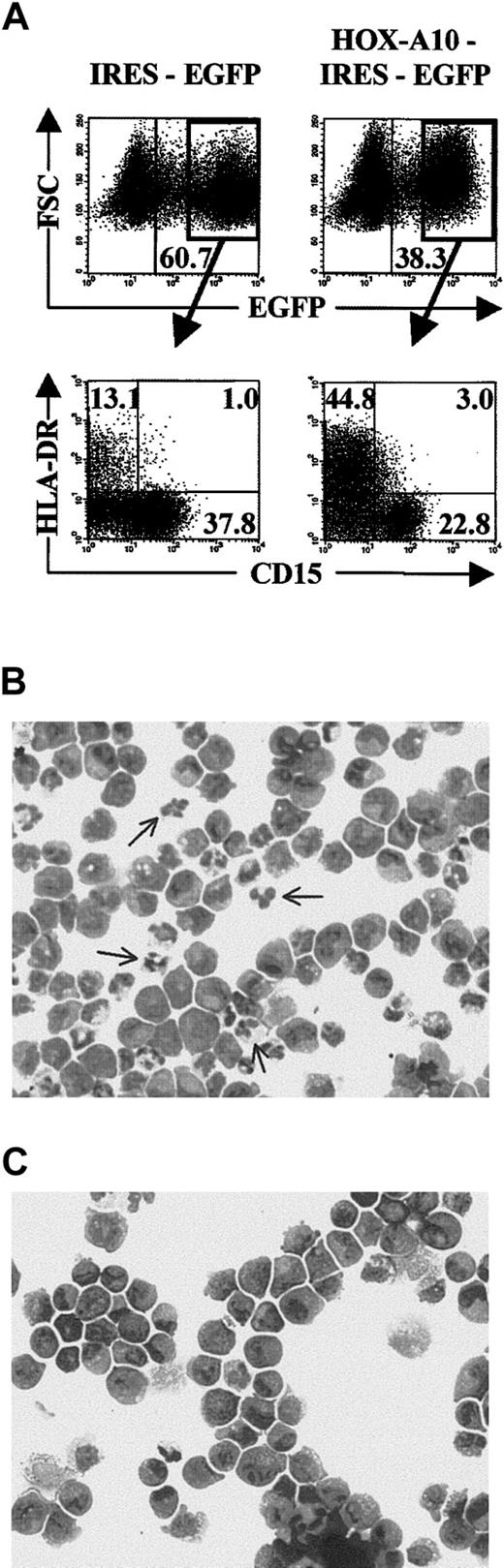
The effect of HOX-A10 overexpression on monocyte differentiation was analyzed by coculturing transduced CB progenitor cells on the MS-5 stromal cell line in the presence of the human cytokines SCF, FL, TPO, and GM-CSF. Analysis was done 2 weeks after initiation of the cultures. There was a slightly higher increase in the percentage of EGFP+ cells in HOX-A10-IRES-EGFP–transduced cultures compared with control transduced cultures, thereby showing that continuous expression of HOX-A10 slightly favors expansion or survival under these culture conditions (Figure3A). Monocytic cells were characterized by expression of CD14, CD4, and HLA-DR. There was always a higher percentage of CD14+ cells in HOX-A10-IRES-EGFP–transduced cells than in control IRES-EGFP–transduced cells (Figure 3A) or untransduced cells (data not shown). Most of these cells expressed low levels of HLA-DR and CD4, which is characteristic of monocytes. The generation of monocytes was confirmed by Wright-Giemsa staining on sorted EGFP+ CD14+ cells; this assessment showed that 95% of IRES-EGFP–transduced CD14+ cells (Figure 3B) and 99% of HOX-A10-IRES-EGFP–transduced CD14+cells (Figure 3C) contained mature monocytes and some macrophages. As a result, the absolute number of CD14+ monocytic cells generated from HOX-A10-IRES-EGFP–transduced CB progenitor cells was, on average, 6-fold higher than that generated from IRES-EGFP–transduced precursor cells (Table 1).
Overexpression of
HOX-A10 enhances differentiation of human monocytes. (A) EGFP-expression profile of IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells at initiation (day 0) and harvesting (day 14) of MS-5 stromal cell cultures in the presence of SCF, FL, TPO, and GM-CSF. Upper dot plots show FSC compared with EGFP. Values in the corresponding dot plots indicate the percentage of EGFP+ cells. Lower dot plots show 4-color flow cytometric analysis of the MS-5 stromal cell cultures on day 14, gated on EGFP+ cells. Dot plots show CD4-APC compared with CD14-PE and HLA-DR-APC compared with CD14-PE. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values indicate the percentage of cells in the corresponding quadrants. Panels B and C show Wright-Giemsa staining of EGFP+ CD14+ cells sorted from IRES-EGFP–transduced cell cultures (B) or HOX-A10-IRES-EGFP–transduced cell cultures (C). Arrows indicates monocytes (m) or macrophages (mφ). Original magnification B-C, × 200.
Overexpression of
HOX-A10 enhances differentiation of human monocytes. (A) EGFP-expression profile of IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells at initiation (day 0) and harvesting (day 14) of MS-5 stromal cell cultures in the presence of SCF, FL, TPO, and GM-CSF. Upper dot plots show FSC compared with EGFP. Values in the corresponding dot plots indicate the percentage of EGFP+ cells. Lower dot plots show 4-color flow cytometric analysis of the MS-5 stromal cell cultures on day 14, gated on EGFP+ cells. Dot plots show CD4-APC compared with CD14-PE and HLA-DR-APC compared with CD14-PE. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values indicate the percentage of cells in the corresponding quadrants. Panels B and C show Wright-Giemsa staining of EGFP+ CD14+ cells sorted from IRES-EGFP–transduced cell cultures (B) or HOX-A10-IRES-EGFP–transduced cell cultures (C). Arrows indicates monocytes (m) or macrophages (mφ). Original magnification B-C, × 200.
Monocyte-derived dendritic cell differentiation.
To test whether the generated monocytes were able to differentiate into mature dendritic cells, we sorted both IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD14+ EGFP+ cells after 9 days of culture under monocyte culture conditions (Figure4A) and then cultured the cells for 4 days in the presence of GM-CSF, TNF-α, and IL-4. Cells with long dendrites were clearly visible in these cultures (data not shown), and as shown in Figure 4B, on maturation toward dendritic cells, these cultured cells lost CD14 expression, as expected, and a subpopulation of the cells acquired high CD80 expression combined with high levels of HLA-DR, which is typical for mature dendritic cells. This occurred in both IRES-EGFP–transduced cells and HOX-A10-IRES-EGFP–transduced cells.
HOX-A10 –transduced monocytes can generate dendritic cells.
(A) Histograms show CD14 staining of sorted EGFP+CD14+ monocytes generated from IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells after 9 days of culture in MS-5 stromal cells in the presence of SCF, FL, TPO, and GM-CSF. (B) Flow cytometric analysis of cells generated from sorted IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD14+ cells shown in panel A after 4 days of culture in the presence of GM-CSF, TNF-α, and IL-4. Histograms show CD14 staining, and bivariate dot plots show CD80-PE compared with HLA-DR-APC. Values indicate the percentage of cells in the corresponding quadrant.
HOX-A10 –transduced monocytes can generate dendritic cells.
(A) Histograms show CD14 staining of sorted EGFP+CD14+ monocytes generated from IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells after 9 days of culture in MS-5 stromal cells in the presence of SCF, FL, TPO, and GM-CSF. (B) Flow cytometric analysis of cells generated from sorted IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD14+ cells shown in panel A after 4 days of culture in the presence of GM-CSF, TNF-α, and IL-4. Histograms show CD14 staining, and bivariate dot plots show CD80-PE compared with HLA-DR-APC. Values indicate the percentage of cells in the corresponding quadrant.
Granulocytic differentiation.
Differentiation of granulocytes from retrovirally transduced CB progenitors was studied by culturing the cells under the same conditions used for monocytic differentiation, except that GM-CSF was replaced with G-CSF. Cultures were initiated with the same progeny of transduced cells used for monocytic differentiation (Figure 3A), and analysis was done after 2 weeks of culture. In contrast to the results under the monocyte culture conditions, the fraction of EGFP+ cells was less increased in HOX-A10-IRES-EGFP–transduced cultures compared with control transduced cultures (Figure 5A). Moreover, the fraction of CD15+ cells was reduced in these cells overexpressing HOX-A10, resulting in a one-third reduction in the absolute number of granulocytes generated fromHOX-A10–transduced CB progenitors compared with controls (Table 1). Wright-Giemsa staining on sorted CD15+EGFP+ cells from both cultures showed that virtually all CD15+ cells were granulocytic cells at different stages of development. However, in HOX-A10-IRES-EGFP–transduced CD15+ cells (Figure 5C), aside from an increase in cells with blastlike morphologic features, virtually no mature granulocytes with segmented nuclei were detected. In contrast, in IRES-EGFP–transduced CD15+ cells, mature granulocytes were clearly present (Figure 5B). In addition, the fraction of HLA-DR–positive cells was significantly higher in the HOX-A10-IRES-EGFP–transduced cells than in control transduced cells (Figure 5A). Most of these cells were CD14+ (data not shown), thus showing that there was an enhanced differentiation ofHOX-A10–overexpressing CB progenitors into the monocytic differentiation pathway in the granulocyte culture conditions. Together, these results show that overexpression of HOX-A10enhances differentiation of CB progenitors into monocytic cells and allows their differentiation into granulocytic precursor cells but that final maturation of the granulocytic cells is blocked.
Overexpression of
HOX-A10 slightly reduces the generation of CD15+ granulocytic cells and blocks their final maturation. (A) Four-color flow cytometric analysis of cells recovered from MS-5 stromal cell cultures in the presence of SCF, FL, TPO, and G-CSF, initiated with IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells. Dot plots show FSC compared with EGFP and HLA-DR-APC compared with CD15-PE for IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells on day 14 of culture. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values in the corresponding dot plots indicate the percentage of cells in the corresponding quadrant. (B) Wright-Giemsa staining of EGFP+CD15+ cells sorted from IRES-EGFP–transduced cell cultures shown in panel A. The arrow indicates mature granulocytes. (C) Wright-Giemsa staining of EGFP+ CD15+ cells sorted from HOX-A10-IRES-EGFP–transduced cell cultures shown in panel A. Original magnification B-C, × 200.
Overexpression of
HOX-A10 slightly reduces the generation of CD15+ granulocytic cells and blocks their final maturation. (A) Four-color flow cytometric analysis of cells recovered from MS-5 stromal cell cultures in the presence of SCF, FL, TPO, and G-CSF, initiated with IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells. Dot plots show FSC compared with EGFP and HLA-DR-APC compared with CD15-PE for IRES-EGFP– and HOX-A10-IRES-EGFP–transduced CD34+ Lin− CB cells on day 14 of culture. Quadrants were set to include at least 99% of cells stained with isotype controls in the lower left quadrants. Values in the corresponding dot plots indicate the percentage of cells in the corresponding quadrant. (B) Wright-Giemsa staining of EGFP+CD15+ cells sorted from IRES-EGFP–transduced cell cultures shown in panel A. The arrow indicates mature granulocytes. (C) Wright-Giemsa staining of EGFP+ CD15+ cells sorted from HOX-A10-IRES-EGFP–transduced cell cultures shown in panel A. Original magnification B-C, × 200.
Discussion
Most studies of homeobox genes have focused on their role in embryogenesis and oncogenesis. In this study, we investigated the effect of overexpression of HOX-A10 on differentiation of human stem cells into the myeloid and lymphoid lineages. We found that development of T, B, and NK cells is severely hampered in cells expressing high levels of HOX-A10, thus showing that continuous expression of this gene is detrimental to lymphoid development. In contrast, overexpression of HOX-A10 had various effects on differentiation of stem cells toward the myeloid lineage. Generation of monocytic cells was greatly enhanced and the cells were able to differentiate into dendritic cells. On the other hand, development of immature granulocytes was reduced only slightly, but their final maturation was blocked completely.
Differentiation of stem cells toward the lymphoid lineage was severely hampered by continuous expression of HOX-A10. This was indicated by 2 different findings. First, there was a reduction in the fraction of EGFP+ cells in HOX-A10-IRES-EGFP–transduced cultures compared with control transduced cultures. This was most pronounced during T-cell differentiation in FTOCs but also clearly occurred under the NK-cell and B-cell culture conditions. Second, of the remaining EGFP+ cells, fewer HOX-A10-IRES-EGFP–transduced cells than control transduced cells differentiated into lymphocytes. This shows that lymphoid development from CB stem cells that overexpress HOX-A10 is reduced by a defect in both proliferation/survival and differentiation.
Different effects on myeloid development were observed. Stem cells transduced with HOX-A10-IRES-EGFP encoding retrovirus showed slightly less proliferation/survival in cultures containing G-CSF compared with control transduced progenitors. This was accompanied by a small reduction in differentiation toward CD15+ cells, suggesting a slight reduction in granulocyte differentiation from HOX-A10-IRES-EGFP–transduced CB precursors. However, granulocytic cells derived from HOX-A10-IRES-EGFP–transduced stem cells were clearly immature, since virtually no cells present had segmented nuclei. Thus, although development of granulocytic precursor cells decreased only slightly, these cells were blocked from undergoing final maturation toward polymorphonuclear leukocytes. Monocytic differentiation was the only hematopoietic pathway analyzed that was not reduced by continuous expression of HOX-A10; in fact, differentiation toward this lineage was enhanced. This resulted from augmentation of proliferation/survival, since the fraction of EGFP+ cells increased more in HOX-A10-IRES-EGFP–transduced cultures than in controls, but also from increases in differentiation, since the percentage of CD14+ cells was higher in cells that continuously expressed HOX-A10. Wright-Giemsa staining showed that these CD14+ monocytic cells contained mature monocytes and some macrophages. Under appropriate culture conditions, these cells were able to differentiate into mature dendritic cells. Therefore, all these results suggest thatHOX-A10 is a transcription factor that can drive hematopoietic precursor cells toward the monocytic differentiation pathway but not toward other hematopoietic lineages.
Using an in vivo model, Buske et al27 showed that HOX-A10-IRES-EGFP–transduced CB progenitors contribute more to the myeloid lineage and less to the lymphoid lineage. In regard to lymphoid development, however, they analyzed only B-cell differentiation. Here, we showed that total lymphoid development was hampered, since differentiation of both T cells and NK cells was also reduced. Buske et al27 used CD15 as a marker for total myeloid differentiation and found that the fraction of CD15+ cells in HOX-A10-IRES-EGFP–transduced cells was greater than that in IRES-EGFP–transduced cells. This finding is in agreement with our results, since we found that lymphoid development was much more affected than myeloid development. However, CD15 alone is not sufficient as a marker for development of all myeloid lineages. Although Buske et al27 showed that terminal myeloid differentiation is inhibited by enforced expression ofHOX-A10, we found that this is indeed the case for granulocytic differentiation, but that monocytic differentiation was not affected; in contrast, it was enhanced by overexpression ofHOX-A10. This finding of enhanced monocytic differentiation is in agreement with results of a study showing that enforced expression of HOX-A10 facilitates differentiation of myelomonocytic U937 cells into monocytes/macrophages.34 It would be interesting to see whether generation of CD14+monocytic cells is also enhanced in vivo.27
Because HOX-A10 belongs to a large family of transcription factors, an intriguing question is which genes are regulated by this protein. However, data on target genes of HOX proteins in general are limited. We showed that HOX-A10-IRES-EGFP–transduced hematopoietic precursor cells respond differently to various combinations of cytokines and that both proliferation/survival and differentiation are affected. Given the high level of responsiveness of HOX-A10-IRES-EGFP–transduced progenitors to GM-CSF, it might be thatHOX-A10 is involved in regulating expression of the corresponding receptor and perhaps of downstream signal-transduction proteins. Alternatively, HOX-A10 itself may be a downstream effector of the GM-CSF receptor and excess HOX-A10 mimics this signaling pathway. It is interesting in this context that on interferon-γ signaling, phosphorylation of tyrosine residues ofHOX-A10 can inhibit DNA binding and therefore its activity.35 Because human CD34+ progenitor cells express HOX-A10, lymphoid-specific cytokines might use this mechanism to inactivate HOX-A10 protein activity and thereby enable the lymphoid differentiation pathway. Since overexpression induces an excess of HOX-A10, this phosphorylation presumably does not occur on all HOX-A10proteins in HOX-A10-IRES-EGFP–transduced CB progenitors and thereby interferes with lymphoid differentiation.
A summary of the effects on hematopoietic differentiation of enforced expression of HOX-A10 in CB precursor cells as observed by Buske et al27 and described here is provided in Figure6. In accordance with the most widely accepted scheme for hematopoiesis,36 the first option for differentiating hematopoietic stem cells is to become either a lymphoid or a myeloid multipotent progenitor. Lymphoid differentiation is severely hampered by enforced expression of HOX-A10. This could be due to inhibition of differentiation of stem cells toward the common lymphoid precursor (CLP) or an inability of the CLP to differentiate into mature lymphocytes. This question is currently difficult to address because the human CLP has not been well characterized, despite several studies in this area.37,38In all situations, however, HOX-A10 seems to direct progenitor cells toward the myeloid lineage. Along this differentiation pathway, the common myeloid precursor can differentiate into the megakaryocyte/erythrocyte pathway or the granulocyte/monocyte pathway. Buske et al27 showed that development of erythrocytes is severely inhibited by overexpression of HOX-A10. The effect of continuous HOX-A10 expression on the development of megakaryocytes in humans has not been analyzed, but studies in mice by Thorsteinsdottir et al30 found that such expression results in an expansion of megakaryocyte blast cells. Whether this also occurs in humans is not known, since differences among species can exist. Indeed, overexpression of HOX-A10 in human stem cells caused an inhibition of erythrocyte differentiation, but this was not the case in murine cells.27 30 Analyses of granulocyte/monocyte differentiation showed that terminal granulocytic differentiation was inhibited whereas monocytic differentiation was enhanced. Therefore, at this lineage-decision point, it appears that HOX-A10 drives the precursor cells toward the monocytic lineage.
Schematic overview of the effects on hematopoietic differentiation of enforced expression of
HOX-A10 in CB precursor cells. HSC indicates hematopoietic stem cells; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; MEP, megakaryocyte-erythrocyte progenitor; and GMP, granulocyte-monocyte progenitor.
Schematic overview of the effects on hematopoietic differentiation of enforced expression of
HOX-A10 in CB precursor cells. HSC indicates hematopoietic stem cells; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; MEP, megakaryocyte-erythrocyte progenitor; and GMP, granulocyte-monocyte progenitor.
All these data and the fact that expression of HOX-A10 is restricted to multipotential precursor cells and early myeloid progenitors show that HOX-A10 can act as a key regulator of hematopoietic stem cell differentiation. In the light of the crucial role of HOX genes during embryonic development, it is clear that this gene family has a similar important function during normal hematopoiesis. However, many members of the family require further investigation and target genes of HOX proteins must be identified.
We thank José De Bosscher, Evelien Naessens, Katrien Goeman, Koen Hugelier, Caroline Van Geyt, Veronique De Backer, Greet De Smet, Caroline Collier, and Achiel Moerman for technical assistance; An De Creus and Tessa Kerre for expert scientific advice; Dr Jan Philippé for help with Wright-Giemsa staining and analysis; the Department of Respiratory Diseases, Ghent University Hospital, for microscopical analysis; the Department of Obstetrics, Ghent University Hospital, for the CB samples; Dr L. Coulembel (Institut Gustave Roussy, Villejuif, France) and Kirin Brewery (Tokyo, Japan) for the MS-5 stromal cell line; Dr H. Spits (The Netherlands Cancer Institute, Amsterdam) for the gift of the IRES-EGFP construct; and Dr G.P. Nolan (Stanford University School of Medicine, CA) for the gift of the Phoenix-NA packaging cell line and the LZRS vector.
Supported by grants from Ghent University; the Flanders Institute for Biotechnology; and the Fund for Scientific Research Flanders, Belgium.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Georges Leclercq, Ghent University Hospital, 4 Blok A, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail:georges.leclercq@rug.ac.be.