Abstract
The translocation (9;22) gives rise to the p190Bcr-Abl and p210Bcr-Abl tyrosine kinase proteins, considered sufficient for leukemic transformation. Philadelphia-positive (Ph+) acute leukemia patients failing to respond to initial induction therapy have a poor prognosis with few effective treatment options. Imatinib is an orally administered, potent inhibitor of the Bcr-Abl tyrosine kinase. We conducted a clinical trial in 56 patients with relapsed or refractory Ph+ acute lymphoblastic leukemia (ALL; 48 patients) or chronic myelogenous leukemia in lymphoid blast crisis (LyBC; 8 patients). Imatinib was given once daily at 400 mg or 600 mg. Imatinib induced complete hematologic responses (CHRs) and complete marrow responses (marrow-CRs) in 29% of ALL patients (CHR, 19%; marrow-CR, 10%), which were sustained for at least 4 weeks in 6% of patients. Median estimated time to progression and overall survival for ALL patients were 2.2 and 4.9 months, respectively. CHRs were reported for 3 (38%) of the patients with LyBC (one sustained CHR). Grade 3 or 4 treatment-related nonhematologic toxicity was reported for 9% of patients; none of the patients discontinued therapy because of nonhematologic adverse reactions. Grade 4 neutropenia and thrombocytopenia occurred in 54% and 27% of patients, respectively. Imatinib therapy resulted in a clinically relevant hematologic response rate in relapsed or refractory Ph+ acute lymphoid leukemia patients, but development of resistance and subsequent disease progression were rapid. Further studies are warranted to test the effects of imatinib in combination with other agents and to define the mechanisms of resistance to imatinib.
Introduction
The Philadelphia translocation (Ph) is the most common cytogenetic abnormality associated with adult acute lymphoblastic leukemia (ALL), occurring in 20% to 40% of patients.1-4 It is also detected in 2% to 5% of children with ALL5 and in approximately 1% of patients with acute myeloid leukemia (AML).6 The Philadelphia chromosome is the result of a t(9;22) (q34; q11) reciprocal translocation leading to the formation of a BCR-ABL fusion gene, the transcription of which results either in an 8.5-kb mRNA that encodes a 210-kDa protein or in a 7.5-kb mRNA that codes for a 190-kDa protein.7,8 These BCR-ABL proteins display constitutively active tyrosine kinase activity that is considerably greater than that of the normal 145-kDa ABL gene product.9,10 Expression of the deregulated BCR-ABL kinase has been shown to induce resistance to apoptosis11 and growth factor independence12and to possess pronounced transforming potential in mouse models, and it accounts for the malignant phenotype of chronic myelogenous leukemia (CML).13
The presence of the BCR-ABL translocation is the single most important adverse prognostic parameter in Ph+ acute leukemia, with long-term survival of less than 10% with current chemotherapy regimens.4,14-16 Conventional induction chemotherapy, as well as recently developed regimens such as hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), generally achieves response rates of 60% to 80% in newly diagnosed patients, which are slightly inferior to those in Ph− ALL, but these responses are not durable.14,16,17 In a recent report, overall complete hematologic response (CHR) duration of patients with Ph+ ALL was 8 months1; adult patients with ALL who relapse after primary chemotherapy or are refractory have a considerably inferior prognosis.18 Intensification of chemotherapy has had no substantial impact on the unfavorable course of de novo Ph+ adult ALL1,19,20 or in patients who fail first-line therapy; these patients generally do not respond well to further intensive treatment18 and may be regarded as candidates for palliative care or experimental therapies. Allogeneic stem cell transplantation (SCT) is the only curative form of treatment to date, with long-term survival of 35% to 65% if it is performed in first complete remission.21-25 In contrast, the outcome with allogeneic SCT in actively relapsed or refractory ALL is poor.18 Although allogeneic transplantation remains the treatment of choice beyond first remission, long-term survival is approximately 17% in second or third remission and only 5% at 2 years from transplantation in primary induction failure or relapse.24,26 27
Imatinib (Glivec, also imatinib mesylate [Gleevec]; formerly STI571; Novartis Pharma, Basel, Switzerland) is a rationally designed, potent selective competitive inhibitor of the BCR-ABL protein-tyrosine kinase.28-30 In an ascending-dose phase 1 study, imatinib induced substantial and durable responses with minimal toxicity, at daily doses of 300 mg and higher, in nearly all patients with chronic phase CML,31 results that were recently confirmed in a phase 2 trial.32 Imatinib was also shown to have significant antileukemic activity in patients with accelerated-phase CML or in myeloid blast crisis, although results were inferior to results in chronic phase.33,34 In patients with lymphoid blast crisis (LyBC) or Ph+ ALL entered in the initial phase 1 study, imatinib at daily doses of 300 to 1000 mg induced hematologic responses in 14 of 20 patients (70%), including 4 complete hematologic responses (20%) and 7 marrow responses (35%).35
In view of the possible therapeutic activity of imatinib, its low toxicity, and the limited options available to relapsed ALL patients, we conducted a phase 2 study of imatinib in patients with Ph+ ALL or LyBC who had failed first-line treatment.
Patients and methods
Patients
Male or female patients were eligible for inclusion in this study if they were at least 18 years of age and had a morphologically confirmed diagnosis of relapsed or refractory Ph+ ALL or LyBC. Relapsed disease was defined as first or subsequent relapse after standard chemotherapy, autologous or allogeneic bone marrow, or peripheral blood SCT. Refractory disease was defined by the failure of conventional induction therapy to induce complete remission after 2 courses. For patients with CML, LyBC was defined as at least 30% blasts in peripheral blood or marrow expressing lymphoid cell surface markers, irrespective of prior therapy.
Patients were required to have alanine aminotransferase (ALT) and aspartate aminotransferase (AST) not higher than 3 × the upper normal limit in cases without suspected leukemic involvement of the liver, or not higher than 5 × the upper normal limit in cases of suspected liver involvement; serum total bilirubin levels not higher than 3 × the upper normal limit; and serum creatinine levels not higher than 2 × the upper normal limit. Women of childbearing potential were required to have a negative pregnancy test prior to starting treatment, and both male and female patients were required to use barrier contraceptive measures throughout therapy with imatinib. Patients were excluded from the trial if they had an Eastern Co-operative Oncology Group (ECOG) performance status of 3 or 4, grade 3/4 cardiac disease, leukemic central nervous system involvement, or any serious concomitant medical condition. Exclusion criteria also included treatment with anthracyclines, mitoxantrone, etoposide, methotrexate, or cyclophosphamide within 21 days before starting therapy; treatment with any other investigational agent or with high-dose cytosine arabinoside (1-3 g/m2every 12 to 24 hours for 6 to 12 doses) within 28 days before starting therapy; treatment with hematopoietic SCT within 6 weeks before starting therapy; or if recovery from SCT was not complete. Patients were also excluded from enrollment if they had a history of noncompliance with therapy or if they were considered by the investigator to be potentially unreliable.
All patients gave written informed consent to participate in the study, and the study was reviewed and approved by a recognized ethics review committee at each trial center. The study was performed in accordance with the Declaration of Helsinki (as amended in Tokyo, Venice, and Hong Kong).
Study design and treatment
This was an open-label, nonrandomized, multicenter, multinational Novartis-sponsored phase 2 trial designed to evaluate the clinical efficacy of imatinib, as determined by the rate of sustained hematologic response (lasting at least 4 weeks), and the safety of treatment.
Patients started treatment with imatinib at daily doses of 400 or 600 mg. For patients who relapsed, dose escalation to a maximum of 400 mg twice daily was permitted at the discretion of the investigator. On a case-by-case basis following discussion between the investigator and sponsor, dose escalation was also permitted for patients who did not achieve hematologic response after at least 1 month of therapy. Patients were scheduled to receive treatment for 24 weeks and then were continued indefinitely in cases where the investigator judged that further treatment was of clinical benefit.
Treatment was interrupted or reduced in response to nonhematologic, hepatic, or hematologic toxicity, which was graded according to National Cancer Institute/National Institutes of Health (NCI/NIH) Common Toxicity Criteria. For patients requiring dose reduction, daily doses were reduced from 600 to 400 mg, or from 400 to 300 mg. Dose reductions below 300 mg per day were not allowed by study design. If grade 2 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 1 or lower and was then resumed at the original dosage. If grade 2 toxicity recurred following resumption, treatment was again interrupted until recovery and was resumed at a reduced dose. If grade 3 or 4 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 1 or lower and then resumed at a reduced dose. Specific dose reduction rules for hepatic toxicity were applied to patients who enrolled with elevated baseline aminotransferase levels (3-fold to 5-fold above upper normal limits). If such patients developed increases in one or more aminotransferase levels of greater than 3-fold, therapy was interrupted until levels returned to baseline and was then resumed at a reduced dose. For patients who experienced clinically relevant, but lower than 3-fold increases in aminotransferase levels, treatment was interrupted until recovery and was then resumed at the same dose. If patients experienced a subsequent clinically relevant increase in aminotransferase levels, treatment was interrupted until recovery and resumed at a reduced dose. Dose reductions for hematologic toxicity were to be considered only for patients with grade 4 neutropenia (neutrophil count below 0.5 × 109/L) lasting at least 2 weeks, after a minimum of 28 days of therapy. In practice, however, imatinib doses were also reduced in cases of grade 4 thrombocytopenia considered to be unrelated to leukemia. Bone marrow examinations were performed routinely, and biopsies were obtained insofar as possible, until recovery from grade 4 neutropenia or thrombocytopenia. For patients with persistent marrow cellularity of less than 10% and blasts exceeding 10%, the daily dosage was successively reduced at 2-week intervals or was interrupted until recovery of neutropenia or thrombocytopenia to at least grade 2. Upon recovery, treatment was resumed at the full initial dose. Treatment was not interrupted or reduced for patients with marrow cellularity or blasts greater than 10%.
No concomitant anticancer drugs were to be administered. Treatment with allopurinol at 300 mg per day was recommended until stabilization of white blood cell (WBC) counts, and patients with febrile neutropenia or infection could receive treatment with colony-stimulating factors at the investigator's discretion.
Evaluation of patients
Patients were evaluated for hematologic and cytogenetic response and relapse at frequent intervals during the initial 24 weeks of imatinib treatment. Peripheral blood samples were obtained and analyzed at baseline, 3 times weekly for the first 4 weeks, weekly from weeks 5 to 13, every 2 weeks after week 13, and on the last day of treatment. Bone marrow aspirates were performed at screening; weeks 5, 9, 13, and 25; and on the last day of treatment; bone marrow biopsies were optional and were obtained as indicated. Physical examination to evaluate liver and spleen size and extramedullary involvement was carried out at screening, every 4 weeks during therapy, and on the last day of treatment. After the initial 24 weeks of treatment, bone marrow aspirates or biopsies for analysis of disease status were obtained every 3 months for the first 6 months and every 4 months thereafter. Following treatment, patients were followed for survival at least every 3 months, until death. Treatment toxicity was evaluated by patient interview at each office visit. Toxicity was graded according to NCI/NIH Common Toxicity Criteria.
The primary efficacy endpoint in this study was sustained hematologic response lasting at least 4 weeks, assessed as (1) complete hematologic response (CHR), defined according to conventional criteria as blast count below 5% in bone marrow, no blasts in peripheral blood, neutrophil count at least 1.5 × 109/L, platelet count at least 100 × 109/L, and no evidence of extramedullary involvement36; (2) complete marrow response (marrow-CR) in patients achieving a blast count of less than 5% in bone marrow, no blasts in peripheral blood, no evidence of extramedullary involvement, a neutrophil count at least 1.0 × 109/L, but no platelet recovery (platelet count at least 20 × 109/L); (3) partial response, defined as fewer than 15% blasts in peripheral blood and bone marrow. Sustained responses were required to be observed at 2 consecutive evaluations at least 4 weeks apart.
Secondary efficacy endpoints were the induction of cytogenetic response, time to disease progression, and overall survival. Complete cytogenetic response was defined as the absence of Ph+metaphases. Time to progression was calculated as the time from treatment start until relapse after initial response, or the interval when treatment was discontinued owing to unsatisfactory therapeutic response, adverse events, or patient death from any cause. Time to progression was censored at the last examination date for patients continuing treatment without progression, or at the time treatment was discontinued for reasons other than adverse events, unsatisfactory therapeutic response, or death. Overall survival was calculated as the time from treatment start to the date of death from any cause. Survival was censored at the time treatment was discontinued to allow bone marrow transplantation, or at the last recorded contact or evaluation when patients were alive at time of analysis.
Statistical analysis
Planned sample sizes of 30 patients with ALL were based upon practical considerations rather than inferential hypothesis testing, as this trial was regarded as a preliminary investigation of imatinib in acute leukemias. Enrollment of patients with CML in LyBC was discontinued before accrual of the 29 patients initially planned, on the basis of emerging phase 1 data suggesting that single-agent therapy with imatinib in patients with LyBC induced less durable responses than in the other Ph+ advanced leukemia disease groups. Response rates are reported as intent-to-treat analyses. Patients who withdrew from treatment before a sustained response was reported were counted as nonresponders. Time to progression and survival were computed by means of standard Kaplan-Meier methods. Safety results are reported for all enrolled patients who received at least one dose of imatinib.
Results
Patients and treatment
A total of 56 patients were enrolled at 18 centers in France, Germany, Italy, Switzerland, the United Kingdom, and the United States from September 1999 to May 2000. During screening examinations for study enrollment, patients had a confirmed diagnosis of Ph+acute leukemia consisting of ALL (48 patients) or LyBC (8 patients).
Table 1 summarizes patient characteristics and disease history at baseline for all 56 enrolled patients. The first 10 patients (5 with ALL and 5 with LyBC) started initial therapy with imatinib at daily doses of 400 mg, and in the subsequent 46 patients, the initial dose was 600 mg/d. However, dose escalation or reduction was permitted for all patients, and no substantial difference in efficacy or toxicity was observed between patients who started therapy at these different doses. Median treatment durations were similar for patients with ALL (62 days; range, 14-343 days) and LyBC (56 days; range, 32-325 days). Of the 56 patients enrolled, 3 were still on treatment at the time of analysis. The remaining patients withdrew from treatment owing to disease progression (72%), adverse events (5%), death during therapy (9%), or elective SCT (9%).
Demographics, disease history, and characteristics of patients at baseline
| Characteristics . | ALL, n = 48 . | LyBC, n = 8 . |
|---|---|---|
| Age, y | ||
| Median (range) | 50 (22-78) | 60 (49-68) |
| Sex, no. (%) | ||
| Male | 24 (50) | 4 (50) |
| Female | 24 (50) | 4 (50) |
| ECOG score, no. (%) | ||
| Grade 0-1 | 26 (54) | 5 (62) |
| Grade 2 | 19 (40) | 3 (38) |
| Grade 3 | 1 (2) | 0 |
| NA | 2 (4) | 0 |
| Prior response, no. (%) | ||
| Relapsed after first remission | 19 (40) | 3 (38) |
| Relapsed after 2 or more remissions | 12 (25) | 3 (38) |
| Refractory | 17 (35) | 2 (25) |
| Prior BMT, no. (%) | 10 (21) | 0 |
| Diagnosis to study entry, mo | ||
| Median | 8 | 5 |
| Range | 2-118 | 0-14 |
| WBC (× 109/L) | ||
| Median | 10 | 13 |
| Range | 0.2-195 | 3-106 |
| Blasts in peripheral blood, % | ||
| Median | 18 | 1 |
| Range | 0-94 | 0-84 |
| Blasts in bone marrow, % | ||
| Median | 83 | 50 |
| Range | 3-100 | 13-95 |
| Platelets (× 109/L) | ||
| Median | 32 | 108 |
| Range | 1-1715 | 9-1059 |
| Characteristics . | ALL, n = 48 . | LyBC, n = 8 . |
|---|---|---|
| Age, y | ||
| Median (range) | 50 (22-78) | 60 (49-68) |
| Sex, no. (%) | ||
| Male | 24 (50) | 4 (50) |
| Female | 24 (50) | 4 (50) |
| ECOG score, no. (%) | ||
| Grade 0-1 | 26 (54) | 5 (62) |
| Grade 2 | 19 (40) | 3 (38) |
| Grade 3 | 1 (2) | 0 |
| NA | 2 (4) | 0 |
| Prior response, no. (%) | ||
| Relapsed after first remission | 19 (40) | 3 (38) |
| Relapsed after 2 or more remissions | 12 (25) | 3 (38) |
| Refractory | 17 (35) | 2 (25) |
| Prior BMT, no. (%) | 10 (21) | 0 |
| Diagnosis to study entry, mo | ||
| Median | 8 | 5 |
| Range | 2-118 | 0-14 |
| WBC (× 109/L) | ||
| Median | 10 | 13 |
| Range | 0.2-195 | 3-106 |
| Blasts in peripheral blood, % | ||
| Median | 18 | 1 |
| Range | 0-94 | 0-84 |
| Blasts in bone marrow, % | ||
| Median | 83 | 50 |
| Range | 3-100 | 13-95 |
| Platelets (× 109/L) | ||
| Median | 32 | 108 |
| Range | 1-1715 | 9-1059 |
NA indicates not available.
Efficacy
Table 2 summarizes hematologic response rates for the 48 ALL and 8 LyBC patients. Values represent the best response observed at any time during therapy. Of the 48 ALL patients, 9 (19%) achieved a CHR; a marrow-CR or partial marrow response was observed in 5 patients (10%) and 15 patients (31%), respectively. Thus, 60% had reductions in blast counts in peripheral blood and in bone marrow corresponding to hematologic response on at least one occasion. Hematologic responses lasting at least 4 weeks were reported for 13 patients (27%), including 3 CHRs (6%). Many patients with an initial hematologic response did not have adequate bone marrow evaluations at later assessments, and sustained response could not be confirmed solely for that reason. The median time to hematologic response was 1 month, corresponding to the first scheduled evaluation of hematologic response; reduction in peripheral blood blasts typically occurred within 1 week after initiation of imatinib therapy. Complete cytogenetic responses were reported for 8 (17%) of the 48 patients with ALL.
Hematologic response in patients with acute lymphoblastic leukemia
| Hematologic response . | ALL . | LyBC . | ||
|---|---|---|---|---|
| All responses, N = 48 . | Sustained responses, N = 48 . | All responses, N = 8 . | Sustained responses, N = 8 . | |
| Overall, no. (%) | 29 (60) | 13 (27) | 4 (50) | 2 (25) |
| 95% CI, % | 45.3-74.2 | 15.3-41.8 | 15.7-84.3 | 3.2-65.1 |
| CR, no. (%) | 9 (19) | 3 (6) | 4 (50) | 1 (12.5) |
| Marrow-CR, no. (%) | 5 (10) | 0 | 0 | 0 |
| Partial marrow response, no. (%) | 15 (31) | 10 (21) | 0 | 1 (12.5) |
| Not evaluable, no. (%) | 7 (15) | 10 (21) | 0 | 0 |
| No response, no. (%) | 12 (25) | 25 (52) | 4 (50) | 6 (75) |
| Hematologic response . | ALL . | LyBC . | ||
|---|---|---|---|---|
| All responses, N = 48 . | Sustained responses, N = 48 . | All responses, N = 8 . | Sustained responses, N = 8 . | |
| Overall, no. (%) | 29 (60) | 13 (27) | 4 (50) | 2 (25) |
| 95% CI, % | 45.3-74.2 | 15.3-41.8 | 15.7-84.3 | 3.2-65.1 |
| CR, no. (%) | 9 (19) | 3 (6) | 4 (50) | 1 (12.5) |
| Marrow-CR, no. (%) | 5 (10) | 0 | 0 | 0 |
| Partial marrow response, no. (%) | 15 (31) | 10 (21) | 0 | 1 (12.5) |
| Not evaluable, no. (%) | 7 (15) | 10 (21) | 0 | 0 |
| No response, no. (%) | 12 (25) | 25 (52) | 4 (50) | 6 (75) |
CI indicates confidence interval; CR, complete remission.
One of the 3 patients with a sustained CHR has maintained an ongoing response 11 months after the start of therapy, with no evidence of Ph+ cells in bone marrow. Of the remaining 2 patients with sustained CHR, 1 relapsed after 4.7 months and withdrew from imatinib therapy. Another was found to have Ph+ cells in a marrow sample after 5.6 months, and started concomitant treatment with interferon alfa 1 month later. Concomitant therapy with interferon alfa and imatinib has been maintained, and the patient remains relapse-free 3 months after starting combined therapy. This patient has been censored from all evaluations of efficacy and safety for this trial, from the start date of concomitant interferon therapy.
Among the 19 patients with no reported hematologic response at any time, 12 had transient (duration of no more than 4 weeks in 8 patients, 5 weeks in 2 patients, and 9 and 10 weeks in 1 patient each) but marked reductions in peripheral blood blasts, indicating some degree of hematologic improvement. Of the remaining 7 patients, 6 were not assessable for hematologic improvement owing to low circulating blasts at study entry.
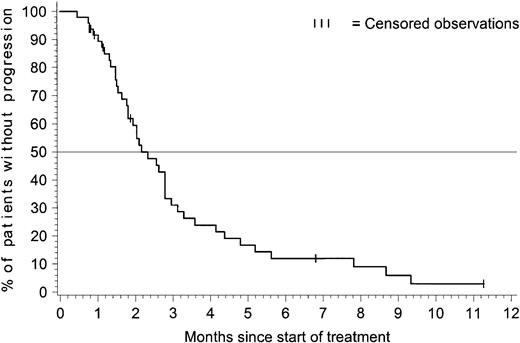
Figure 1 presents the time to progression for patients with ALL. The estimated median time to progression was 2.2 months (95% CI = 1.8-2.8 months), and the estimated progression-free rate at 6 months was 12% (95% CI = 2%-22%).
Time to progression for patients with acute lymphoblastic leukemia.
The time-to-progression analysis included all 48 patients with ALL. At the time of analysis, 41 patients had documented progression, and 7 were censored: 5 patients discontinued therapy within the first 2 months to undergo bone marrow transplantation (BMT) procedures and were censored at the time of discontinuation; 1 patient was censored after receiving interferon alfa after 7 months on the study, and 1 patient was progression-free and receiving treatment after 11 months on the study. The estimated median time to progression was 2.2 months with 95% CI = 1.8-2.8 months.
Time to progression for patients with acute lymphoblastic leukemia.
The time-to-progression analysis included all 48 patients with ALL. At the time of analysis, 41 patients had documented progression, and 7 were censored: 5 patients discontinued therapy within the first 2 months to undergo bone marrow transplantation (BMT) procedures and were censored at the time of discontinuation; 1 patient was censored after receiving interferon alfa after 7 months on the study, and 1 patient was progression-free and receiving treatment after 11 months on the study. The estimated median time to progression was 2.2 months with 95% CI = 1.8-2.8 months.
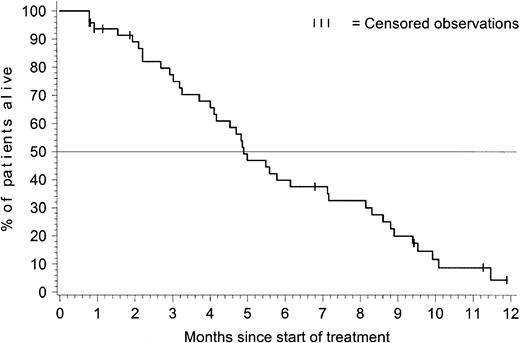
Figure 2 presents overall survival (OS) for the 48 patients with ALL. The Kaplan-Meier estimate for median OS was 4.9 months (95% CI = 4.1-7.1 months), and the estimated overall survival rate at 6 months was 40% (95% CI = 25% to 54%).
Overall survival for patients with acute lymphoblastic leukemia.
At the time of analysis, 39 of the 48 ALL patients died, and 9 were censored for the analysis of overall survival: 5 discontinued therapy with imatinib to undergo BMT (3 subsequently died by the time of analysis); 1 was censored after 7 months after receiving interferon alfa; and 3 patients remained alive at the time of analysis, 9 to 12 months after starting treatment with imatinib (treatment is ongoing for only 1 of these 3 patients). The estimated median survival was 4.9 months with 95% CI = 4.1-7.1 months.
Overall survival for patients with acute lymphoblastic leukemia.
At the time of analysis, 39 of the 48 ALL patients died, and 9 were censored for the analysis of overall survival: 5 discontinued therapy with imatinib to undergo BMT (3 subsequently died by the time of analysis); 1 was censored after 7 months after receiving interferon alfa; and 3 patients remained alive at the time of analysis, 9 to 12 months after starting treatment with imatinib (treatment is ongoing for only 1 of these 3 patients). The estimated median survival was 4.9 months with 95% CI = 4.1-7.1 months.
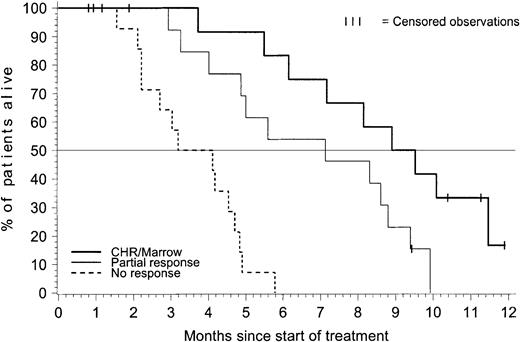
To determine whether patients achieving any form of hematologic response had a survival advantage compared with nonresponders, we compared patients with a CHR or marrow-CR (n = 14), those with a partial response (n = 15), and the 14 patients who were treated for at least 4 weeks but did not have documented response. Whereas median survival was 9.2 months for patients who had CHR/marrow-CR and 7.1 months for patients with a partial response, all of the nonresponders died and their median survival was only 3.6 months (P < .001) (Figure 3).
Overall survival for patients with acute lymphoblastic leukemia—by hematologic response.
This analysis of overall survival by best achieved hematologic response on treatment included 14 patients with CHR or marrow response, 15 patients with partial response, and 14 patients who were treated for at least 4 weeks but did not have documented response (5 patients were excluded). Whereas median survival was 9.2 months for patients who had CHR/marrow response and 7.1 months for patients with partial response, all of the nonresponder died and their median survival was only 3.6 months (P < .001).
Overall survival for patients with acute lymphoblastic leukemia—by hematologic response.
This analysis of overall survival by best achieved hematologic response on treatment included 14 patients with CHR or marrow response, 15 patients with partial response, and 14 patients who were treated for at least 4 weeks but did not have documented response (5 patients were excluded). Whereas median survival was 9.2 months for patients who had CHR/marrow response and 7.1 months for patients with partial response, all of the nonresponder died and their median survival was only 3.6 months (P < .001).
When we analyzed other factors potentially affecting treatment outcome, only the WBC count at baseline was shown to have a significant effect on survival, with a longer median survival (7.2 months) in patients with a WBC count below 10 × 109/L compared with patients with a baseline WBC count exceeding 10 × 109/L (median survival, 4.1 months) (P = .0163 by log-rank test). None of the other clinical or laboratory parameters—initial imatinib dose, age, sex, time since diagnosis, prior response, prior BMT, presence of hepatomegaly or splenomegaly, WBC count, platelets, blast cells in peripheral blood, hemoglobin and additional chromosomal abnormalities—were statistically significant in terms of predicting initial response to imatinib or survival.
Among 11 patients who had undergone prior SCT procedures, treatment with imatinib induced sustained hematologic responses in 3 (27%; 2 CHRs and 1 partial response), and a further 5 patients (45%) met criteria for hematologic response on at least one occasion (1 CHR and 4 partial responses). These rates of response are comparable to those observed for all 48 patients with ALL (Table 2).
Five patients with ALL discontinued treatment with imatinib in order to undergo allogeneic SCT. Of these, 3 discontinued within 1 month, with blood counts consistent with CHR or marrow response (1 patient each), or without a valid response evaluation (1 patient); and 2 discontinued within 2 months, with CHR and marrow response (1 patient each). Sustained marrow response was documented for one patient who discontinued after 1.9 months of therapy, while other patients had too few evaluations to determine response duration. Of these 5 patients, 1 died of treatment-related complications; 2 have relapsed and died of leukemia; and 2 were alive at the time of analysis 10 months after they had started imatinib.
Efficacy results for patients with LyBC were generally similar to those for patients with ALL (Table 2). Sustained hematologic responses (lasting at least 4 weeks) were reported for 2 of the 8 patients with LyBC (one CHR and one partial response). Of the other 6 LyBC patients, 2 had unsustained CHRs and 4 had reductions in blast counts in peripheral blood and/or bone marrow, but did not satisfy criteria for hematologic response. Complete cytogenetic responses were reported for one with LyBC. Median overall survival for the 8 patients with LyBC was 6.6 months (95% CI = 2.4-9.9 months). One LyBC patient who achieved CHR and complete cytogenetic response remained progression-free and receiving imatinib therapy after 11 months.
Toxicity
The toxicity of imatinib in this trial was generally similar to that observed in patients with CML.31 33-35 Table3 presents treatment-related nonhematologic adverse reactions observed in 10% of patients or more. The most frequently reported events were nausea, vomiting, and edema, with few of these events described as grade 3 or 4. Table4 presents the incidence of grade 3 or 4 hematologic toxicity and abnormal laboratory findings. The most common grade 3 or 4 findings were leukopenia or neutropenia.
Nonhematologic adverse events related to treatment with imatinib
| Adverse event3-150 . | All enrolled patients, N = 56 . | |
|---|---|---|
| All grades no. (%) . | Grades 3 or 4 no. (%) . | |
| Nausea | 43 (77) | 1 (2) |
| Vomiting | 35 (63) | 0 |
| Lower limb edema | 16 (29) | 1 (2) |
| Periorbital edema | 15 (27) | 1 (2) |
| Abdominal pain | 14 (25) | 0 |
| Muscle cramps | 8 (14) | 0 |
| Skin rash | 6 (11) | 1 (2) |
| Face edema | 6 (11) | 0 |
| Diarrhea | 6 (11) | 0 |
| Adverse event3-150 . | All enrolled patients, N = 56 . | |
|---|---|---|
| All grades no. (%) . | Grades 3 or 4 no. (%) . | |
| Nausea | 43 (77) | 1 (2) |
| Vomiting | 35 (63) | 0 |
| Lower limb edema | 16 (29) | 1 (2) |
| Periorbital edema | 15 (27) | 1 (2) |
| Abdominal pain | 14 (25) | 0 |
| Muscle cramps | 8 (14) | 0 |
| Skin rash | 6 (11) | 1 (2) |
| Face edema | 6 (11) | 0 |
| Diarrhea | 6 (11) | 0 |
Events reported in at least 10% of patients.
Hematologic and nonhematologic abnormalities during treatment with imatinib
| Parameter and grade4-150 . | All enrolled patients, N = 56 no. (%) . |
|---|---|
| Anemia | |
| Grade 3 | 17 (30) |
| Grade 4 | 4 (7) |
| Thrombocytopenia | |
| Grade 3 | 12 (21) |
| Grade 4 | 15 (27) |
| Leukocytopenia | |
| Grade 3 | 15 (27) |
| Grade 4 | 23 (41) |
| Neutrocytopenia | |
| Grade 3 | 7 (13) |
| Grade 4 | 30 (54) |
| Alkaline phosphatase increase | |
| Grade 3 | 1 (2) |
| Grade 4 | 0 |
| ALT increase | |
| Grade 3 | 2 (4) |
| Grade 4 | 0 |
| Bilirubin increase | |
| Grade 3 | 2 (4) |
| Grade 4 | 0 |
| Parameter and grade4-150 . | All enrolled patients, N = 56 no. (%) . |
|---|---|
| Anemia | |
| Grade 3 | 17 (30) |
| Grade 4 | 4 (7) |
| Thrombocytopenia | |
| Grade 3 | 12 (21) |
| Grade 4 | 15 (27) |
| Leukocytopenia | |
| Grade 3 | 15 (27) |
| Grade 4 | 23 (41) |
| Neutrocytopenia | |
| Grade 3 | 7 (13) |
| Grade 4 | 30 (54) |
| Alkaline phosphatase increase | |
| Grade 3 | 1 (2) |
| Grade 4 | 0 |
| ALT increase | |
| Grade 3 | 2 (4) |
| Grade 4 | 0 |
| Bilirubin increase | |
| Grade 3 | 2 (4) |
| Grade 4 | 0 |
Abnormalities occurring during treatment or worsening from baseline to the indicated grade according to the NCI/NIH Common Toxicity Criteria.
None of the 56 patients enrolled in this study discontinued therapy with imatinib because of treatment-related nonhematologic adverse events. Treatment-related adverse events regarded as serious were reported for 9 patients (16%), and comprised neutropenia or febrile neutropenia (4 patients), elevated liver aminotransferases (2 patients), fever of unknown origin, cerebral edema, headache, anorexia, cachexia, nausea, vomiting, and generalized rash (1 patient each; some patients experienced multiple serious adverse events). No treatment-related deaths were reported.
Discussion
The treatment of patients with Ph+ acute leukemia who have failed primary therapy poses a difficult clinical challenge. Patients who relapse after intensive combination chemotherapy or allogeneic transplantation, or who are refractory to treatment, have few therapeutic options.16,18,27 We conducted this phase 2 trial to determine whether imatinib, a potent inhibitor of the oncogenic Bcr-Abl tyrosine kinase, when administered at well-tolerated doses as defined in an earlier trial,31,35 could induce meaningful and durable hematologic responses in patients with relapsed or refractory Ph+ ALL or LyBC. In the present study, the rates of CHR and of marrow-CR with imatinib given orally at 400 mg or 600 mg per day were 19% and 10% in patients with relapsed or refractory ALL. In the vast majority of patients, these responses were of short duration, with a median time to disease progression of only 2.2 months, an estimated progression-free rate of 12% at 6 months, and an overall survival rate of 40% at 6 months by Kaplan-Meier analysis. Thus, CHRs were sustained for at least 4 weeks in only 6% of patients with ALL. Overall, these findings are similar to those of a recently published phase 1 study, which demonstrated an overall response rate of 70%, a marrow response with blasts fewer than 15% in 50%, and a complete hematologic response rate of 20% in patients with a lymphoid phenotype.35 Reasons for the slightly inferior results in our phase 2 study may be (1) patient selection, particularly as the number of patients with Ph+ALL in the phase 1 study was small and the results of ALL and LyBC patients were combined, and (2) slightly different definitions of response.
Published results of conventional salvage chemotherapy in adult ALL have shown poor treatment outcome independently of the presence of the BCR-ABL translocation, with median disease-free survival (DFS) ranging from 2 to 7.5 months18,37; in patients with adverse risk factors, some series have reported the chance of long-term DFS to be less than 5%.38 Therefore, DFS and OS observed in the present study of single-agent imatinib is within the range of previous experience with aggressive chemotherapy regimens. While the initial CHR rates of 30% to 60% that have been reported for salvage chemotherapy appear higher than the 19% achieved in our study, more detailed analyses have demonstrated that the presence of additional unfavorable clinical features has a profound negative impact on the response to chemotherapy. In a multivariate analysis, worse survival was observed with an increasing numbers of adverse features, permitting stratification into risk groups with median survival as low as 2 months and corresponding CR rates of 9% in patients with 3 unfavorable features.39 As a large proportion of patients in our study had several unfavorable risk factors in addition to the Philadelphia chromosome, eg, higher age, second or subsequent relapse, relapse after SCT and short (less than 1 year) duration of first CR, the results achieved with imatinib are comparable to those observed with intensive chemotherapy regimens. In contrast to chemotherapy, which is associated with significant morbidity and induction mortality of up to 40%,18 imatinib was associated with comparatively few severe adverse events and an overall death rate during study treatment of 9%. Imatinib was well tolerated despite pronounced hematologic toxicity in many patients, which did not require discontinuation of therapy, however, and was associated with remarkably few serious infectious or bleeding complications. Nonhematologic adverse reactions were grade 1 or 2 in the majority of cases. These observations are most likely attributable to the more selective mechanism of action of imatinib as opposed to conventional cytotoxic agents, which induce considerably greater organ damage, such as mucositis, which predisposes to subsequent complications of therapy. The low rate of complete absolute neutrophil count and platelet recovery that we observed even in patients demonstrating a good marrow response and clearance of circulating blasts is unclear. Inhibition of c-kit kinase activity by imatinib could conceivably compromise normal hematopoietic recovery, but this is largely speculative.40
Development of resistance to imatinib has become the central issue concerning the use of imatinib in advanced BCR-ABL+ leukemias. In Ph+ ALL, secondary resistance has been shown to be associated with point mutations in the adenosine triphosphate binding domain or activation loop of the BCR-ABL oncoprotein in a large proportion, and possibly the majority, of patients with lymphoid BCR-ABL+leukemias.41,42 No point mutations have so far been identified prior to imatinib therapy in patients showing primary resistance to this agent, suggesting that mechanisms of resistance are multifactorial. On the basis of in vitro models, genomic amplification of the BCR-ABL fusion gene, increased expression of the BCR-ABL protein, enhanced drug efflux due to up-regulation of multidrug resistance proteins, or decreased cellular bioavailability of imatinib have been postulated to play a role in resistance.43-46 Further investigation of resistance mechanisms is likely to suggest treatment strategies able to circumvent or delay resistance in patients with advanced Ph+ acute leukemia. This outlook is supported by the recent demonstration that gene expression profiling of Ph+ ALL blasts by microarray analysis is able to discriminate between imatinib-sensitive and imatinib-refractory leukemias, and that genes related to apoptosis pathways and cell cycle control—among others—are differentially expressed in sensitive and resistant cells.47
While the lack of long-term efficacy of imatinib is at first glance sobering, the inability of a single agent to cure patients with the most unfavorable leukemic subtype following failure of primary therapy should come as no surprise. Rather, our observation of pronounced, albeit brief, hematologic responses in the majority of relapsed and refractory patients, in conjunction with imatinib's favorable safety profile encourages further investigation of imatinib as an adjunct to established antileukemic therapy. For example, the antileukemic activity of imatinib may open a window of opportunity for patients who are resistant to chemotherapy but are otherwise eligible for allogeneic SCT. In our study, 2 of 5 patients who were transferred to allogeneic SCT exhibit prolonged DFS; these encouraging but preliminary results need more extensive testing in prospective clinical trials.
Because of its low toxicity, imatinib may also be useful as palliative treatment for patients who are not candidates for aggressive salvage therapy, and incorporation of this selective agent in trials in the setting of first-line treatment of Ph+ acute leukemia seems justified.
Recent reports of additive or synergistic in vitro activity of imatinib in combination with cytotoxic agents active against ALL, such as daunorubicin or cytarabine,48-50 or in combination with other novel signal transduction modulators, such as farnesyl transferase inhibitors,51 52 support clinical trials to test the feasibility of combination regimens. Our current study provides the basis for investigations aimed at identifying combination therapies most appropriate in the various clinical settings of Ph+ acute leukemias.
We wish to thank the numerous coinvestigators, nursing staff, and clinical trial monitors who participated in this study. The contributions of data managers and programmers at Novartis Pharmaceuticals are gratefully acknowledged. Dr David Parkinson and Dr Greg Burke (Novartis) provided invaluable support, and we thank Marianne Rosamilia, Dr John Ford, and Dr Elisabeth Wehrle (Novartis) for their collaboration in implementing the protocol and reporting of the study results. Dr Thomas Brown provided valuable assistance in preparing the manuscript. We are also indebted to Ann Bolton for assisting in data analysis and preparation of the manuscript.
In addition to the authors, the following investigators participated in this trial: B. Wassmann, MD, Medizinische Klinik III, Johann Wolfgang Goethe University, Frankfurt, Germany; M. O'Dwyer, MD, Portland, OR; Ron Paquette, Department of Medicine and Molecular Biology Institute, University of California at Los Angeles; J. F. Apperly, MD, London, United Kingdom; F. X. Mahon, MD, Laboratoire de Greffe de Moelle, Université Victor Segalen, Bordeaux, France; M. Schuster, MD, New York, NY; G. Rosti, MD, Bologna, Italy; Joachim Beck, MD, Universitätsklinikum, 3 Medizinische Klinik und Poliklinik, Mainz, Germany; Haifa-Kathrin Al-Ali, MD, Abteilung Hämatologie/Onkologie, Universität Leipzig, Germany; D. Russo, MD, Udine, Italy; Dr Monika Ebnoether, Division of Hematology, Universitätsklinik, Kantonspital, Basel, Switzerland; Dr Tanja Lahaye, III Medizinische Universitätsklinik Mannheim der Universität Heidelberg, Mannheim, Germany; and Professor S. J. Proctor and Dr A. L. Lennard, Department of Haematology, Royal Victoria Infirmary, University of Newcastle, United Kingdom.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2001-12-0181.
Supported by a grant from Novartis.
S. F.-R., I. G., and R. C. are employees of Novartis Pharma.
This study was presented in part at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2000, San Francisco, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
O. G. Ottmann, Medizinische Klinik III/Abteilung Hämatologie, Johann Wolfgang Goethe University, 60590 Frankfurt, Germany; e-mail: ottmann@em.uni-frankfurt.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal