Abstract
The effect of 2-methoxyestradiol, 2ME2, an endogenous metabolite of 17β-estradiol (E2), on cell growth and cytoskeletal functions in a BCR-ABL—transformed cell line model was investigated. We determined the interaction of 2ME2 with STI571 (Gleevec, imatinib mesylate) in STI571 drug-sensitive and -resistant cell lines. In cells expressing BCR-ABL, STI571 cooperated with 2ME2 in reducing cell growth, and STI571-resistant cells were sensitive to 2ME2 treatment. 2ME2 also inhibited growth of several cancer cell lines by a mechanism independent of BCR-ABL. BCR-ABL transformation leads to altered motility, increased adhesion, and spontaneous migration in different in vitro model systems. 2ME2 was found to specifically inhibit the spontaneous motility of BCRABL—transformed Ba/F3 cells and to change the morphology and volume of treated cells. Cells attached to fibronectin-coated surfaces showed a reduced number of filipodia and lamellipodia. In addition, 2ME2 significantly reduced BCRABL—mediated adhesion to fibronectin. The spontaneous migration of BCR-ABL—transformed cells through a transwell membrane also was found to be significantly decreased by 2ME2. Cytoskeletal changes were accompanied by alteration of tubulin formation, distinct from paclitaxel treatment. These results demonstrate that 2ME2 treatment of transformed cells strongly reduces cytoskeletal functions and may also be useful for the treatment of cancers with high metastatic potential. Combination of 2ME2 with other anticancer drugs may be beneficial to treatment of drug-resistant cancers. (Blood. 2003;102:289-296)
Introduction
Transformation of hematopoietic stem cells by oncogenic tyrosine kinases includes activation of signaling pathways normally activated by hematopoietic growth factors (HGFs) and usually leads to clonal expansion of hematopoietic progenitor cells. Many oncogenic tyrosine kinases share common downstream signaling proteins such as SHP-2, STATs, PI3K, and others.1 Such common pathways are likely to lead to common biologic events, including the regulation of proliferation, viability, or adhesion. Several oncogenic tyrosine kinases in hematopoietic cells have been extensively studied, including BCR-ABL, TEL-ABL, TEL-ARG, TEL-JAK2, and TEL-PDGFβR. BCR-ABL is the transforming protein in chronic myelogenous leukemia (CML), a clonal myeloproliferative disorder caused by the t(9;22) Philadelphia chromosome translocation, which fuses BCR to the c-ABL tyrosine kinase. The ABL tyrosine kinase in the BCR-ABL fusion protein is constitutively activated, and this activity may increase over time as the disease progresses from stable phase to blast crisis.2 The TEL (ETV6) gene has been found to be fused to several tyrosine kinases, including ABL,3-6 ARG (ABL-related gene, ABL2),7,8 JAK2,9,10 PDGFβR,11 and TRKC12 in hematopoietic disorders. We have previously shown that intracellular levels of reactive oxygen species are regulated by some of these tyrosine kinases.13
Reactive oxygen species (ROS) are formed in cells transformed by oncogenic tyrosine kinases and in response to a variety of stimuli, including UV irradiation14 or binding of cytokines such as transforming growth factor-β (TGF-β),15 epidermal growth factor (EGF),16 platelet-derived growth factor (PDGF),17 or steel factor.18 ROS include intermediates that are generated by reduction of molecular oxygen through stepwise electron transfer starting with the superoxide radical (O2•-), to hydrogen peroxide (H2O2), and the hydroxyl radical (•OH), which can be reduced to water. There are additional ROS, such as nitric oxide (NO•) and peroxynitrate (ONOO•) that can be produced within the cell and display a variety of biologic activities.19,20
Increased levels of ROS in transformed cells have recently been used to induce apoptosis by inhibiting the ROS-reducing enzyme superoxide dismutase. Huang et al demonstrated that inhibition of superoxide dismutase by 2ME2 specifically inhibits growth of leukemic (especially CML) cells.21 The mechanism is likely to involve damage to mitochondria and induction of apoptosis through elevated levels of O2•-. 2ME2 did not inhibit growth of normal hematopoietic cells, suggesting that the regulation of superoxide dismutase activity may be an important mechanism in transformation and a potential target for drug treatment. However, it is possible that 2ME2 uses additional mechanisms of action.
2ME2 is a natural 17β-estradiol (E2) metabolite generated by hydroxylation through cytochrome P450 and methylation through catechol-O-methyltransferase.22,23 Despite the structural similarity with 17β-estradiol, 2ME2 has a low binding affinity to the estrogen receptor.24 The plasma levels of 2ME2 in women are high compared with men and can be even more elevated during pregnancy.25 Currently, 2ME2 (Panzem) has been introduced into clinical trials by Entremed (Rockville, MD) for a variety of cancers. 2ME2 induces apoptosis in transformed but not normal resting cells characterized by caspase activation and DNA fragmentation as well as G2/M cell cycle arrest.21,26,27 It has also been suggested that 2ME2 regulates apoptosis through elevated expression of the death receptor 5 protein and therefore likely increases the sensitivity to its ligand TRAIL (tumor necrosis factor—related apoptosis-inducing ligand).28 Even though 2ME2-dependent wild-type p53 induction enhances apoptosis, p53 may not be necessary for the biologic effects of this drug.21,29,30 2ME2 also has been suggested to inhibit angiogenesis by regulating capillary tube formation in vitro.31,32
The goal of this study was to use in vitro models to determine the biologic effects of 2ME2 on cytoskeletal functions in cells transformed by BCR-ABL or other tyrosine kinase oncogenes. Since 2ME2 acts by increasing intracellular superoxide levels, we initially sought to determine the interaction of 2ME2 with the tyrosine kinase inhibitor STI571, which lowers ROS levels in cells transformed by BCR-ABL.13 Despite the apparent different effects of 2ME2 and STI571 on ROS, in combination the drugs weakly cooperate and enhance inhibition of cell growth in a BCR-ABL—transformed cell line. Additionally, 2ME2 effectively inhibits growth of an STI571-resistant cell line. We found that 2ME2 treatment of hematopoietic cell lines transformed by BCR-ABL results in inhibition of several biologic functions that are normally tightly regulated through integrins and the cytoskeletal molecules, including spontaneous cell motility, adhesion to fibronectin, and transwell migration. 2ME2 reduces adhesion and transwell migration in Ba/F3 cells transformed by TEL-ABL, TEL-JAK2, or TEL-PDGFβR. There is also inhibition of cell growth in a wide variety of cancer cell lines, including leukemia, lymphoma, lung cancer, breast cancer, and melanoma cell lines. Disregulation of adhesion, migration, and motility through altered superoxide levels may contribute to abnormal signaling events in cancer cells.
Materials and methods
Cells
The murine pre—B-cell line Ba/F3 was grown in RPMI 1640 containing 10% fetal calf serum and 10% WEHI-3B—conditioned medium as a source of murine interleukin-3. Ba/F3 cell lines transfected with a BCR-ABL, TEL-ABL, TEL-JAK2, or TEL-PDGFβR cDNA were grown in the absence of growth factors. An STI571 (Gleevec, imatinib mesylate)—resistant cell line was generated by treatment of BCR-ABL—transfected Ba/F3 cells with a single dose of STI571 (1 μM, Gleevec; Novartis Pharmaceuticals, Basel, Switzerland), and subsequent outgrowth of the cell population was then treated for an additional 2 weeks with STI571 (1 μM). The CML (BV173, KU812), small-cell lung cancer (H661, Calu, H209, and H69), breast cancer (MCF-7 and MDA), melanoma (G361, U008AT, and K019AX), leukemia, and lymphoma cell lines (DHL4, DHL6, DHL7, DHL10, Namalwa, Blin-1, Jurkat, MM1) were grown in RPMI 1640 containing 10% fetal calf serum. The cell viability was determined using an MTT assay (In Vitro Toxicology Assay Kit, Sigma, St Louis, MO) or trypan blue exclusion after treatment with 2-methoxyestradiol (2ME2) or 17β-estradiol (E2) (Calbiochem, La Jolla, CA). Cells from CML blast crisis patients were obtained through the Connell and O'Reilly Families Cell Manipulation Core at the Dana-Farber Cancer Institute with informed consent and Dana-Farber Cancer Institute institutional review board—approved protocol.
Time-lapse video microscopy
Cells were washed and resuspended in culture medium (0.1 × 106 cells/mL) and transferred to a cell culture plate (60 × 15 mm, Becton Dickinson Labware, Franklin Lakes, NJ). The plate was placed in an incubator (Omega temperature control device, Washington, PA) with 5% (vol/vol) CO2 supply and humidity control on an Olympus IX70 inverted microscope (Olympus, Lake Success, NY) with Hoffman optics (10/20/40×), mounted on a vibration-free base. The microscope was operated in a darkened room, protected from outside light sources, and images were captured with an Optronics DEI-750 3CCD digital color video camera (Optronics Engineering, Galeta, CA). The magnification on the microscope was adjusted so that a few single cells or clusters of cells were clearly visible.
Cell adhesion assay
96-well tissue-culture plates (Costar 96-well polystyrene tissue-culture—treated flat-bottom plate, Corning, Corning, NY) were coated with 50 μL 5 μg/mL human fibronectin solution (Gibco BRL, Life Technologies, Rockville, MD) for 18 hours at 4°C. The plates were washed twice with phosphate-buffered saline (PBS). Plates were then blocked with RPMI 1640 containing 0.2% (wt/vol) bovine serum albumin (BSA) to inhibit nonspecific binding. Cells were preincubated in 1 μg/mL calcein-am (Molecular Probes, Eugene, OR) for 30 minutes at 37°C, subsequently washed twice with culture medium, and resuspended at 5 × 105 cells/mL. 100 μL cell suspension was added per well (n = 4) to 96-well plates and the plate briefly spun for 10 seconds at 200g. 2ME2-treated and vehicle (DMSO)—treated cells were incubated for 2 hours at 37°C. In control experiments the adhesion of BCR-ABL—transformed Ba/F3 cells to fibronectin was determined in the presence of an RGD (Arg-Gly-Asp) or control peptide (Biomol Research Laboratories, Plymouth Meeting, PA). The medium was carefully removed and the plate washed 3 times. The number of labeled cells bound to the bottom of the microtiter plates was analyzed at λexcitation = 494 nm and λemission = 517 nm with a 96-well fluorescence plate reader (SPECTRAmax GEMINI XS, Molecular Devices, Sunnyvale, CA) connected to a Pentium II PC and controlled by SOFTmax Pro Version 3.1 software (Molecular Devices). The change in cell adhesion correlates directly with the change in fluorescence, and a percent decrease in adhesion was calculated as the percent in change of fluorescence compared with the control.
Transwell migration assay
The lower chamber of a transwell plate (8 μm pore size polycarbonate membrane, Corning Costar, Cambridge, MA) was filled with 600 μL medium. Starved Ba/F3 cells or CML patient cells were counted using a Coulter particle counter (Coulter Counter Z2, Beckman Coulter, Fullerton, CA) and resuspended at 2 × 106 cells/mL or 4 × 106 cells/mL, respectively. 100 μL of this cell suspension was transferred to the upper chamber. The medium contained either 2ME2, E2, or DMSO. Cells in the lower compartment were resuspended and counted after different time periods using a Coulter particle counter. The spontaneous transwell migration of cells was expressed as the percentage of migrating cells compared with control-treated cells. The standard error of the mean was calculated from independently performed experiments. The statistical significance of the data were analyzed using the Student t test.
Immunofluorescence staining and confocal laser scanning microscopy
NIH3T3.p210 or MCF-7 cells grown on coverslips were treated with 1 μg/mL nocodazole (Sigma), 1 μM taxol, or 5 μM 2ME2 for 24 hours at 37°C. For visualization of microtubules, cells were incubated with the cross-linking agent, dithiobis-(succinimidyl) propionate (DSP, 0.2 mg/mL) for 5 minutes at room temperature, and washed in PBS and microtubule stabilizing buffer (MTSB: 100 mM PIPES [piperazine diethanesulfonic acid], 1 mM EGTA [ethylene glycol tetraacetic acid], 4% polyethylene glycol 8000). Cells were then permeabilized with 0.5% Triton X-100, washed twice in PBS, and fixed in ice-cold methanol for 5 minutes. The anti—α-tubulin monoclonal antibody (Sigma) was applied at 37°C for 1 hour, followed by incubation with secondary rhodamine-conjugated anti-mouse antibody (Jackson ImmunoResearch) for 30 minutes at 37°C. Nuclei were counterstained with 4′,6-diamidino-2 phenylindole (DAPI), and coverslips were mounted in glycerol/gelatin (Sigma). Cells were examined using an LSM410 confocal laser scanning microscope (Carl Zeiss, Jena, Germany), and images were processed for reproduction using Adobe PhotoShop software (Adobe Systems, Mountain View, CA).
Apoptosis assays
The activity of caspase-3 was measured in cell lysates (CaspACE Assay System; Promega, Madison, WI), and annexin V—positive staining was determined by FACS (fluorescence-activated cell-sorter) analysis (Annexin-V—Fluos Staining Kit; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's directions in cells that were treated with 2ME2, E2, or the solvent DMSO.
Measurement of intracellular superoxide
Fluorescence image analysis has been used to determine the relative levels of superoxide (O2•-) in response to 3 μM 2ME2 and E2 or DMSO. Cells were treated for 18 hours before FACS analysis. The relative levels of intracellular O2•- were analyzed using the cell permeable redox-sensitive fluorochrome HET (2′,7′-diamino-10-ethyl-9-phenyl-9,10-dihydrophenanthridine; Sigma). Cells were incubated with 60 ng/mL HET (dihydroethidium) for 1 hour at 37°C and subsequently washed twice in cold PBS before analysis using a Coulter Epics XL flow cytometer (Coulter, Miami, FL).
Results
2ME2 cooperates with STI571 in cell line models
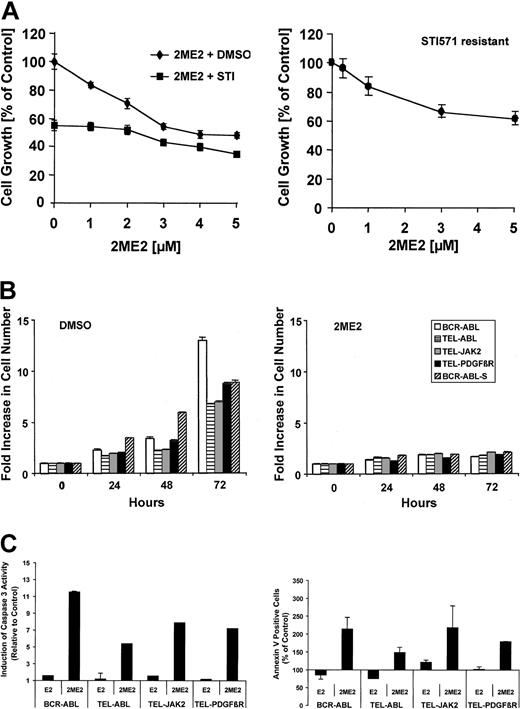
2ME2 has been suggested to inhibit superoxide dismutase activity and to lead to cell death by increasing levels of the ROS superoxide, produced in transformed cells.21 On the other hand, we have previously shown that the novel tyrosine kinase inhibitor STI571 leads to inhibition of ROS in BCR-ABL—expressing hematopoietic cells.13 We therefore initially sought to determine if 2ME2 could cooperate with STI571 in inhibiting cell growth. Ba/F3 cells transformed by the BCR-ABL oncogene were treated with 0-5 μM 2ME2 alone or in combination with STI571 (Figure 1A, left panel). The concentration of STI571 (0.5 μM) was chosen to induce an approximately 50% reduction in cell growth. The solvent DMSO did not affect cell growth of BCR-ABL—transformed Ba/F3 cells (data not shown). 2ME2 led to a dose-dependent reduction in cell growth, which was further reduced by 0.5 μM STI571 treatment. Interestingly, STI571 (0.5 μM) reduced cell growth by 45% in the absence of 2ME2, but in the presence of 5 μM 2ME2, cell growth was reduced by only 27% (equivalent to a 13% reduction in absolute cell number). This suggests that both drugs weakly cooperate, and it is also likely that STI571 and 2ME2 inhibit overlapping pathways. Similar data with a dose-dependent reduction in cell growth by 2ME2 alone or in combination with STI571 were found in the small-cell lung cancer (SCLC) cell line H209 (data not shown), which expresses c-Kit and can be inhibited by STI571.33 Recently, STI571 resistance has been seen in BCR-ABL—transformed cells.34-36 We determined the efficacy of 2ME2 in an STI571-resistant leukemic cell line. Similar to the BCR-ABL—transformed STI571-sensitive Ba/F3 cell line, 2ME2 also inhibited cell growth in an STI571-resistant cell line (Figure 1A, right panel). The reduction in cell growth was comparable to the sensitive cell line with a significant reduction in cell growth at 1 μM 2ME2. In contrast to 2ME2, the parent compound E2 (5 μM) does not have a significant effect on cell growth under these conditions (Huang et al21 and data not shown). The inhibitory effect of 2ME2 was augmented upon a longer treatment period, and cell growth was reduced by more than 75% in a 72-hour culture compared with DMSO-treated cells (Figure 1B). These data suggest that 2ME2 can be combined with drugs that down-regulate intracellular ROS levels like STI571, and 2ME2 has the potential to overcome STI571 drug resistance. To determine potential effects of 2ME2 on cells transformed by tyrosine kinase oncogenes, we also used Ba/F3 cell lines transformed by tyrosine kinase oncogenes different from BCR-ABL, including TEL-ABL, TEL-JAK2, and TEL-PDGFβR. Cells were treated at a concentration of 2ME2 (5 μM) that was shown above to significantly reduce cell growth in BCR-ABL—transformed cells (Figure 1A) and compared with untreated cells. Consistent with the above data, 2ME2 inhibited cell growth after 72 hours in the Ba/F3 cells transformed by all tyrosine kinase oncogenes tested by 70%-87% (Figure 1B). E2 (5 μM) did not inhibit proliferation compared with DMSO-treated cells over a 96-hour period (not shown).
2ME2 inhibits cell growth of STI571-sensitive and -resistant cells. STI571-sensitive (BCR-ABL) or STI571-resistant (BCR-ABL-S) Ba/F3 cells transformed by BCR-ABL (A) and Ba/F3 cell lines transformed by tyrosine kinase oncogenes (B-C) were used. (A) Cells were treated with the indicated amounts of 2ME2 in combination with either STI571 (STI571-sensitive cells, left panel) (0.5 μM; ▪) or the solvent DMSO (♦) as indicated. (B) Cell growth also was determined in a 72-hour culture in response to 2ME2 (5 μM, right panel) or the solvent DMSO (left panel) as indicated. (C) Induction of caspase-3 activity (typical experiments, n = 3, left panel) and annexin V—positive cell staining (n = 2, right panel) was measured after 24 hours of treatment with 2ME2 (5 μM) or E2 (5 μM) and compared to cells treated with DMSO. Values represent means ± SEM.
2ME2 inhibits cell growth of STI571-sensitive and -resistant cells. STI571-sensitive (BCR-ABL) or STI571-resistant (BCR-ABL-S) Ba/F3 cells transformed by BCR-ABL (A) and Ba/F3 cell lines transformed by tyrosine kinase oncogenes (B-C) were used. (A) Cells were treated with the indicated amounts of 2ME2 in combination with either STI571 (STI571-sensitive cells, left panel) (0.5 μM; ▪) or the solvent DMSO (♦) as indicated. (B) Cell growth also was determined in a 72-hour culture in response to 2ME2 (5 μM, right panel) or the solvent DMSO (left panel) as indicated. (C) Induction of caspase-3 activity (typical experiments, n = 3, left panel) and annexin V—positive cell staining (n = 2, right panel) was measured after 24 hours of treatment with 2ME2 (5 μM) or E2 (5 μM) and compared to cells treated with DMSO. Values represent means ± SEM.
Consistent with previous published data,21,26,27 we found that 2ME2 specifically induced G2/M cell cycle arrest (not shown). We further sought to determine if the 2ME2-dependent reduction in cell growth was also in part associated with increased apoptosis. Activation of caspase-3, a downstream effector of the proapoptotic caspase-9, was found to be induced by 2ME2. Ba/F3 cells transformed by tyrosine kinase oncogenes and treated with 2ME2 showed an increase in caspase-3 activity compared with E2- or DMSO-treated cells (Figure 1C, left panel). This suggests that 2ME2 is likely to lead to the cleavage and activation of procaspase-9, which consecutively cleaves and activates procaspase-3. In addition, treatment of the transformed Ba/F3 cell lines with 2ME2 also increased annexin V—positive staining (Figure 1C, right panel), an indication for increased exposure of phosphatidylserine to the outer cell membrane during apoptosis. The number of annexin V—positive cells was compared with DMSO-treated cells with an average of 9.7% annexin V—positive cells (early apoptosis) and 3.6% annexin V/propidium iodide—positive cells (late apoptosis). 2ME2 treatment increased the percentage of annexin V as well as annexin V/propidium iodide—stained cells. These data demonstrate that 2ME2 induces cell cycle arrest as well as apoptosis, and both events in combination are likely to contribute to the reduced cell growth of 2ME2-treated cells.
2ME2 regulates growth in leukemia, lymphoma, and solid tumor cell lines
We also compared the effect of 2ME2 and E2 on cell lines transformed by a mechanism different from a tyrosine kinase fusion-protein, including leukemia (BV173, KU812, Blin-1, Jurkat) and lymphoma (DHL4, DHL6, DHL7, DHL10, Namalwa) cell lines (Figure 2A) as well as solid tumor cell lines (H661, Calu, H209, H69 MCF-7, MDA, G361, U008AT, K019AX) (Figure 2B). In all cell lines tested, 2ME2, in contrast to E2, reduced cell growth over a 72-hour time period. However, 2ME2 was less effective in growth inhibition of some lymphoma cell lines, including DHL10 (26% reduction) and DHL6 (24% reduction), as compared with other lymphoma cell lines, including DHL4 (56% reduction), DHL7 (81% reduction), and Namalva (66% reduction). In the BCR-ABL—expressing cell lines BV173, KU812, and K562, cell growth by 2ME2 was inhibited by 43%, 48%, and 20%, respectively. In the Blin-1 B-cell line and the Jurkat T-cell line, cell growth was reduced by 79% and 98%, respectively. Similarly, in the solid tumor cell lines 2ME2 significantly inhibited cell growth compared with DMSO or E2 treatment, with the exception of the small-cell lung cancer (SCLC) line H69, where 2ME2 showed only minimal effectiveness under these conditions (7% reduction). In other lung cancer cell lines, including H661, Calu, and H209, as well as the melanoma cell lines G361, U008AT, and K019AX or the breast cancer cell lines MCF-7 and MDA, growth was reduced by approximately 23%-63%.
2ME2 inhibits cell growth in hematologic and other malignancies. Cell growth of leukemia and lymphoma cell lines (A) and solid tumor cell lines, including small cell lung cancer, melanoma, and breast cancer (B) was determined. Cell growth was determined in a 3-day culture (n = 4) after treatment with DMSO (▦), 5 μM E2 (▪), or 5 μM 2ME2 (□). Values represent means ± SEM. *Significant differences (P < .005) were observed between 2ME2- and DMSO-treated cells (**P = .05 for the H69 cell line).
2ME2 inhibits cell growth in hematologic and other malignancies. Cell growth of leukemia and lymphoma cell lines (A) and solid tumor cell lines, including small cell lung cancer, melanoma, and breast cancer (B) was determined. Cell growth was determined in a 3-day culture (n = 4) after treatment with DMSO (▦), 5 μM E2 (▪), or 5 μM 2ME2 (□). Values represent means ± SEM. *Significant differences (P < .005) were observed between 2ME2- and DMSO-treated cells (**P = .05 for the H69 cell line).
2ME2 regulates cell shape, size, and motility of BCR-ABL—transformed Ba/F3 cells
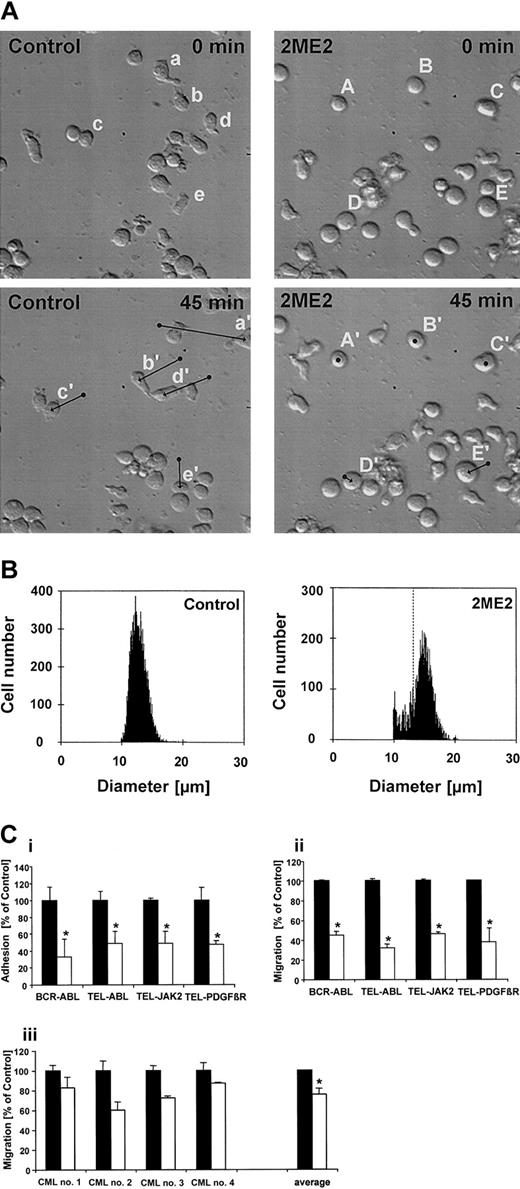
BCR-ABL—transformed cells display multiple cytoskeletal abnormalities including altered adhesion, motility, and migration.37 To determine potential effects of 2ME2 on cells transformed by tyrosine kinase oncogenes, we monitored the spontaneous in vitro motility in BCR-ABL—transformed Ba/F3 cells. In the absence of 2ME2, these cells typically exhibited an irregularly shaped morphology with increased formation of lamellipodia and filopodia as well as high spontaneous migration reflecting enhanced motility as monitored by time-lapsed video microscopy (Salgia et al37 and Figure 3A, left panels, cells a, b, d, and e). Occasionally, round cells changed to an irregularly shaped form and still showed high motility (Figure 3A, left panels, cell c). In contrast, 2ME2 treatment (5 μM, 150-minute pretreatment) dramatically decreased the spontaneous motility of BCR-ABL—transformed Ba/F3 cells and altered their morphology to a round shape without pseudopodia (Figure 3A, right panels, cells A-E). Interestingly, the size of the cells also increased after 2ME2 treatment. The average diameter of 2ME2-treated Ba/F3.p210 cells increased from 13 μm to maximally 15 μm under these conditions (Figure 3B), which represents an approximate 52% increase in cell volume.
2ME2 alters morphology, adhesion, and motility of cells transformed with tyrosine kinase oncogenes. (A) The motility of individual cells was determined by time-lapsed video microscopy (original magnification, × 20). Ba/F3 cells transformed with BCR-ABL were either pretreated for 150 minutes with the solvent DMSO (control) or with 5 μM 2ME2. Cells were then monitored for 45 minutes and typical cells were marked by lowercase (control) or capital (2ME2) letters at the beginning (A-E; a-e) and the end (A′-E′; a′-e′) of the time period. The arrows indicate the centroid movement of typical cells (bottom panels). (B) The cell size of BCR-ABL—transformed Ba/F3 cells was determined before (control, left panel) or after 2ME2 treatment (2ME2, right panel) using a Coulter particle counter. Dashed line indicates peak position of the control. (Ci-ii) Ba/F3 cells transformed with BCR-ABL, TEL-ABL, TEL-JAK2, or TEL-PDGFβR were treated either with the solvent DMSO (▪) or with 5 μM 2ME2 (□). The adhesion of calcein-labeled cells to fibronectin (i) or transwell migration (ii) was measured after a 4-hour pretreatment with DMSO or 2ME2. The data are presented as percentage of DMSO-treated cells (n = 4). (iii) CML cells were pretreated for 2 hours either with 20 μME2(▪) or with 20 μM 2ME2 (□), and the transwell migration was measured after 1 hour. The data are presented individually as percentage of E2-treated cells (n = 2) as well as the average of 4 independent experiments. Values represent means ± SEM. *Significant differences (P < .05) were observed between 2ME2 and control-treated cells.
2ME2 alters morphology, adhesion, and motility of cells transformed with tyrosine kinase oncogenes. (A) The motility of individual cells was determined by time-lapsed video microscopy (original magnification, × 20). Ba/F3 cells transformed with BCR-ABL were either pretreated for 150 minutes with the solvent DMSO (control) or with 5 μM 2ME2. Cells were then monitored for 45 minutes and typical cells were marked by lowercase (control) or capital (2ME2) letters at the beginning (A-E; a-e) and the end (A′-E′; a′-e′) of the time period. The arrows indicate the centroid movement of typical cells (bottom panels). (B) The cell size of BCR-ABL—transformed Ba/F3 cells was determined before (control, left panel) or after 2ME2 treatment (2ME2, right panel) using a Coulter particle counter. Dashed line indicates peak position of the control. (Ci-ii) Ba/F3 cells transformed with BCR-ABL, TEL-ABL, TEL-JAK2, or TEL-PDGFβR were treated either with the solvent DMSO (▪) or with 5 μM 2ME2 (□). The adhesion of calcein-labeled cells to fibronectin (i) or transwell migration (ii) was measured after a 4-hour pretreatment with DMSO or 2ME2. The data are presented as percentage of DMSO-treated cells (n = 4). (iii) CML cells were pretreated for 2 hours either with 20 μME2(▪) or with 20 μM 2ME2 (□), and the transwell migration was measured after 1 hour. The data are presented individually as percentage of E2-treated cells (n = 2) as well as the average of 4 independent experiments. Values represent means ± SEM. *Significant differences (P < .05) were observed between 2ME2 and control-treated cells.
2ME2 reduces adhesion and transwell migration of BCR-ABL, TEL-ABL, TEL-JAK2, and TEL-PDGFβR—transformed cells
Cell motility is a complex cellular function that is altered through changes in cell adhesion and is also reflected through cellular migration. As another cytoskeletal function, the adhesion of Ba/F3 cells transformed by tyrosine kinase oncogenes was measured on fibronectin-coated surfaces before and after 2ME2 treatment. BCR-ABL—transformed Ba/F3 and 32Dcl3 cells have increased adhesion to extracellular matrix proteins.38 Using BCR-ABL—transformed Ba/F3 cells, we found that treatment with 2ME2 (2.5 hours, 5 μM) led to a 66% reduction in adhesion to fibronectin compared with DMSO-treated cells (Figure 3C, top left). DMSO did not affect adhesion compared with untreated cells (not shown). 2ME2 also reduced adhesion to fibronectin to a comparable degree in cells transformed by other oncogenic tyrosine kinase oncogenes, including TEL-ABL (52% reduction), TEL-JAK2 (51% reduction), and TEL-PDGFβR (53% reduction) (Figure 3Ci). A peptide containing the integrin ligand motif RGD (Arg-Gly-Asp) specifically inhibited the adhesion to fibronectin (not shown). Since adhesion to fibronectin in this type of assay is mediated by β1-integrins,38 these results suggest that 2ME2 decreases integrin function in the transformed cell lines.
The effect of 2ME2 on transwell migration also was determined. We have shown previously that BCR-ABL—transformed Ba/F3 cells show elevated spontaneous transwell migration.37 Again, cells were treated either with 2ME2 (4 hours, 5 μM) or DMSO as a control. Consistent with the previous experiments, 2ME2 also led to a reduction in transwell migration compared with DMSO treatment in BCR-ABL, TEL-ABL, TEL-JAK2, and TELPDGFβR—transformed cells (Figure 3Cii). DMSO did not affect transwell migration compared with untreated cells (not shown). 2ME2 reduced migration in Ba/F3 cells transformed by BCR-ABL to 45%, by TEL-ABL to 32%, by TEL-JAK2 to 46%, or by TEL-PDGFβR—transformed cells to 38% of the control. In control experiments, Ba/F3 cells transformed with BCR-ABL, TEL-ABL, TEL-JAK2, or TEL-PDGFβR were treated with 5 μM E2 (not shown). We did not find a difference in inhibition of adhesion between E2 and 2ME2. In contrast, cell motility was not affected by E2 or the solvent DMSO. These data suggest that 2ME2 has a similar effect on adhesion and motility in cell lines transformed by different tyrosine kinase oncogenes.
In addition, the effect of 2ME2 on transwell migration of primary CML blast crisis patient samples was determined. Cells were pretreated with 2ME2 (2 hours, 20 μM) and compared with E2 (2 hours, 20 μM) pretreated samples as a control (Figure 3Ciii). Similar to the Ba/F3 cell line model, 2ME2 was more efficient in reducing the migration of the CML cells compared with the control treatment. 2ME2 treatment led to a significant decrease of migration, with an average of 24% in relation to E2-treated samples (P = .01). These data are consistent with the biologic effects of 2ME2 on migration observed in the BCR-ABL—transfected Ba/F3 cell lines.
Effects of 2ME2 on tubulin organization
The above data suggest that 2ME2 can regulate multiple cellular functions related to the cytoskeleton. We next tested whether 2ME2 had an effect on the organization of the cytoskeleton. Therefore, we examined the pattern of actin and tubulin distribution in the absence and presence of 2ME2 and drugs that disrupt the cytoskeleton as controls. We used NIH3T3 cells transformed by BCR-ABL (NIH3T3.p210), which we have previously shown to display BCR-ABL—dependent cytoskeletal abnormalities.37 In control experiments, cells were treated with a concentration of either nocodazole or paclitaxel, which are known to have an effect on microtubules, and then stained for α-tubulin. Antitubulin staining in untreated interphase cells showed the ubiquitous, fine filament structure of the microtubules in NIH3T3.p210 cells (Figure 4A). 2ME2 treatment as well as nocodazole and paclitaxel resulted in a significant disruption of the filamentous structure and the overall organization of microtubules (Figure 4B-D). Treatment with paclitaxel promoted the stabilization of microtubules and led to the formation of dense tubulin bundles (Figure 4C). In contrast, nocodazole-treated cells showed disrupted microtubules composed of small fragments (Figure 4D). Treatment with 2ME2 led in part to depolymerization of tubulin and the appearance of disorganized microtubules (Figure 4B). DAPI staining showed a significant increase of multinucleated cells after all drug treatments, indicating improperly completed mitoses (Figure 4A-D). In additional experiments, microfilaments were visualized, but 2ME2 had no significant effect on the actin cytoskeleton (data not shown). Our results suggest that 2ME2 disrupts cytoskeletal function in part through depolymerization of tubulin and may therefore also affect normal mitosis.
2ME2 alters tubulin organization in a BCR-ABL—transformed cell line. NIH3T3.p210 cells were treated with DMSO (A,E), 5 μM 2ME2 (B,F), 1 μM paclitaxel (C,G), or 1 μg/mL nocodazole (D,H) for 24 hours, washed, and fixed as described. Immunofluorescence analysis was performed using anti—α-tubulin antibodies (A-D) and DAPI (E-H). Bars = 10 μm.
2ME2 alters tubulin organization in a BCR-ABL—transformed cell line. NIH3T3.p210 cells were treated with DMSO (A,E), 5 μM 2ME2 (B,F), 1 μM paclitaxel (C,G), or 1 μg/mL nocodazole (D,H) for 24 hours, washed, and fixed as described. Immunofluorescence analysis was performed using anti—α-tubulin antibodies (A-D) and DAPI (E-H). Bars = 10 μm.
Effects of 2ME2 on intracellular superoxide levels
2ME2 has been reported to elevate intracellular ROS levels by inhibiting superoxide dismutase.21 In order to determine the direct effect of 2ME2 on superoxide levels in our cell system, we measured the intracellular levels of superoxide in response to 2ME2 by dihydroethidium (HET) staining. Whereas 2ME2 increased superoxide levels in the MM1 multiple myeloma cell line by a maximum of 56%, we did not consistently observe an overall increase in superoxide in Ba/F3 cells transformed by the tyrosine kinase oncogenes. However, 2ME2-treated transformed Ba/F3 cells were more sensitive than MM1 cells and rapidly underwent apoptosis (Chauhan et al39 ). This would imply that different cell lines show different sensitivity to 2ME2. The FACS analysis also indicated that 2ME2 treatment resulted in the appearance of larger cells with high ethidium staining (likely cells with altered tubulin organization) and smaller cells with low ethidium staining (such as late apoptotic cells).
Discussion
We have compared the effect of E2 to 2ME2 on different leukemia, lymphoma, and solid tumor cell lines. Consistent with previously published data, 2ME2, but not E2, inhibits cell growth in different sensitive cell lines and cell types.40 The inhibition of cell growth was noted to be approximately 20%-98% over a course of 72 hours. The mechanism of growth inhibition is likely to involve damage to mitochondria and induction of apoptosis through elevated levels of O2•-.21 It should be noted that ROS generation cannot only cause apoptosis, but also can lead to enhancement of transformation. There is a fine balance of ROS generation that regulates cell survival and cell death. Signaling intermediates such as ROS are likely to contribute to transformation by activating pathways that influence cell growth, viability, or other biologic activities. One of the most interesting signaling pathways includes the induction of ROS by BCR-ABL.13 The potential role of ROS in transformation has been shown by overexpression of the superoxide-generating NADPH (nicotinamide adenine dinucleotide phosphate) oxidase Mox1 in NIH3T3 fibroblasts, which increases cell growth and induces tumors in athymic mice.41 On the other hand, inhibition of superoxide dismutase (SOD), an enzyme that catalyzes the reduction of superoxide to H2O2, by 2ME2 kills leukemia cells but not normal lymphocytes.21 This suggests that ROS levels in hematopoietic cells are regulated to maintain a fine balance between ROS-supported cell survival and free-radical—mediated cell death. Regulation of superoxide dismutase activity may be an important mechanism in transformation and an important target for drug treatment. It has been suggested that ROS also may act as second messengers to regulate activities of redox-sensitive enzymes, including protein phosphatases42,43 or Ras.44 It is likely that changes in the oxidative state in the cell through inhibition of superoxide dismutase by 2ME2 would lead to an imbalance in the activity of some of these enzymes or the activity of other redox-sensitive proteins and processes that are either direct targets of ROS or downstream effectors.
The cytoskeleton is an important target for oncogenes, and abnormal cytoskeletal function previously has been linked to the transformed phenotype in CML and other diseases. We have shown previously that BCR-ABL induces cytoskeletal abnormalities that affect morphology, motility, and adhesion.37 In this study, we found that 2ME2 inhibits several cytoskeletal functions, normally activated by oncogenic tyrosine kinases, including adhesion, transwell migration, and cell motility. In particular, the inhibition of migration and adhesion was similar in all oncogenic transformed cell lines tested and did not appear to be specific toward a single tyrosine kinase oncogene. In contrast to the selected reduction of cell growth through 2ME2, but not E2, we did not observe a difference in the effects of these 2 compounds on inhibition of cell adhesion. It is therefore likely that adhesion does not contribute to the reduction of cell growth in vitro but may contribute in part to altered motility or migration. The significant effect of 2ME2 on the cytoskeleton is apparent in the motility assay, where even a short treatment of BCR-ABL—transformed cells with 2ME2 led to a drastic inhibition of cell movement. We would hypothesize that 2ME2, during early events, leads in part to decreased adhesion through the alteration of function of β1 integrins. Integrins are key elements of cell adhesion, cell motility, and migration. Once the β1 integrins are disrupted, downstream events of cell motility also are affected. It would now be useful to see the direct link between 2ME2 treatment, motility, and integrin function.
2ME2 had been described previously to display antiangiogenic activity, and it has been suggested that it may inhibit tubulin polymerization.31,45,46 The inhibition of tubulin polymerization, as reflected by another effect on cytoskeletal function of 2ME2, may occur through a mechanism that is independent of inhibition of SOD activity. In vitro experiments have demonstrated that 2ME2 can inhibit tubulin polymerization through competing with colchicin for its binding site at a micromolar concentration.45,47 Disruption of the microtubuli may contribute to the formation of multinucleated cells and consequently enlarged cell volume, similar to paclitaxel.26,32,48,49 Microtubules are formed by α/β-tubulin monomers and normally regulate cell shape, motility, transport, or division.50 The structurally related γ-tubulin is required for initiating the polymerization of microtubules.51 There are also 2 forms of α-tubulin, tyrosinated and detyrosinated tubulin, that are generated by posttranslational modification and are present in distinct subsets of microtubules.52 It will be interesting to determine if 2ME2 has a preference for either tubulin, especially considering that the microtubules containing the detyrosinated tubulin resemble the shape of the 2ME2-treated tubulin. A small amount of detyrosinated tubulin is usually found in interphase microtubules and is absent from the astral fibers of the metaphase spindle.52
It also remains to be determined if the regulation of microtubular function, adhesion, and cell motility or migration by 2ME2 is in part due to altered levels of ROS through inhibition of SOD. 2ME2 is expected to increase intracellular levels of superoxide and reduce hydrogen peroxide levels. This is of interest, since we have previously shown that hydrogen peroxide can specifically lead to an increase of transwell migration in hematopoietic cell lines.18 We also observed reduced adhesion in cells transformed by tyrosine kinase oncogenes. It is possible that BCR-ABL, TEL-ABL, TEL-PDGFβR, and TEL-JAK2 activate an ROS-sensitive pathway that affects cytoskeletal function. Altered levels of ROS in cells transformed by tyrosine kinase oncogenes could potentially contribute to the reduced transwell migration or adhesion induced by 2ME2 through an as-yet-unknown mechanism that may involve redox-sensitive signaling mechanisms.
Since transformation by activated tyrosine kinases leads to an increase in intracellular ROS, we investigated the potential drug interaction of 2ME2 with STI571, a known inhibitor of oncogenic tyrosine kinases.53 STI571-related drug resistance in CML is of clinical relevance, and it will limit its therapeutic efficacy.34-36 This novel tyrosine kinase inhibitor leads to a reduction of intracellular ROS in cells transformed by activated ABL tyrosine kinase.13 However, 2ME2 likely acts by elevating intracellular ROS levels through inhibition of SOD. Consistent with these data, we found that inhibition of BCR-ABL kinase activity by STI571 only weakly cooperated with the growth inhibitory effects of 2ME2. In addition, 2ME2 overcame drug resistance in an STI571-resistant cell line. In the absence of 2ME2, STI571 was more effective in reducing cell growth than in the presence of this drug, and one possibility is that BCR-ABL activates a pathway that is required for the inhibitory effect of 2ME2, such as increased levels of ROS.
Overall, our data suggest that 2ME2 is a potent anticancer drug that demonstrates some cooperative effects with STI571 but can also cause apoptosis of STI571 drug-resistant cells. Identifying targets for other signal transduction inhibitors that are complementary to STI571 without adding toxicity to normal blood cells is critical, and 2ME2 may be beneficial in the treatment of this or other resistant cancers. In addition to the antiproliferative effect and inhibition of adhesion, migration, or motility by 2ME2, it may significantly reduce the tumorigenic potential of cancer cells. In particular, reduced cytoskeletal function could inhibit premature release of leukemia cells from the marrow and accumulation in the blood, or in the case of solid tumors, prevent metastasis by inhibiting cell motility. Down-regulation of cytoskeletal functions by 2ME2 may therefore contribute to reduced metastasis and invasion of cancer cells, and thus it would be useful to test this drug further.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-03-0729.
Supported by the Leukemia Research Foundation (M.S.) and the American Cancer Society (M.S., R.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal