Abstract
The immunosuppressive drugs, cyclosporine A (CsA), tacrolimus, or sirolimus, were analyzed as single agents and in combination with anti-CD40L monoclonal antibody (mAb) for their effects on alloengraftment in mice conditioned with minimal total body irradiation (TBI). Whereas anti-CD40L mAb facilitated chimerism, neither sirolimus nor CsA resulted in substantial alloengraftment. However, sirolimus was synergistic with anti-CD40L mAb for inducing donor chimerism. Contrary to expectations, CsA, a T-cell receptor (TCR) signaling inhibitor, did not abrogate anti-CD40L mAb-facilitated engraftment but rather increased engraftment in anti-CD40L mAb-treated mice. Although tacrolimus alone or with anti-CD40L mAb resulted in similar levels of donor chimerism, donor T-cell reconstitution was very low in tacrolimus-treated mice. At 1 week after transplantation, CsA decreased thymic numbers more profoundly than sirolimus or tacrolimus in anti-CD40L mAb-treated recipients. In contrast, only sirolimus resulted in a decrease in host splenic T-cell numbers in anti-CD40L mAb-treated recipients. Importantly, sirolimus and anti-CD40L mAb induced profound donor tolerance with 100% acceptance of donor skin grafts placed early after bone marrow transplantation (BMT). In contrast, anti-CD40L mAb alone or in combination with CsA resulted in 12% or less donor skin graft acceptance early (1 month) and 60% or less later (3 months) after BMT. These data have clinical relevance and indicate that immunosuppressive pharmacologic agents enhance anti-CD40L mAb-facilitated alloengraftment and tolerance induction under nonmyeloablative conditioning.
Introduction
Productive T-cell activation and proliferation require 2 signaling events. The first, antigen recognition, is the engagement of the T-cell receptor (TCR) with the major histocompatibility complex (MHC) peptide ligand complex on the surface of the antigen-presenting cell (APC). The second, provided by costimulatory signals, is required for interleukin 2 (IL-2) production, T-cell proliferation, and full effector function. Signal 1 in the absence of signal 2 renders a T cell nonresponsive or tolerant to antigen. Blockade of the CD40L/CD40 costimulatory pathway via anti-CD40L monoclonal antibody (mAb) has been effective in the promotion of alloengraftment of bone marrow (BM) and solid organs in several murine transplant models.1-7 Although anti-CD40L mAb as a single agent has been shown to facilitate BM and solid organ engraftment in mice, it has been less effective in nonhuman primate transplant models.8-10 Therefore, in these studies, we evaluated the efficacy of the pharmacologic immune suppressive agents, sirolimus, cyclosporine A (CsA), and tacrolimus as single agents and in combination with anti-CD40L mAb for their effects on alloengraftment under a nonmyeloablative-conditioning regime.
The macrolide antibiotic sirolimus binds to molecular target of rapamycin (mTOR) and blocks a late stage of T-cell activation downstream of both TCR engagement (signal 1) and costimulation (signal 2). Sirolimus mediates immunosuppression by inhibiting IL-2 responsiveness. Previously, we reported that sirolimus potently inhibited graft-versus-host disease (GVHD) mortality11-14and also increased donor chimerism in sublethally (600 cGy total body irradiation [TBI]) irradiated murine recipients.15Studies by Li et al16 indicated that sirolimus was additive or synergistic with costimulatory blockade for the acceptance of donor skin allografts in unconditioned mice. Additionally, a recent clinical trial concluded that sirolimus had substantial activity in the treatment of steroid-refractory acute GVHD, indicating that sirolimus is a beneficial immunosuppressive agent in bone marrow transplantation (BMT).17 These data and the mode of action suggested that sirolimus might be additive or synergistic with costimulatory blockade strategies for the promotion of alloengraftment by preventing the clonal expansion of alloreactive T cells that escaped costimulatory blockade.
CsA and tacrolimus are calcineurin inhibitors that block TCR signaling (signal 1) at an early stage in T-cell activation. Although CsA and tacrolimus are proven immunosuppressive reagents for the promotion of engraftment and amelioration of GVHD in rodents, these agents may antagonize tolerance induction when used in combination with costimulatory blockade strategies by inhibiting TCR signals necessary for tolerance induction. Additionally, the administration of CsA has been shown to inhibit CD40L expression on CD4+ T cells induced by TCR signaling,18 albeit not CD40L expression induced by multiple cytokines sharing the IL-2 receptor common γ chain.19 Alternatively, it is possible that CsA and tacrolimus may facilitate engraftment mediated by costimulatory blockade by reducing the intensity of TCR signaling, allowing for a more readily attainable blockade of costimulation. Literature reports have indicated that these immune suppressive agents can either inhibit or enhance solid organ graft acceptance when used in combination with costimulatory pathway blockade.16,18,20-23 In contrast to the restricted expression of alloantigen on a solid organ graft, alloantigenic targets of an immune response are widely distributed throughout the recipient or on donor BM cells that are systemically infused. In addition, patients are conditioned for BMT with chemotherapy and/or radiotherapy that can induce proinflammatory responses resulting in increased T-cell alloreactivity.24Thus, studies in solid organ recipients cannot be directly extrapolated to BM transplant recipients. The effect of combined calcineurin inhibitors and costimulatory blockade strategies on alloengraftment was not readily predictable. The purpose of the current study was to determine the effect of sirolimus, CsA, and tacrolimus on anti-CD40L mAb-facilitated alloengraftment under a nonmyeloablative-conditioning regime. These data have potentially important clinical ramifications in the development of costimulatory blockade approaches for patients receiving BM transplants.
Materials and methods
Mice
C57BL/6 (H2b) (termed B6) and BALB/c (H2d) female mice were purchased from the National Institutes of Health (Bethesda, MD). B10.BR (H2k) female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were used at 8 to 12 weeks of age and housed in a specific pathogen-free facility in microisolator cages.
Bone marrow transplantation
B6 mice were subjected to TBI by x-ray (39 cGy/min) at the indicated dosage on day −1. On day 0, 40 × 106 whole BM cells from BALB/c mice were injected intravenously by lateral tail vein injection into irradiated B6 recipients. This BM cell dose (2 × 109/kg) is within the range achievable clinically by the combined infusion of BM plus granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells. Survival was monitored daily, and mice were weighed twice weekly for the first month after transplantation, then once weekly thereafter. Anti-CD40L mAb (hybridoma MR1, hamster immunoglobulin G [IgG]) was obtained by culturing the hybridoma in 10% fetal bovine serum–Dulbecco minimum essential medium in a hollow fiber bioreactor (Accucyst JR; Cellex Biosciences, Minneapolis, MN). Supernatant was purified by ammonium sulfate precipitation. Anti-CD40L mAb or irrelevant hamster IgG (hIgG) (Rockland, Gilbertsville, PA) was administered intraperitoneally at a dosage of 200 μg daily from days −1 through +5, then twice weekly until day 14 after BMT. Sirolimus (Rapamycin; Wyeth-Ayerst, Princeton, NJ) was administered intraperitoneally as a suspension in 0.5% carboxy methyl cellulose (CMC) (Sigma, St Louis, MO) at a dosage of 1.5 mg/kg daily from days −1 through +13 after BMT.11,15Cyclosporine A (CsA) (Sandoz Research Institute, East Hanover, NJ) was administered intraperitoneally as a suspension in CMC at a dosage of 80 mg/kg daily from days −1 through +13 after BMT.13Tacrolimus (FK506; Fujisawa Pharmaceuticals, Osaka, Japan) was administered intraperitoneally as a suspension in CMC at a dosage of 24 mg/kg daily from days −1 through +13 after BMT.25Previously published data comparing drug vehicles and drug dose titrations determined that these dosages of sirolimus, CsA, and tacrolimus as drug suspensions in CMC were optimal in reducing or delaying GVHD mortality.11,13 25 Drug dosage reductions of 2-fold resulted in greatly diminished therapeutic efficacy. Anti-CD4 (hybridoma GK1.5, rat IgG2) and anti-CD8 (hybridoma 2.43, rat IgG2b) depleting mAbs were administered intraperitoneally at a dosage of 400 μg on days −2, 1, 5, and 9 to cohorts of mice in some experiments as indicated.
Flow cytometric analysis
Peripheral blood leukocytes (PBLs) were obtained by retro-orbital venipuncture and harvested over a Ficoll gradient. Single cell suspensions were made of thymi and spleens. Cells were stained with fluorochrome-conjugated antibodies (anti-CD8, anti-CD4, anti-MAC-1, anti-CD19, anti-H2d, anti-H2b, and irrelevant isotype controls) purchased from PharMingen (San Diego, CA). Stained cells were analyzed using CellQuest software on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Skin grafting
Full-thickness skin grafts (approximately 1 cm2) obtained from BALB/c and B10.BR donor mice were transplanted on the lateral thorax of recipient mice and secured with a bandage for 7 days. Grafts were monitored daily for the first 3 weeks and then twice weekly thereafter. Rejection was defined as the complete loss of viable donor graft tissue.
Statistics
Group comparisons of percentage donor chimerism were made by Student t test. Group comparisons of rates of engraftment were made by chi-squared test. P ≤ .05 was considered significant.
Results
Anti-CD40L mAb and sirolimus are synergistic for the promotion of alloengraftment under a nonmyeloablative-conditioning regime
To determine the effect of sirolimus on engraftment as a single agent, and in combination with anti-CD40L mAb, B6 mice were conditioned with 200 cGy TBI and administered a single dose (40 × 106) of BALB/c BM. Recipients received a 2-week course of irrelevant hIgG, anti-CD40L mAb, sirolimus, or a combination of anti-CD40L mAb and sirolimus. Mice were evaluated for percentage of donor chimerism at 6 weeks (data not shown) and 3.5 months after transplantation by PBL phenotyping (Table1, experiment 1). All 10 anti-CD40L–treated mice had donor engraftment (mean of 69% ± 5%) in contrast to 0 of 10 hIgG-treated mice. Although sirolimus has been shown to substantially increase donor engraftment under conditions of heavier TBI,15 no engraftment was evident in any sirolimus-treated mice conditioned with 200 cGy TBI. Combined anti-CD40L mAb and sirolimus resulted in similar rates and only a modestly higher level of engraftment as compared with anti-CD40L mAb administered as a single agent (75% ± 6% versus 69% ± 5%;P = .038, Table 1, experiment 1). These data indicated that anti-CD40L mAb was a more potent engraftment-enhancing agent than sirolimus, and sirolimus only modestly enhanced the potent engraftment-promoting effect of anti-CD40L mAb in the context of 200 cGy TBI.
Anti-CD40L alone or in combination with sirolimus or CsA results in high levels of donor chimerism in mice conditioned with 200 cGy TBI
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| Experiment 1 | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/10* | 69 ± 5* | 64 -76 |
| Sirolimus | 0/10† | 0 ± 0† | NA |
| Anti-CD40L + sirolimus | 9/9* | 75 ± 6*,† | 70 -83 |
| Experiment 2 | |||
| Hamster IgG | 0/15 | 0 ± 0 | NA |
| Anti-CD40L | 9/10* | 64 ± 24* | 61 -85 |
| CsA | 3/9*,† | 6 ± 14† | 3 -43 |
| Anti-CD40L + CsA | 17/17* | 62 ± 13* | 28 -78 |
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| Experiment 1 | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/10* | 69 ± 5* | 64 -76 |
| Sirolimus | 0/10† | 0 ± 0† | NA |
| Anti-CD40L + sirolimus | 9/9* | 75 ± 6*,† | 70 -83 |
| Experiment 2 | |||
| Hamster IgG | 0/15 | 0 ± 0 | NA |
| Anti-CD40L | 9/10* | 64 ± 24* | 61 -85 |
| CsA | 3/9*,† | 6 ± 14† | 3 -43 |
| Anti-CD40L + CsA | 17/17* | 62 ± 13* | 28 -78 |
B6 mice were irradiated with 200 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day 14. PBLs were typed for percentage of donor-host at 3.5 months after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is defined as average percentage donor cells of all mice in the group ± 1 SD. Range indicates lowest to highest level of percentage donor cells in engrafted mice. NA indicates not applicable.
P ≤ .017 compared with hamster IgG.
P ≤ .011 compared with anti-CD40L as a single agent.
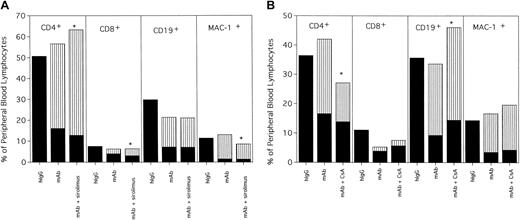
PBLs from hIgG-treated rejected control mice and anti-CD40L–treated and anti-CD40L plus sirolimus–treated engrafted mice were phenotyped for percentages of T cells, B cells, and myeloid cells (Figure1A). Mice receiving anti-CD40L mAb plus sirolimus had a modest but significantly higher proportion of donor CD4+ T cells compared with mice receiving only anti-CD40L mAb (51% versus 41%; P = .0007). There was also a higher proportion of donor CD8+ T cells in mice receiving both anti-CD40L mAb and sirolimus. Total donor PBL chimerism remained stable or increased in each engrafted mouse over the course of a year with no mortality, apparent morbidity, or weight loss associated with either treatment (data not shown).
Anti-CD40L mAb alone or in combination with (A) sirolimus or (B) CsA results in long-term multilineage engraftment.
Mice from Table 1 were phenotyped at 3.5 months after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells and MAC-1+ myeloid cells. On the y-axis are shown the host and donor proportions of each of the lineages. The solid part of the bar indicates the proportion of each lineage that is of host origin; the striped part of the bar indicates the proportion of each lineage that is of donor origin. Irrelevant hIgG-treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. * Indicates P < .05 compared with donor proportion in mice receiving anti-CD40L mAb as a single agent.
Anti-CD40L mAb alone or in combination with (A) sirolimus or (B) CsA results in long-term multilineage engraftment.
Mice from Table 1 were phenotyped at 3.5 months after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells and MAC-1+ myeloid cells. On the y-axis are shown the host and donor proportions of each of the lineages. The solid part of the bar indicates the proportion of each lineage that is of host origin; the striped part of the bar indicates the proportion of each lineage that is of donor origin. Irrelevant hIgG-treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. * Indicates P < .05 compared with donor proportion in mice receiving anti-CD40L mAb as a single agent.
Although there was not an effect on engraftment by the addition of sirolimus to anti-CD40L mAb, the baseline engraftment rate and level were relatively high in mice receiving anti-CD40L mAb as a single agent. To increase the likelihood of finding an effect by sirolimus on anti-CD40L-facilitated engraftment, the TBI dose was reduced. At 100 cGy TBI, 9 of 19 (47%) anti-CD40L–treated mice had evidence of chimerism with a group mean of 19% donor at 3.5 months after BMT (Table 2, experiment 1). Mice receiving sirolimus as a single agent had no evidence of donor engraftment even if the drug dose was escalated 3-fold to 4.5 mg/kg/dose (data not shown). In contrast, 20 of 20 (100%) mice receiving anti-CD40L mAb and sirolimus engrafted with a mean of 48% donor (P < .001 compared with anti-CD40L mAb alone). Interestingly, donor chimerism was higher in mice receiving anti-CD40L mAb and sirolimus than in mice receiving depleting anti-CD4 mAb and sirolimus (Table 2, experiment 1,P = .009). Sirolimus in conjunction with CD8 depletion did not result in donor chimerism.
Sirolimus and CsA facilitate donor engraftment in anti-CD40L–treated mice conditioned with 100 cGy TBI
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| Experiment 1 | |||
| Hamster IgG | 0/20 | 0 ± 0 | NA |
| Anti-CD40L | 9/19* | 16 ± 18* | 9 -46 |
| Sirolimus | 0/10† | 0 ± 0† | NA |
| Anti-CD40L + sirolimus | 20/20*,† | 48 ± 5*,† | 37 -59 |
| Sirolimus + anti-CD4 | 9/10*,† | 38 ± 14*,† | 35 -53 |
| Sirolimus + anti-CD8 | 0/10† | 0 ± 0† | NA |
| Experiment 2 | |||
| Hamster IgG | 0/20 | 0 ± 0 | NA |
| Anti-CD40L | 12/19* | 24 ± 20* | 30 -45 |
| CsA | 1/19† | 1 ± 3† | 15 |
| Anti-CD40L + CsA | 18/19*,† | 31 ± 11* | 10 -40 |
| CsA + anti-CD4 | 10/10*,† | 30 ± 11* | 3 -43 |
| CsA + anti-CD8 | 0/10† | 0 ± 0† | NA |
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| Experiment 1 | |||
| Hamster IgG | 0/20 | 0 ± 0 | NA |
| Anti-CD40L | 9/19* | 16 ± 18* | 9 -46 |
| Sirolimus | 0/10† | 0 ± 0† | NA |
| Anti-CD40L + sirolimus | 20/20*,† | 48 ± 5*,† | 37 -59 |
| Sirolimus + anti-CD4 | 9/10*,† | 38 ± 14*,† | 35 -53 |
| Sirolimus + anti-CD8 | 0/10† | 0 ± 0† | NA |
| Experiment 2 | |||
| Hamster IgG | 0/20 | 0 ± 0 | NA |
| Anti-CD40L | 12/19* | 24 ± 20* | 30 -45 |
| CsA | 1/19† | 1 ± 3† | 15 |
| Anti-CD40L + CsA | 18/19*,† | 31 ± 11* | 10 -40 |
| CsA + anti-CD4 | 10/10*,† | 30 ± 11* | 3 -43 |
| CsA + anti-CD8 | 0/10† | 0 ± 0† | NA |
B6 mice were irradiated with 100 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day 14. PBLs were typed for percentage of donor-host at 3.5 months after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is defined as average percentage of donor cells of all mice in the group ± 1 SD. Range indicates lowest to highest level of percentage donor cells in engrafted mice. NA indicates not applicable.
P < .001 compared with hamster IgG.
P ≤ .044 compared with anti-CD40L as a single agent.
Control mice and mice receiving anti-CD40L mAb or sirolimus or the combination were evaluated at 8.5 months after BMT for PBLs, as well as thymic and splenic cellularity and phenotype. There were no abnormalities suggestive of GVHD such as thymic aplasia or peripheral T or B lymphopenia, and no substantial phenotypic differences were noted between groups (n = 4/group, data not shown). The combination of sirolimus and anti-CD40L mAb promoted high levels of stable, long-term donor chimerism with no apparent treatment-associated morbidity in recipients receiving 100 cGy TBI.
CsA is additive with anti-CD40L mAb in increasing the proportion of chimeric nonmyeloablated recipients
To determine the effect of CsA on alloengraftment as a single agent, and in combination with anti-CD40L mAb, B6 mice were conditioned with 200 cGy TBI and administered a single dose (40 × 106) of BALB/c BM. Reagents were administered for 2 weeks after transplantation. Mice were evaluated for donor chimerism 6 weeks (data not shown) and 3.5 months after BMT. Anti-CD40L mAb resulted in a high level of engraftment in 9 of 10 mice (mean of 64%; Table 1, experiment 2). CsA did not abrogate engraftment in anti-CD40L mAb-treated mice. All 17 mice receiving anti-CD40L mAb plus CsA engrafted with a mean of 62% donor cells (Table 1, experiment 2). Phenotyping analysis of PBLs revealed that recipients of anti-CD40L mAb and CsA had a reduced percentage of donor CD4+ T cells as compared with recipients of anti-CD40L mAb alone (Figure 1B; 14% versus 26%, P = .028). All other lineages were proportionately similar between these 2 groups. CsA as a single agent resulted in engraftment in 3 of 9 mice. However, all 3 mice had higher engraftment levels when evaluated for donor chimerism 2 months earlier, and an additional mouse lost donor chimerism entirely (Table 1, experiment 2 and data not shown), indicating that CsA alone did not result in stable, long-term chimerism. Although donor chimerism was stable and long term (> 8 months) in mice receiving both anti-CD40L mAb and CsA, the proportion of T cells that were of donor origin was significantly lower than in mice receiving only anti-CD40L mAb (53% versus 82%, P = .036, data not shown). Although CsA does not abrogate engraftment promotion achieved by anti-CD40L mAb, it may lower donor T-cell representation.
To increase the likelihood of finding an effect on anti-CD40L–facilitated engraftment, CsA was evaluated in mice conditioned with only 100 cGy TBI (Table 2, experiment 2). The combination of CsA and anti-CD40L mAb resulted in a substantially increased incidence of donor chimerism compared with anti-CD40L mAb as a single agent. Twelve of 19 (63%) anti-CD40L–treated mice versus 18 of 19 (95%) anti-CD40L mAb plus CsA-treated mice engrafted (P = .017; Table 2, experiment 2). Although donor chimerism was present in 4 of 20 (25%) CsA-treated mice at 6 weeks after transplantation, 3 of the 4 mice lost donor chimerism and the fourth mouse had a declining chimerism level at 3.5 months after BMT (28% at 6 weeks to 15% at 3.5 months). Although one anti-CD40L mAb plus CsA-treated mouse lost donor chimerism between 6 weeks and 3.5 months, engraftment was stable and durable in the remaining 18 mice for 1 year (data not shown). Similar to sirolimus, CsA in conjunction with CD4+, but not CD8+ T-cell depletion, resulted in engraftment of all mice (Table 2, experiment 2).
Unlike sirolimus, CsA-treated mice transiently lost approximately 10% of their body weight during the 2-week course of CsA administration. At approximately 3 months after transplantation, anti-CD40L mAb and CsA-treated mice again lost weight and about 30% to 50% died 3 to 6 months after BMT with signs consistent with CsA-induced GVHD (weight loss, erythema, and alopecia, data not shown).26 Because some CsA-treated mice with no donor chimerism experienced the same signs, we conclude that autologous GVHD occurred likely because of calcineurin inhibitor–mediated thymic injury with a subsequent failure to control autoaggression.26
Because these data indicated that sirolimus or CsA could substantially increase anti-CD40L–facilitated alloengraftment, these agents were evaluated in nonirradiated recipients. We have reported previously that anti-CD40L mAb does not result in substantial macrochimerism in the absence of TBI in mice receiving a relatively modest allogeneic BM cell dose (40 × 106). Other studies have found that a much higher BM dose can facilitate engraftment under nonmyeloablative conditions.6 27-29 Consistent with these studies, increasing the BM dosage to 80 × 106 cells or administering 2 doses of 40 × 106 cells 2 weeks apart resulted in low-level donor chimerism (3%-15%) in most mice receiving anti-CD40L mAb alone or in combination with either sirolimus or CsA (Table 3). Neither sirolimus nor CsA alone or in combination resulted in significant engraftment (Table 3). Although long-term donor chimerism was achieved in a substantial proportion of anti-CD40L mAb-treated mice (alone or in combination with sirolimus or CsA) in the absence of TBI, donor T-cell engraftment was very low (≤ 1%) in all mice (data not shown). The low donor T-cell reconstitution and lack of donor tolerance as measured by prompt donor skin graft rejection (data not shown) suggest that these low levels of donor chimerism achieved in the absence of TBI may be of limited therapeutic value for most patients.
Anti-CD40L mAb alone or in combination with sirolimus or CsA results in low levels of donor engraftment in nonirradiated mice
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| BM 80 (day 0)3-150 | |||
| Hamster IgG | 1/15 | 0 ± 0 | 3 |
| Anti-CD40L | 7/103-151 | 4 ± 33-151 | 4 -8 |
| Anti-CD40L + sirolimus | 20/203-151,3-152 | 8 ± 23-151,3-152 | 6 -15 |
| Anti-CD40L + CsA | 9/103-151 | 7 ± 33-151 | 3 -11 |
| Sirolimus + CsA | 3/9 | 2 ± 3 | 3 -8 |
| BM 40 (day 0, 14) | |||
| Anti-CD40L | 6/10 | 5 ± 4 | 6 -13 |
| Sirolimus | 0/103-152 | 0 ± 03-152 | NA |
| CsA | 0/103-152 | 0 ± 03-152 | NA |
| Anti-CD40L + sirolimus | 10/103-152 | 10 ± 23-152 | 8 -14 |
| Anti-CD40L + CsA | 10/103-152 | 8 ± 4 | 5 -16 |
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| BM 80 (day 0)3-150 | |||
| Hamster IgG | 1/15 | 0 ± 0 | 3 |
| Anti-CD40L | 7/103-151 | 4 ± 33-151 | 4 -8 |
| Anti-CD40L + sirolimus | 20/203-151,3-152 | 8 ± 23-151,3-152 | 6 -15 |
| Anti-CD40L + CsA | 9/103-151 | 7 ± 33-151 | 3 -11 |
| Sirolimus + CsA | 3/9 | 2 ± 3 | 3 -8 |
| BM 40 (day 0, 14) | |||
| Anti-CD40L | 6/10 | 5 ± 4 | 6 -13 |
| Sirolimus | 0/103-152 | 0 ± 03-152 | NA |
| CsA | 0/103-152 | 0 ± 03-152 | NA |
| Anti-CD40L + sirolimus | 10/103-152 | 10 ± 23-152 | 8 -14 |
| Anti-CD40L + CsA | 10/103-152 | 8 ± 4 | 5 -16 |
Nonirradiated B6 mice were infused with 80 × 106 BALB/c BM on day 0 or 40 × 106 BALB/c BM on days 0 and 14 as indicated. Reagents were administered from day − 1 through day 14. PBLs were typed for percentage donor-host at 3.5 months after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is defined as average percentage of donor cells of all mice in the group ± 1 SD. Range indicates lowest to highest level of percentage donor cells in engrafted mice. NA indicates not applicable.
Refers to BM cell dose (× 106) and day(s) of BM administration.
P < .001 compared with hamster IgG for BM administered only on day 0.
P ≤ .004 compared with anti-CD40L as a single agent.
Tacrolimus promotes alloengraftment as a single agent but impairs donor T-cell reconstitution
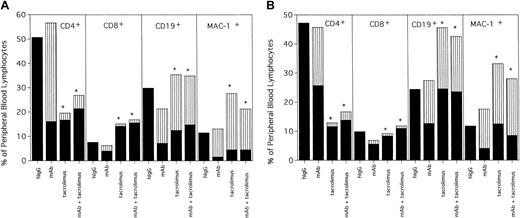
Although structurally related to sirolimus, tacrolimus disrupts calcium-dependent signaling pathways, thereby blocking signal 1 similar to CsA. Unlike either sirolimus or CsA, tacrolimus as a single agent facilitated engraftment in all recipients conditioned with 200 or 100 cGy TBI (Table 4). However, in contrast to sirolimus or CsA, tacrolimus modestly decreased donor chimerism in anti-CD40L mAb-treated mice (Table 4). Multilineage phenotyping of PBLs at 3.5 months after transplantation revealed that tacrolimus administration, either as a single agent or in combination with anti-CD40L mAb, resulted in a dramatic inhibition of donor T-cell reconstitution (Figure 2A,B). Sixty-eight percent of peripheral blood (PB) T cells were of donor origin in anti-CD40L–treated mice conditioned with 200 cGy TBI. In contrast, only 11% or 16% of PB T cells were of donor origin in mice receiving tacrolimus as a single agent or in combination with anti-CD40L mAb, respectively (Figure 2A). The reduction in anti-CD40L mAb-facilitated engraftment by tacrolimus may have been due to the fact that donor T-cell reconstitution was severely compromised in mice receiving tacrolimus. Overall, the proportion of T cells, particularly CD4+ T cells, of either donor or host origin in the PB of mice receiving tacrolimus alone or with anti-CD40L mAb was substantially lower than in mice not receiving tacrolimus. Similar effects were observed in tacrolimus-treated mice conditioned with only 100 cGy TBI (Figure 2B). PB phenotyping at 7 months after transplantation indicated that, although donor chimerism was stable in tacrolimus-treated mice, donor T-cell reconstitution remained very poor (data not shown), suggesting a more prolonged thymic defect than seen in CsA-treated mice. However, it may be difficult to fully extrapolate the comparative differences between CsA and tacrolimus on murine T-cell recovery to humans.
Tacrolimus as a single agent facilitates donor engraftment in mice conditioned with 200 or 100 cGy TBI
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| TBI 200 cGy | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/104-150 | 69 ± 54-150 | 64 -76 |
| Tacrolimus | 5/54-150 | 50 ± 44-150,4-151 | 46 -56 |
| Anti-CD40L + tacrolimus | 9/94-150 | 44 ± 64-150,4-151 | 39 -55 |
| TBI 100 cGy | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/104-150 | 50 ± 64-150 | 40 -56 |
| Tacrolimus | 10/104-150 | 44 ± 74-150 | 33 -53 |
| Anti-CD40L + tacrolimus | 9/94-150 | 42 ± 74-150,4-151 | 28 -50 |
| Reagent . | No. chimeric . | % donor . | Range . |
|---|---|---|---|
| TBI 200 cGy | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/104-150 | 69 ± 54-150 | 64 -76 |
| Tacrolimus | 5/54-150 | 50 ± 44-150,4-151 | 46 -56 |
| Anti-CD40L + tacrolimus | 9/94-150 | 44 ± 64-150,4-151 | 39 -55 |
| TBI 100 cGy | |||
| Hamster IgG | 0/10 | 0 ± 0 | NA |
| Anti-CD40L | 10/104-150 | 50 ± 64-150 | 40 -56 |
| Tacrolimus | 10/104-150 | 44 ± 74-150 | 33 -53 |
| Anti-CD40L + tacrolimus | 9/94-150 | 42 ± 74-150,4-151 | 28 -50 |
B6 mice were irradiated with 200 or 100 cGy TBI as indicated on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day 14. PBLs were typed for percentage of donor-host at 3.5 months after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is defined as average percentage donor cells of all mice in the group ± 1 SD. Range indicates lowest to highest level of percentage donor cells in engrafted mice. NA indicates not applicable.
P < .001 compared with hamster IgG.
P ≤ .02 compared with anti-CD40L as a single agent.
Tacrolimus results in long-term multilineage engraftment at the expense of donor T-cell reconstitution.
Mice conditioned with (A) 200 cGy or (B) 100 cGy from Table 4 were phenotyped at 3.5 months after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells and MAC-1+ myeloid cells. On the y-axis are shown the host and donor proportions of each of the lineages. The solid part of the bar indicates the proportion of each lineage that is of host origin; the striped part of the bar indicates the proportion of each lineage that is of donor origin. Irrelevant hIgG-treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. * Indicates P < .05 compared with donor proportion in mice receiving anti-CD40L mAb as a single agent.
Tacrolimus results in long-term multilineage engraftment at the expense of donor T-cell reconstitution.
Mice conditioned with (A) 200 cGy or (B) 100 cGy from Table 4 were phenotyped at 3.5 months after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells and MAC-1+ myeloid cells. On the y-axis are shown the host and donor proportions of each of the lineages. The solid part of the bar indicates the proportion of each lineage that is of host origin; the striped part of the bar indicates the proportion of each lineage that is of donor origin. Irrelevant hIgG-treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. * Indicates P < .05 compared with donor proportion in mice receiving anti-CD40L mAb as a single agent.
Sirolimus, CsA, and tacrolimus have differential effects on central and peripheral host T-cell numbers early after transplantation
To further investigate the differential effects of sirolimus, CsA, and tacrolimus on anti-CD40L–facilitated alloengraftment, thymic and splenic cellularity and phenotypes were evaluated 7 days after transplantation in mice conditioned with 200 cGy TBI (Tables5 and 6). Previous studies have determined that thymic and splenic cellularity reach their nadir,7 and the rejection process is complete by 7 to 10 days after sublethal TBI and BMT.30 Although anti-CD40L mAb-treated mice had high numbers of thymocytes, the coadministration of sirolimus, CsA, or tacrolimus resulted in a significant reduction in total thymocyte number as compared with anti-CD40L mAb alone (Table 5). CsA resulted in substantially greater reductions in mature CD4 and CD8 single-positive (SP) and intermediate double-positive (DP) thymic populations compared with either anti-CD40L mAb alone or combined with sirolimus. Mice receiving anti-CD40L mAb as a single agent had 5.9-fold more total thymocytes, 8.8-fold more CD4 SP cells, 4-fold more CD8 SP cells, and 6.5-fold more DP cells than mice receiving anti-CD40L mAb plus CsA (Table 5). The coadministration of tacrolimus and anti-CD40L mAb resulted in less thymic reduction than CsA but more than sirolimus.
CsA results in a more profound reduction in thymocyte numbers in anti-CD40L mAb-treated mice more than sirolimus or tacrolimus
| Group . | Total no. . | CD4 SP . | CD8 SP . | DP . | DN . |
|---|---|---|---|---|---|
| Non-BMT controls | 85.3 ± 16.2 | 4.6 ± 0.6 | 1.1 ± 0.2 | 71.3 ± 15.2 | 8.5 ± 2.1 |
| Hamster IgG | 55.8 ± 27.9 | 2.0 ± 1.2 | 0.6 ± 0.3 | 48.1 ± 23.1 | 5.2 ± 3.6 |
| Anti-CD40L | 91.5 ± 19.2 | 3.5 ± 0.8 | 0.8 ± 0.4 | 73.6 ± 12.5 | 13.7 ± 7.3 |
| Anti-CD40L + sirolimus | 35.7 ± 6.35-151 | 1.3 ± 0.55-151 | 0.5 ± 0.2 | 27.6 ± 4.65-151 | 6.3 ± 1.5 |
| Anti-CD40L + CsA | 15.4 ± 4.15-150,5-151 | 0.4 ± 0.15-151 | 0.2 ± 0.25-151 | 11.3 ± 3.15-150,5-151 | 3.4 ± 1.2 |
| Anti-CD40L + tacrolimus | 25.4 ± 2.45-151 | 0.6 ± 0.15-151 | 0.3 ± 0.15-151 | 17.8 ± 6.75-151 | 6.7 ± 4.8 |
| Group . | Total no. . | CD4 SP . | CD8 SP . | DP . | DN . |
|---|---|---|---|---|---|
| Non-BMT controls | 85.3 ± 16.2 | 4.6 ± 0.6 | 1.1 ± 0.2 | 71.3 ± 15.2 | 8.5 ± 2.1 |
| Hamster IgG | 55.8 ± 27.9 | 2.0 ± 1.2 | 0.6 ± 0.3 | 48.1 ± 23.1 | 5.2 ± 3.6 |
| Anti-CD40L | 91.5 ± 19.2 | 3.5 ± 0.8 | 0.8 ± 0.4 | 73.6 ± 12.5 | 13.7 ± 7.3 |
| Anti-CD40L + sirolimus | 35.7 ± 6.35-151 | 1.3 ± 0.55-151 | 0.5 ± 0.2 | 27.6 ± 4.65-151 | 6.3 ± 1.5 |
| Anti-CD40L + CsA | 15.4 ± 4.15-150,5-151 | 0.4 ± 0.15-151 | 0.2 ± 0.25-151 | 11.3 ± 3.15-150,5-151 | 3.4 ± 1.2 |
| Anti-CD40L + tacrolimus | 25.4 ± 2.45-151 | 0.6 ± 0.15-151 | 0.3 ± 0.15-151 | 17.8 ± 6.75-151 | 6.7 ± 4.8 |
B6 mice were irradiated with 200 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day + 6. Thymus counts and phenotype were evaluated on day 7 after BMT. Shown are absolute numbers (× 106). n = 4/group. SP indicates single positive; DP, double positive; DN, double negative.
P ≤ .05 compared with hamster IgG.
P ≤ .05 compared with anti-CD40L mAb as a single agent.
Sirolimus, but neither CsA nor tacrolimus, reduces peripheral host CD4+ and CD8+ T-cell numbers in anti-CD40L mAb-treated mice
| Group . | Total no. . | Total donor . | Host CD4 . | Host CD8 . | Host CD19 . |
|---|---|---|---|---|---|
| Non-BMT controls | 111.3 ± 10.9 | 0 ± 0 | 30.5 ± 2.1 | 20.6 ± 2.5 | 58.3 ± 6.3 |
| Hamster IgG | 37.2 ± 5.5 | 0 ± 0 | 10.5 ± 1.9 | 9.3 ± 1.5 | 7.7 ± 1.2 |
| Anti-CD40L | 34.4 ± 6.7 | 10.4 ± 8.66-150 | 6.8 ± 1.76-150 | 3.8 ± 1.16-150 | 5.8 ± 1.1 |
| Anti-CD40L + sirolimus | 28.1 ± 8.0 | 16.1 ± 5.06-150 | 3.0 ± 1.06-150,6-151 | 1.6 ± 0.56-150,6-151 | 3.9 ± 1.86-150 |
| Anti-CD40L + CsA | 58.3 ± 10.56-150,6-151 | 30.5 ± 7.36-150,6-151 | 9.8 ± 2.0 | 4.6 ± 1.06-150 | 6.9 ± 1.9 |
| Anti-CD40L + tacrolimus | 47.9 ± 7.56-151 | 26.3 ± 0.46-150 | 7.8 ± 0.8 | 4.5 ± 0.26-150 | 5.3 ± 0.26-150 |
| Group . | Total no. . | Total donor . | Host CD4 . | Host CD8 . | Host CD19 . |
|---|---|---|---|---|---|
| Non-BMT controls | 111.3 ± 10.9 | 0 ± 0 | 30.5 ± 2.1 | 20.6 ± 2.5 | 58.3 ± 6.3 |
| Hamster IgG | 37.2 ± 5.5 | 0 ± 0 | 10.5 ± 1.9 | 9.3 ± 1.5 | 7.7 ± 1.2 |
| Anti-CD40L | 34.4 ± 6.7 | 10.4 ± 8.66-150 | 6.8 ± 1.76-150 | 3.8 ± 1.16-150 | 5.8 ± 1.1 |
| Anti-CD40L + sirolimus | 28.1 ± 8.0 | 16.1 ± 5.06-150 | 3.0 ± 1.06-150,6-151 | 1.6 ± 0.56-150,6-151 | 3.9 ± 1.86-150 |
| Anti-CD40L + CsA | 58.3 ± 10.56-150,6-151 | 30.5 ± 7.36-150,6-151 | 9.8 ± 2.0 | 4.6 ± 1.06-150 | 6.9 ± 1.9 |
| Anti-CD40L + tacrolimus | 47.9 ± 7.56-151 | 26.3 ± 0.46-150 | 7.8 ± 0.8 | 4.5 ± 0.26-150 | 5.3 ± 0.26-150 |
B6 mice were irradiated with 200 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day + 6. Spleen counts and phenotype were evaluated on day 7 after BMT. Shown are absolute numbers (× 106). n = 4/group.
P ≤ .05 compared with hamster IgG.
P ≤ .05 compared with anti-CD40L mAb as a single agent.
Quantification of splenic T-cell numbers 7 days after BMT revealed that the coadministration of sirolimus, but neither CsA nor tacrolimus, resulted in a significant decrease (2-fold) in both host CD4+ and CD8+ T-cell numbers in anti-CD40L–treated mice (Table 6). Despite having no effects on host T-cell number, CsA-treated mice had greater numbers of donor MHC class I+ cells compared with mice receiving only anti-CD40L mAb, suggesting that the ability of CsA to facilitate engraftment was not due to effects on host T-cell depletion (Table 6). These data indicated that CsA and tacrolimus administration resulted in thymic but not peripheral host T-cell depletion. In contrast, sirolimus administration resulted in both central and peripheral reductions in host T-cell numbers.
Anti-CD40L mAb in combination with sirolimus results in a more profound degree of in vivo donor tolerance than anti-CD40L mAb alone or in combination with CsA or tacrolimus
Despite a differential targeting of central versus peripheral T-cell depletion by the immunosuppressive agents, the combinations of anti-CD40L mAb with sirolimus, CsA, or tacrolimus resulted in approximately equivalent high levels of donor alloengraftment, albeit with differing levels of donor T-cell reconstitution. To investigate potential differences in donor tolerance conferred by these immunosuppressive reagents, donor skin grafts were placed early (1 month) or later (3 months) after BMT (Table7). Twelve of 27 (44%) anti-CD40L–treated mice accepted donor grafts if skin grafting was delayed for 3 months, although none of 3 mice accepted donor grafts placed at 1 month after BMT (Table 7). The combination of anti-CD40L mAb and CsA resulted in similar donor skin graft acceptance compared with anti-CD40L mAb alone when grafts were placed at 3 months (7 of 18 [39%] versus 12 of 27 [44%], P = .27). Similar to anti-CD40L mAb monotherapy, only a low proportion (1 of 8 [12%]) of anti-CD40L mAb and CsA-treated mice accepted donor grafts placed 1 month after BMT. In contrast, all 6 anti-CD40L mAb plus sirolimus-treated mice accepted donor skin grafts placed as early as 1 month, and all 5 accepted donor grafts placed 3 months after BMT. Few tacrolimus-treated mice survived long term for evaluation of long-term donor graft survival, but tacrolimus did not promote early skin graft acceptance in any of the 4 skin-grafted recipients. As expected, none of the hIgG-treated control mice accepted donor skin grafts placed early (0 of 6) or later (0 of 20) after BMT. Additionally, all mice in all groups promptly rejected third-party B10.BR skin grafts by 3 weeks after grafting (data not shown). Collectively, these data indicate anti-CD40L mAb combined with sirolimus results in an earlier and more profound donor tolerance than when combined with either CsA or tacrolimus or administered as a single agent.
The combination of anti-CD40L mAb and sirolimus promotes early donor skin graft tolerance
| Treatment . | Donor graft acceptance rate when skin grafted at . | |
|---|---|---|
| 1 mo . | 3 mo . | |
| Hamster IgG | 0/6 | 0/20 |
| Anti-CD40L | 0/3 | 15/277-150 |
| Anti-CD40L + sirolimus | 6/67-150,7-151 | 5/57-150 |
| Anti-CD40L + CsA | 1/8 | 7/187-150 |
| Anti-CD40L + tacrolimus | 0/4 | 1/27-152 |
| Treatment . | Donor graft acceptance rate when skin grafted at . | |
|---|---|---|
| 1 mo . | 3 mo . | |
| Hamster IgG | 0/6 | 0/20 |
| Anti-CD40L | 0/3 | 15/277-150 |
| Anti-CD40L + sirolimus | 6/67-150,7-151 | 5/57-150 |
| Anti-CD40L + CsA | 1/8 | 7/187-150 |
| Anti-CD40L + tacrolimus | 0/4 | 1/27-152 |
B6 mice were irradiated with 200 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Reagents were administered from day − 1 through day 14. Peripheral blood leukocytes of all mice were typed for percentage of donor engraftment prior to skin grafting. No hamster IgG–treated mice had any evidence of donor engraftment. All other mice had similar engraftment levels (approximately 50%-60% donor) at the time of skin grafting. Skin grafts were placed at either 1 month or at greater than 3 months after transplantation. Shown is the number of mice accepting donor skin grafts for greater than 6 months. There were no differences between groups in time to rejection in those mice that did not accept grafts. All mice promptly rejected third-party B10.BR skin grafts by 3 weeks after grafting (data not shown).
P < .001 compared with hamster IgG.
P = .003 compared with anti-CD40L as a single agent.
In contrast to other groups, tacrolimus-treated mice had poor long-term survival, greatly limiting the numbers of healthy mice available for late skin grafting. Anti-CD40L versus anti-CD40L + sirolimus at 3 months; P = .059.
Discussion
These studies are the first to compare the effects of CsA, sirolimus, and tacrolimus administered after BMT, as single agents and in combination with anti-CD40L mAb, on alloengraftment using a nonmyeloablative-conditioning regimen. Unlike anti-CD40L mAb, neither CsA nor sirolimus promoted engraftment as single agents in a nonmyeloablative-conditioning regime. However, either sirolimus or CsA increased donor engraftment in anti-CD40L mAb-treated mice under conditions of limited baseline engraftment. Although tacrolimus resulted in donor chimerism as a single agent, it modestly decreased anti-CD40L mAb-mediated engraftment and resulted in very poor donor T-cell reconstitution. The combination of sirolimus and anti-CD40L mAb induced an early and profound degree of donor tolerance.
Sirolimus was predicted to be a useful adjunct to costimulatory blockade for the promotion of alloengraftment. However, the use of calcineurin inhibitors in conjunction with costimulatory blockade strategies has been controversial. One study found that sirolimus induced anergy in a T-cell clone, and CsA inhibited sirolimus-induced anergy.22 Consistent with our studies in a nonmyeloablative, allogeneic BMT model, Li et al16 found that sirolimus and costimulatory blockade were synergistic for skin allograft acceptance. In contrast to our data in BM transplant recipients, the addition of CsA to costimulatory blockade resulted in the abrogation of donor tolerance in both cardiac and skin murine allograft models in non-BM transplant recipients.16,20 The mechanism responsible for the antagonistic effects of CsA on costimulatory blockade appeared to be the prevention of apoptosis of alloreactive T cells.16,20 Moreover, Smiley et al18 found that CsA, but not sirolimus, inhibited activation-induced CD40L expressed on CD4+ T cells and abrogated anti-CD40L mAb-induced cardiac allograft survival. However, a recent study indicates that IL-2, IL-4, IL-7, and IL-15 can induce CD40L expression on human CD4+ T cells via the common receptor γ chain in a TCR-independent fashion.19Importantly, CD40L induction via this pathway was CsA resistant.19 These data suggest that the CD40/CD40L pathway might still be a potential therapeutic target in CsA-treated individuals under conditions in which these cytokines were available to circumvent CsA inhibition of CD40L induction.
Other data indicate that CsA, as a single agent, can induce immunologic tolerance to a solid organ allograft31-33 and be combined successfully with costimulatory blockade. One study determined that CsA administration did not adversely affect renal allograft survival in primates receiving anti-B7 mAbs and may even have resulted in less severe histologic signs of rejection.21 Another study found that CsA did not abrogate renal allograft survival facilitated by CTLA4 immunoglobulin-mediated CD28/B7 blockade.23 Our data indicate that CsA did not abrogate donor BM engraftment mediated by anti-CD40L mAb and, under some conditions, increased the proportion of recipients that engrafted (Table 2, experiment 2). We hypothesize that CsA reduced the intensity of TCR signaling or clonally reduced the alloreactive T-cell population that may allow for a more readily attainable costimulatory blockade in a greater proportion of recipients. Complete block of signal 1, which might have been expected to preclude engraftment mediated by costimulatory blockade, may be difficult to achieve in BMT in which the antigens that trigger a TCR response are very widely distributed. Because the induction of tissue injury resulting in the release of proinflammatory cytokines can occur in conditioned mice, the strength of TCR signals resulting from alloantigen recognition may need to be dampened by immunosuppressive agents such as CsA or tacrolimus to permit costimulatory pathway blockade to be effective. Additionally, CD40L expression induced by cytokines in a proinflammatory environment would be CsA resistant19 and potentially susceptible to blockade by anti-CD40L mAb.
As well as being a general immunosuppressive agent, CsA also has been used successfully before transplantation to create “thymic space” to facilitate alloengraftment with minimal conditioning.34Our studies indicate that CsA administration beginning the day prior to transplantation resulted in significant thymic, but not peripheral, T-cell reduction by day 7 after BMT that may have contributed to the increase in the rate of engraftment mediated by CsA in anti-CD40L mAb-treated mice (Table 5).
The most profound degree of early donor tolerance, as measured by donor skin graft acceptance, was in mice treated with the combination of anti-CD40L and sirolimus. The rejection of donor skin grafts in anti-CD40L mAb-treated mice not receiving sirolimus may be due to the expansion of alloreactive host T cells that escape costimulatory blockade and are primed by the donor graft. Additionally, sirolimus, unlike CsA, does not interfere with the apoptosis of alloreactive T cells that become primed by the donor skin allograft.16
Studies by Umemura et al35 also found a dissociation of BM chimerism and allograft tolerance and indicated that donor T-cell engraftment was required for allograft tolerance but not for durable donor BM chimerism. Consistent with this idea, one possible explanation for the higher rate of early donor skin graft acceptance in mice receiving anti-CD40L mAb and sirolimus may be that this group of mice had the highest proportion of donor T-cell representation. Despite having similar overall chimerism levels (37%-43%) at 8 months after BMT, 28% of PBL T cells were of donor origin in mice receiving anti-CD40L mAb and sirolimus versus 18% in mice receiving only anti-CD40L mAb versus 7% in mice receiving anti-CD40L mAb and CsA (data not shown). Superior donor T-cell reconstitution in mice receiving anti-CD40L mAb and sirolimus might contribute to early donor skin graft acceptance. The reduction in host splenic T-cell numbers by sirolimus, but not CsA or tacrolimus, early after transplantation also may have aided engraftment promotion and early donor tolerance in mice receiving the combination of anti-CD40L mAb and sirolimus.
Despite rejection of donor skin grafts by some anti-CD40L mAb-treated mice not receiving sirolimus, donor BM chimerism remained stable with no apparent perturbations in phenotype or signs of morbidity in those mice even when evaluated several months after the rejection episode (data not shown). Although donor skin graft acceptance with third-party graft rejection is a stringent test for donor tolerance, our data suggest that donor skin graft rejection does not necessarily portend an inevitable loss of donor chimerism. Donor skin graft rejection might indicate only a lack of tolerance to specific donor-type skin antigens or suggest that tolerance is not yet complete. The finding of lower donor skin graft acceptance in mice receiving CsA, rather than sirolimus, in combination with anti-CD40L mAb, might not preclude the use of CsA with costimulatory blockade strategies in BMT patients, although sirolimus may be the preferred pharmacologic agent for this purpose.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-03-0872.
Supported by National Institutes of Health Grants RO1 AI 3449, RO1 HL 63452, R37 HL56067, and PO1 AI-35225 (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce Blazar, University of Minnesota, MMC 109, 420 Delaware St SE, Minneapolis, MN 55455; e-mail:blaza001@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal