Abstract
Mismatch repair deficiency is associated with carcinogenesis, increased spontaneous and induced mutagenesis, and resistance to methylating agents. In humans, leukemias and lymphomas arise in the background of mismatch repair deficiency, raising the possibility that hematopoiesis is abnormal as well. To address hematopoiesis in MSH2–/– mice, we collected marrow and performed serial transplantations of these cells, alone or mixed with wild-type cells, into lethally irradiated healthy mice. Transplant recipients were observed or treated with the methylating agent, temozolomide (TMZ). Methylating agent tolerance was evident by the competitive survival advantage of MSH2–/– marrow progenitors compared with wild-type cells after each TMZ exposure. However, serial repopulation by MSH2–/– cells was deficient compared with wild-type cells. In recipients of mixed populations, the MSH 2–/– cells were lost from the marrow, and mice receiving MSH2–/– cells plus TMZ could not be reconstituted in the third passage, whereas all wild-type cell recipients survived. No differences in telomere length, cell cycle distribution, or homing were observed, but an increase in microsatellite instability was seen in the MSH2–/– early progenitor colony-forming unit (CFU) and Sca+Kit+lin–-derived clones. Thus, mismatch repair deficiency is associated with a hematopoietic repopulation defect and stem cell exhaustion because of accumulation of genomic instability.
Introduction
Hematopoietic stem cells self renew and maintain long-term repopulation. To fulfill this exceptional capacity for replication, regulated cell proliferation, and differentiation, cells must maintain an intact genome. It is inevitable that during the lifetime of a cell, the DNA will accumulate errors from either environmental factors or DNA replication errors. The complex cellular response to this damage includes cell cycle arrest, transcription induction, and DNA repair mechanisms that maintain genomic stability. In the absence of repair, apoptotic pathways lead to cell death, or persistent DNA damage can lead to loss of genetic material, genomic and chromosomal instability, mutagenesis, and ultimately malignant transformation or cell death.
DNA methyl adducts are considered some of the most toxic and mutagenic lesions produced by endogenous and environmental methylating agents.1 One lesion, O6mG, is both cytotoxic and mutagenic. During replication, DNA polymerase preferentially inserts a T opposite O6mG. This is recognized by the mismatch repair (MMR) complex that preferentially removes the newly synthesized strand containing the erroneous T. During re-synthesis, a DNA polymerase again inserts a T opposite to the O6mG, and the process is reinitiated. This futile cycle of repair ultimately leads to strand breaks2 and induction of apoptosis.3 In the absence of MMR, cells are resistant (“tolerant”) to methylating agents,4 have increased cell survival, and an accumulation of mutations.5,6 These mutations are characterized by point mutations, insertions, or deletions in simple repeat sequences throughout the genome, resulting in what has become the hallmark of mismatch repair deficiency, microsatellite instability (MSI).7,8
MMR gene defects have been most closely associated with hereditary nonpolyposis colorectal cancer.9 More recently, MMR mutations and MSI have been identified in hematopoietic malignancies and leukemia and lymphoma cell lines.10-13 A low incidence of MSI has been detected in de novo acute myelogenous leukemia (AML) and myelodysplastic syndrome,14 and a greater incidence has been reported in secondary and treatment-related AML.13,15 Recently, 6 cases of hematologic malignancy have been reported in children homozygous for MMR deficiency16 because of either MLH1 (MutL homolog 1) or MSH2 (MutS homolog 2), most of whom also had germ line neurofibromatosis-1 gene (NF-1) mutations. These reports led to our interest in understanding the role of MMR in hematopoietic stem cell maintenance.
The role of DNA repair processes in the maintenance of the stem cell phenotype, characterized by stem cell longevity and long-term repopulation capacity after DNA damage, has not been evaluated. In studies reported here, we hypothesized that MMR machinery was required for maintenance of stem cell function and that its absence would lead to accumulation of genomic damage and loss of repopulating capacity. For these studies, we used primary murine hematopoietic cells from mice deficient in the mismatch repair gene MSH2–/–, and asked whether they had a selection bias when competed against wild-type cells in long-term repopulating serial bone marrow transplantation assays. Because of the known methylation tolerance of MMR-defective cells, we evaluated competitive progenitor and stem cell survival with and without in vivo temozolomide (TMZ) exposure. Despite an early survival advantage in vivo, after TMZ, MSH2–/– stem cells exhibited a progressive loss in primary and secondary recipients, leading to bone marrow failure in tertiary recipients associated with an increase in microsatellite instability. These studies suggest that MMR-defective progenitors have abnormal repopulating capacity resulting from methylating agent exposure which leads to stem cell exhaustion.
Materials and methods
Generation of mice
MSH2–/– mice, generated in a C57Bl/6 background strain, were kindly provided by T. W. Mak (Amgen Research Institute, University of Toronto, Toronto, ON). Mice used for experiments were generated by interbreeding heterozygotes. The resulting progeny (wild-type and homozygous knock-out) were used as donor mice.
Serial bone marrow transplants
Bone marrow was flushed from the femur, tibia, and pelvis of 6- to 8-week-old wild-type or MSH2–/– siblings using RPMI containing 10 U/mL heparin. Cells (2 × 106 per mouse) were washed and infused or mixed at a ratio of either 50:50 or 10:90 (–/– to wild type, respectively) and infused via tail vein into recipients lethally irradiated with 850 cGy using a 57Co source. Wild-type C57 Bl/6 recipient mice were monitored for 8 weeks, then killed to obtain bone marrow for use as donor cells. Harvested marrow was washed as described earlier, and 2 × 106 cells were transplanted into irradiated secondary recipients. This procedure was repeated for tertiary and quaternary recipients.
TMZ treatment
TMZ (Drug Synthesis and Chemistry Branch, National Cancer Institute [NCI], Bethesda, MD) was dissolved in 10% dimethyl sulfoxide (DMSO) and 90% sterile phosphate-buffered saline (PBS) and used within 10 minutes. This mixture (80 mg/kg) was injected intraperitoneally for 3 consecutive days into mice 3 weeks after bone marrow transplantation.
Isolation of Sca+Kit+lin– cells
To obtain clonal Sca+kit+/lin– cells, marrow was obtained from mice as described in “Serial bone marrow transplants.” Cells were blocked with normal rat serum for 10 minutes, treated with Fc block (CD16/32; BD-Pharmingen, San Diego, CA) for 5 minutes, then stained with phycoerythrin (PE)–conjugated antimurine lineage markers Ter119, CD3, CD4, CD11b, B220; allophycocyanin (APC)–conjugated anti–c-kit; and fluorescein isothiocyanate (FITC)–conjugated anti–Sca-1 for 20 minutes at 4°C. The appropriate immunoglobulin G (IgG) isotype controls (BD-Pharmingen) were performed simultaneously. Sca+Kit+lin– cells were identified in the lineage-negative population as those positive for both markers and were sorted as single cells into 96-well plates containing α-mem supplemented with murine interleukin 3 (m-IL3), m-IL6, rat stem cell factor (SCF), and spleen cell-conditioned medium. Fourteen days later, colonies consisting of more than 1000 cells were removed from the wells, and DNA was isolated for polymerase chain reaction (PCR)–based genotyping.
Colony-forming unit (CFU) assay and genotyping
The origin of the colonies derived from both methylcellulose and Sca+kit+lin– sorting was determined by PCR using primers specific for the MSH2 and neomycin genes. PCR was performed under conditions previously described.17
Cell cycle analysis
Flow cytometry was used to assess the cell cycle status of bone marrow stem cells. Bone marrow mononuclear cells were labeled for 20 minutes at 4°C with biotinylated Ter119, CD3, B220, and CD11b, followed by APC-conjugated strepavidin and FITC-conjugated c-kit, also for 20 minutes at 4°C. Cells were washed with buffer (1 × Hanks balanced salt solution containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1 g/L glucose, and 10% fetal calf serum [FCS]) and incubated with Hoechst dye no. 33342 (1.67 μM) at 37°C for 45 minutes each as previously described.18 Cells were then washed twice and analyzed on Becton Dickinson LSR and CellQuest software (San Jose, CA); G1-S-G2-M populations were identified by Hoechst staining of lineage-negative and c-kit–positive cells.
Microsatellite instability assay
CFU and Sca+Kit+lin– clones derived from the marrow of mice reconstituted with either MSH2–/– or wild-type cells were isolated, and DNA was extracted by treatment with Triton-X 100 buffer containing 10 mg/mL Proteinase K. Two microsatellite loci (D3mit203 and D7mit62) were evaluated for each clone, and PCR primers (MAPPAIRS) were obtained from Research Genetics (Huntsville, AL). The forward primer of each loci was end-labeled using PNK (Roche Biochemicals, Indianapolis, IN) and 32P or 33P (Dupont-NEN, Boston, MA). The end-labeled primer was then used in a PCR reaction containing an unlabeled reverse primer. PCR conditions were performed as recommended by the manufacturer. PCR samples were run on a 6% denaturing Long Ranger gel (Biowhittacre Molecular Applications, Rockland, ME) for approximately 3 hours. The gel was dried and exposed to autoradiography film overnight.
Bone marrow homing assay
Bone marrow mononuclear cells were harvested from the hind limbs of a sibling pair of MSH2–/– and wild-type mice. MSH2–/– cells were stained with 5 μM CSFE (5- and 6-carboxyfluorescein diacetate succinimidyl ester) (Molecular Probes, Eugene, OR) and wild-type cells were stained with 5 μM DiD (Molecular Probes) both according to manufacturer's directions. The reverse-labeling scenario was also performed (MSH2–/– stained with DiD and wild-type cells stained with CSFE). Cells were then mixed at a 50:50 ratio of MSH2–/– to wild-type cells, and 5 × 106 of the mixed cells were injected via tail vein into mice irradiated with 8.5 Gy. Forty-eight hours later, bone marrow was harvested and stained with biotin-labeled lineage markers CD3, CD4, B220, Ter119, and CD11b followed by strepavidin-PerCP-Cy5.5 conjugate (all from BD Biosciences, San Jose, CA). CSFE- and DiD-labeled cells in the bone marrow were detected in the FL1 and SSC-W channels, respectively, using an LSR flow cytometer (Becton Dickinson) in the lineage-negative gate.
Spleen colony-forming unit (CFU-S) assay
C57Bl/6 recipients were lethally irradiated with 850 cGy 57Co and received transplants of 5 × 104 wild-type or MSH2–/– bone marrow mononuclear cells. Twelve days after transplantation, mice were killed, and spleens were harvested and fixed with Bouin fixative. Colonies were counted using a dissecting microscope.
Telomere length
The average length of telomere repeat sequences at chromosome ends in individual cells was measured using fluorescence in situ hybridization (FISH) as described.19 Bone marrow was harvested from mice and washed in PBS with 0.1% bovine serum albumin (BSA). Cells (2 × 105) were resuspended in 200 μL hybridization buffer (in triplicate) containing 0.3 μg/mL FITC-labeled telomere probe (C3TA2) (Applied Biosystems, Framingham, MA). Samples were denatured at 80°C for 10 minutes and hybridized for 2 hours at room temperature. Cells were washed and resuspended in 300 μL PBS 0.1% BSA containing RNase and 7-AAD (7-aminoactinomycin D) (BD-Pharmingen). Analysis was performed on a LSR flow cytometer (Becton Dickinson), and FITC fluorescence was assessed on the low forward and low side scatter 7-AAD–positive population.
Results
Selection advantage of MSH2–/– bone marrow progenitors
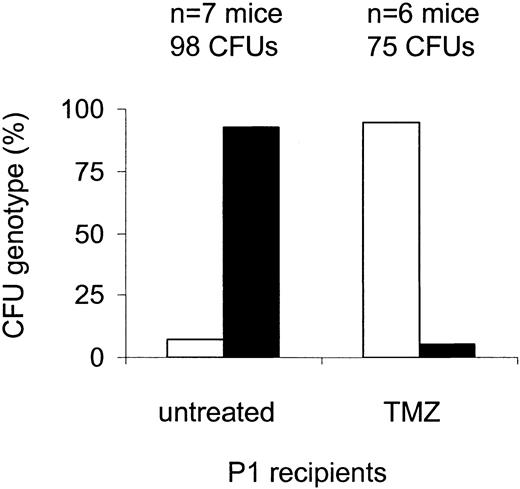
We first evaluated whether TMZ-resistant MSH2–/– bone marrow progenitors had a selective advantage in vivo. Mice that received transplants of 10% MSH2–/– and 90% wild-type marrow were treated with 80 mg/kg TMZ 3 times and harvested 8 weeks after transplantation. Enrichment was determined by genotype in both Sca+Kit+lin– cells and methylcellulose progenitors (CFU). We found that MSH2–/– CFUs were markedly enriched after drug treatment compared with untreated cells: at 8 weeks, 9 (9.8%) of 92 CFUs from 7 untreated primary recipients were MSH2–/–, whereas in treated mice MSH2–/– CFUs collected from 6 primary recipients increased 10-fold to 71 (95%) of 75 (P = <.0001, Fisher exact test) (Figure 1). Similarly, 2 (6%) of 33 Sca+Kit+lin– clones obtained from untreated primary recipients were MSH2–/– (n = 3 mice) compared with 22 (92%) of 24 MSH2–/– Sca+Kit+lin– clones in treated recipients (n = 3 mice) (P < .0001, Fisher exact test). This finding indicates that mismatch repair–defective hematopoietic progenitor cells can be enriched by drug treatment in vivo.
Enrichment of MSH2–/– CFU after TMZ treatment. Lethally irradiated wild-type mice received transplants of a mixture of 10% MSH2–/– (□) and 90% wild-type (▪) whole bone marrow, followed by treatment 3 weeks later with 80 mg/kg TMZ daily times 3. Bone marrow was harvested 8 weeks after transplantation, and enrichment of MSH2–/– cells was determined by PCR of individual CFUs. There was a significant increase in the proportion of MSH2–/– CFUs recovered from treated versus untreated mice; P < .0001 (Fisher exact test).
Enrichment of MSH2–/– CFU after TMZ treatment. Lethally irradiated wild-type mice received transplants of a mixture of 10% MSH2–/– (□) and 90% wild-type (▪) whole bone marrow, followed by treatment 3 weeks later with 80 mg/kg TMZ daily times 3. Bone marrow was harvested 8 weeks after transplantation, and enrichment of MSH2–/– cells was determined by PCR of individual CFUs. There was a significant increase in the proportion of MSH2–/– CFUs recovered from treated versus untreated mice; P < .0001 (Fisher exact test).
Competitive survival of long-term repopulating MSH2–/– stem cells
Previous studies have shown that MSH2–/– embryonic fibroblast cells are genomically unstable.17 This information led us to ask whether MSH2–/– hematopoietic progenitor cells would have altered repopulation function, and, if so, whether this alteration was related to genomic instability. To expose a defect that might result during stem cell stress, we subjected MSH2–/– hematopoietic cells alone and in competition with normal cells, using a mixture of 10% MSH2–/– and 90% wild-type cells, to 3 serial transplantations. Marrow cells were transplanted into lethally irradiated recipient mice, sequentially into the first recipient (P1), second recipient (P2), and third recipient (P3) at 8-week intervals. MSH2–/– cells were 10% of the initial transplant mixture, but in cohorts of mice transplanted sequentially, the proportion of MSH2–/– hematopoietic progenitors recovered in the marrow declined with each transplant. In P1 mice, the percentage of MSH2–/– CFUs and Sca+Kit+lin– clones declined to 7% (n = 98 colonies from 7 mice) and 6.8% (n = 43 colonies from 3 mice), respectively. MSH2–/– CFUs decreased further to 1% (n = 36 colonies from 3 mice) in P2 and 0% (n = 60 colonies from 4 mice) in P3 recipient mice (Table 1). Sca+Kit+lin– clones also decreased to 0% by P2 (n = 33 colonies from 3 mice).
Genotype of CFUs harvested from untreated or TMZ-treated mice that received transplants of 10% MSH2–/– and 90% wild-type bone marrow mononuclear cells
Serial transplant recipient . | TMZ (n) . | No TMZ (n) . |
|---|---|---|
| P1 | MSH2-/-, 95% | MSH2-/-, 7% |
| wild type, 5% | wild type, 93% | |
| (7) | (7) | |
| P2 | MSH2-/-, 90% | MSH2-/-, 1% |
| wild type, 10% | wild type, 99% | |
| (4) | (3) | |
| P3 | No CFU | Wild type, 100% |
| (13) | (4) |
Serial transplant recipient . | TMZ (n) . | No TMZ (n) . |
|---|---|---|
| P1 | MSH2-/-, 95% | MSH2-/-, 7% |
| wild type, 5% | wild type, 93% | |
| (7) | (7) | |
| P2 | MSH2-/-, 90% | MSH2-/-, 1% |
| wild type, 10% | wild type, 99% | |
| (4) | (3) | |
| P3 | No CFU | Wild type, 100% |
| (13) | (4) |
To exclude the possibility that MSH2–/– cells were lost with sequential transplantation because the absolute number initially transplanted was too few, we transplanted 1 × 106 MSH2–/– cells at 50:50 ratio with wild-type cells and evaluated repopulation capacity as described earlier. Similar to the observation in mice that received transplants of 10% MSH2–/– and 90% wild type, an approximate 30% decline in MSH2–/– cells was observed in bone marrow obtained from P1 mice. P1 marrow contained 32% MSH2–/– CFUs (n = 36 colonies from 3 mice) and 26% Sca+Kit+lin– clones (n = 18 colonies from 2 mice). CFUs from P2 mice reverted to 100% wild type (n = 21 CFU from 2 mice) 8 weeks after transplantation, and subsequently mice receiving transplants survived P3 (n = 4) and P4 transplantations (n = 3) (Table 2). These data indicate that MSH2–/– cells have a repopulation defect and are thus lost during serial transplantation.
Genotype of CFUs harvested from untreated or TMZ-treated mice that received transplants of 50% MSH2–/– and 50% wild-type bone marrow mononuclear cells
Serial transplant recipient . | TMZ (n) . | No TMZ (n) . |
|---|---|---|
| P1 | MSH2-/-, 95% | MSH2-/-, 32% |
| wild type, 5% | wild type, 68% | |
| (3) | (3) | |
| P2 | MSH2-/-, 100% | wild type, 100% |
| (2) | (2) | |
| P3 | No CFU | Wild type, 100% |
| (6) | (4) |
Serial transplant recipient . | TMZ (n) . | No TMZ (n) . |
|---|---|---|
| P1 | MSH2-/-, 95% | MSH2-/-, 32% |
| wild type, 5% | wild type, 68% | |
| (3) | (3) | |
| P2 | MSH2-/-, 100% | wild type, 100% |
| (2) | (2) | |
| P3 | No CFU | Wild type, 100% |
| (6) | (4) |
Competitive survival of MSH2–/– long-term repopulating cells after TMZ
A different profile was observed in TMZ-treated mice subjected to the same serial bone marrow transplantation schema because there was initial selection in favor of the MSH2–/– progenitor cells after TMZ. After serial transplantation, however, we again noted loss of MSH2–/– progenitor and repopulating cells measured as both MSH2–/– CFUs and Sca+kit+lin– clones. As predicted, when the marrow of primary mice recipients of bone marrow transplanted with a mixture of 10% MSH2–/–:90% wild-type cells and treated with TMZ was analyzed 5 weeks after treatment, the proportion of 10% MSH2–/– CFUs (75 colonies from 7 mice) and Sca+Kit+lin– clones (48 colonies from 4 mice) increased to 95%. When these marrow cells were collected from the primary mice and transplanted into secondary recipients, and these mice were treated again with TMZ, the proportion of MSH2–/– marrow CFUs (n = 60 colonies from 4 mice) and Sca+Kit+lin– clones (n = 25 colonies from 2 mice) were maintained at 90% and 80%, respectively (n = 4 mice). Thus, with TMZ treatment, it was possible to maintain the selective advantage of the MSH2–/– cells. However, 12 of 13 mice that received transplants of marrow from P2 recipients that had themselves been repopulated with TMZ-treated P1 marrow cells died of bone marrow failure 3 weeks after transplantation, whereas mice recipients of marrow from TMZ-treated wild-type cells survived tertiary (P3) and quaternary (P4) transplantations. The one surviving mouse recipient of TMZ-treated marrow from the P3 cohort was killed at 8 weeks, but the marrow was very hypocellular and produced no CFUs after methylcellulose culture so genotyping was not possible. From this information we conclude that, despite a drug resistance phenotype, TMZ treatment of MSH2–/– cells results in a repopulating defect during sequential transplantation.
We performed similar transplantations (P1 through P3) using a mixture of 50% wild-type and 50% MSH2–/– marrow cells and TMZ treatment on week 3 of both P1 and P2 recipients. The CFUs in bone marrow harvested from mice 5 weeks after TMZ treatment also increased to 95% MSH2–/– at P1 (n = 30 CFU from 3 mice) and 100% at P2 (n = 30 CFU from 2 mice) (Table 2). As earlier, P3 mice died (n = 6 mice) shortly after transplantation. These data suggest that mismatch repair–defective hematopoietic progenitors have an early selection advantage after TMZ and appear to replace the marrow of recipients but develop late stem cell exhaustion that is not observed in wild-type cells.
Maintenance of selection advantage of MSH2–/– progenitors
Because our data indicated that MSH2–/– bone marrow cells had a selection advantage in the presence of TMZ, we asked whether repeat selection was necessary to maintain MSH2–/– progenitors. In 2 additional cohorts of mice recipients of the 10% MSH2–/– and 90% wild-type mixture, we treated at P1 only and observed for the proportion of MSH2–/– cells after P2 and P3 transplantation. There was a decrease in MSH2–/– CFUs from 95% to 77% by P2 (n = 36 colonies from 3 mice) and to 0% by P3 (n = 14 mice). Similarly, mice treated in P2 only resulted in 7% MSH2–/– CFUs (n = 101 colonies from 6 mice [compared with > 90% selection in mice treated during P1]), and the genotype reverted to 100% wild type by P3 (n = 6 mice) (data not shown). This observation suggests that maintenance of MSH2–/– cells is dependent on early and repeated selection with TMZ but that these cells fail to engraft in the P3 mouse.
Bone marrow failure in tertiary recipients
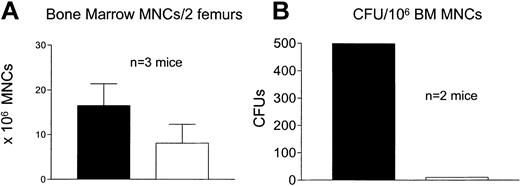
Because TMZ-treated mice repopulated with MSH2–/– cells in the earlier experiments did not survive tertiary transplantation, we examined hematopoietic engraftment to determine whether they had marrow failure as the cause of death. Mice were initially transplanted with 100% MSH2–/– cells, and serial transplant recipients were treated with TMZ at P1 and P2 or left untreated. Tertiary recipients of both cohorts were killed 18 days after transplantation. The total number of bone marrow mononuclear cells (MNCs) were not significantly different in treated versus untreated mice (n = 3) (Figure 2A). However, the number of CFUs was 599 per 106 bone marrow MNCs in untreated mice, which was drastically reduced to 10 CFU per 106 bone marrow MNCs in treated mice (P < .0001, Fisher exact test) (n = 2 mice, untreated range, 549-648; treated range, 6-14) (Figure 2B). Thus, TMZ treatment of mice that received transplants of MSH2–/– marrow cells is associated with bone marrow failure. Such a decrease was not observed in wild-type treated mice (“Maintenance of selection advantage of MSH2–/– progenitors”).
Bone marrow failure in tertiary recipients. Lethally irradiated wild-type mice received transplants of 100% MSH2–/– cells (P1). Three weeks later mice were treated with TMZ (□) at 80 mg/kg daily times 3 or left untreated (▪). Bone marrow was harvested from P1 recipients and transplanted into P2 and P3 recipients at 8-week intervals. Mice were treated again at P2 with TMZ or left untreated. P3 recipients were killed 18 days after transplantation. Bone marrow mononuclear cells (A) were measured, and CFUs were assessed by methylcellulose assay (B). There was a significant decrease in total cells and CFUs recovered from the marrow of treated versus untreated mice; P < .0001 (Fisher exact test).
Bone marrow failure in tertiary recipients. Lethally irradiated wild-type mice received transplants of 100% MSH2–/– cells (P1). Three weeks later mice were treated with TMZ (□) at 80 mg/kg daily times 3 or left untreated (▪). Bone marrow was harvested from P1 recipients and transplanted into P2 and P3 recipients at 8-week intervals. Mice were treated again at P2 with TMZ or left untreated. P3 recipients were killed 18 days after transplantation. Bone marrow mononuclear cells (A) were measured, and CFUs were assessed by methylcellulose assay (B). There was a significant decrease in total cells and CFUs recovered from the marrow of treated versus untreated mice; P < .0001 (Fisher exact test).
Cell cycle status of MSH2–/– cells
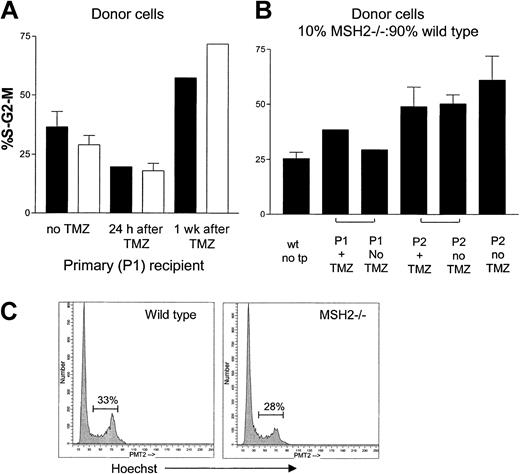
Because previous studies have suggested that mismatch repair–deficient tumor cells have a proliferative advantage in vitro, we asked whether the bone marrow failure we observed was due to exhaustion of quiescent cells resulting from an increased number of MSH2–/– cells in active cell cycle. To measure the percentage of cycling cells, we stained with Hoechst DNA dye no. 33342. For this analysis, we first used mice reconstituted with 100% wild-type or 100% MSH2–/– cells and measured the percentage of S-G2-M cells in the lineage-negative fraction of bone marrow mononuclear cells recovered from the marrow of mice receiving transplants. Analyses were done at 24 hours and 1 week after TMZ and in untreated mice at the same time points. We found no significant difference in the percentage of cells in S-G2-M between wild-type and MSH2–/– cells (Figure 3A,C). The percentage of S-G2-M cells in untreated mice was 36% in wild-type and 28.8% MSH2–/– mice. This percentage initially decreased to 19% and 18%, respectively, 24 hours after TMZ but then increased to more than 50% for both strains of mice at 1 week after transplantation We next measured the frequency of cycling cells in serial transplant recipient mice initially receiving transplants of 10% MSH2–/– and 90% wild-type cells compared with 100% wild-type cells. As expected, the percentage of lineage-negative cells in S-G2-M increased with successive transplantations, but there was no difference between treated and untreated mice that were repopulated with MSH2–/– or wild-type cells (Figure 3B). Thus, MSH2–/– cells do not have a shift in the distribution of cell cycle compared with wild-type cells after bone marrow transplantation.
Cell cycle distribution among lineage-negative cells recovered from P1 and P2 marrow transplant recipient mice. Bone marrow MNCs were stained with PE-conjugated lineage-specific antibodies (“Materials and methods”) followed by Hoechst dye. The percentage of lineage-negative cells in the S-G2-M phase of the cells cycle was identified. (A) P1 mice that received transplants of either 100% MSH2–/– (□) or 100% wild-type (▪) marrow. Three weeks after transplantation, mice were treated with TMZ or left untreated. Mice were killed at 24 hours or 1 week after treatment. (B) Marrow cells collected from P1 and P2 serial transplant recipients. P1 mice received transplants of a mixture of 10% MSH2–/– and 90% wild-type cells and were treated with TMZ or left untreated. Marrow cells were harvested from P1 and P2 recipient mice 8 weeks after transplantation. (C) Representative histograms comparing the percentage of S-G2-M populations from wild-type and MSH2–/– mice.
Cell cycle distribution among lineage-negative cells recovered from P1 and P2 marrow transplant recipient mice. Bone marrow MNCs were stained with PE-conjugated lineage-specific antibodies (“Materials and methods”) followed by Hoechst dye. The percentage of lineage-negative cells in the S-G2-M phase of the cells cycle was identified. (A) P1 mice that received transplants of either 100% MSH2–/– (□) or 100% wild-type (▪) marrow. Three weeks after transplantation, mice were treated with TMZ or left untreated. Mice were killed at 24 hours or 1 week after treatment. (B) Marrow cells collected from P1 and P2 serial transplant recipients. P1 mice received transplants of a mixture of 10% MSH2–/– and 90% wild-type cells and were treated with TMZ or left untreated. Marrow cells were harvested from P1 and P2 recipient mice 8 weeks after transplantation. (C) Representative histograms comparing the percentage of S-G2-M populations from wild-type and MSH2–/– mice.
Homing of MSH2–/– cells
To investigate whether the repopulating defect was due to abnormal homing of MSH2–/– progenitors, we directly compared bone marrow migration by transplanting 4 × 106 cells each of dye-labeled MSH2–/– and wild-type cells into the same recipient mouse. In one cohort of mice, MSH2–/– cells were labeled with the intracellular dye CFSE, and wild-type cells were labeled with the lipophilic membrane dye DiD. To control for staining artifact, the reverse-labeling scenario was also performed in a second cohort of mice. Forty-eight hours after transplantation of labeled cells homing was analyzed. By combining the results of both cohorts, the percentage of lin– MSH2–/– cells in the marrow was 1.97% ± 0.75% compared with 1.75% ± 0.47% wild-type cells (n = 4 mice; P = .23) and was independent of which dye was used. Additionally, day 12 CFU-Ss were enumerated in recipients of MSH2–/– or wild-type whole bone marrow transplants. Wild-type mice had 7.8 ± 1.0 colonies per spleen (n = 7) per 1 × 104 infused cells, whereas MSH2–/– mice had 8.8 ± 1.6 colonies per 1 × 104 infused cells (n = 8) (P = .1309). These data suggest that MSH2–/– cells have an equivalent homing efficiency to that of wild-type cells.
Genomic instability of hematopoietic progenitors
Microsatellite instability (MSI) represents alterations in length of simple repeat sequences and is a phenotype of mismatch repair–associated genetic instability. MSI is most frequently detected in tumor cells7 and other mitotically active cells.20 To determine whether hematopoietic progenitors had evidence of genomic instability, we analyzed 2 different informative microsatellite loci. These loci were selected from 4 loci evaluated during initial experiments and are representative of sites susceptible for detection of MSI in MSH2–/– mice.21 First, we measured the frequency of MSI in CFUs from wild-type and MSH2–/– murine bone marrow after treatment in vitro with 800 μM TMZ (IC50 [concentration that inhibits 50%]). No MSI was detected in wild-type CFUs (n = 45), but MSI was found in 5.4% (2 of 37) of the CFUs from MSH2–/–-treated marrow cells.
On the basis of this result, we then measured MSI in CFUs recovered from the marrow of mice that received transplants of either 100% wild-type or 100% MSH2–/– marrow cells. In CFUs recovered from mice that received transplants of wild-type marrow cells, no MSI was detected in CFUs from untreated primary (n = 26), TMZ-treated primary (n = 26) or TMZ-treated secondary recipients (n = 26). In mice receiving MSH2–/– marrow cells, MSI was also not detected in CFUs from untreated primary recipients (0 of 30). However, 2.9% (2 of 68) CFUs from secondary recipients had evidence of MSI. In addition, in mice receiving MSH2–/– cells that were then treated with TMZ, marrow CFUs recovered 6 weeks later had a very high MSI incidence of 10.6% (10 of 94) (P = .0152 compared with wild type, Fisher exact test) (Table 3 and Figure 4).
Microsatellite instability in CFUs from mice that received transplants of either wild-type or MSH2–/– bone marrow
. | Untreated primary recipient . | TMZ-treated primary recipient* . | TMZ-treated secondary recipient*† . | Untreated secondary recipient† . |
|---|---|---|---|---|
| Wild type | 0 of 26 | 0 of 24 | 0 of 26 | not done |
| MSH2-/- | 0 of 26 | 0 of 24 | 8 of 70 | 2 of 63 |
. | Untreated primary recipient . | TMZ-treated primary recipient* . | TMZ-treated secondary recipient*† . | Untreated secondary recipient† . |
|---|---|---|---|---|
| Wild type | 0 of 26 | 0 of 24 | 0 of 26 | not done |
| MSH2-/- | 0 of 26 | 0 of 24 | 8 of 70 | 2 of 63 |
Wild-type—treated versus MSH2-/-—treated recipients, P = .0152.
TMZ-treated secondary versus untreated secondary recipients, P = .09.
Microsatellite instability in CFUs isolated from mice receiving MSH2–/– bone marrow. Individual CFUs were harvested from mice receiving transplants of 100% MSH2–/– cells and subjected to PCR using a 33P-labeled primer specific for the informative locus D3mit 203. PCR products were electrophoresed on a 7% denaturing gel. The representative gel depicts characteristic microsatellite instability in 2 of 13 CFUs (lanes 3 [insertion] and 12 [deletion]) from secondary recipient mice. Similar evaluations of wild-type marrow from primary and secondary recipients failed to show evidence of microsatellite instability.
Microsatellite instability in CFUs isolated from mice receiving MSH2–/– bone marrow. Individual CFUs were harvested from mice receiving transplants of 100% MSH2–/– cells and subjected to PCR using a 33P-labeled primer specific for the informative locus D3mit 203. PCR products were electrophoresed on a 7% denaturing gel. The representative gel depicts characteristic microsatellite instability in 2 of 13 CFUs (lanes 3 [insertion] and 12 [deletion]) from secondary recipient mice. Similar evaluations of wild-type marrow from primary and secondary recipients failed to show evidence of microsatellite instability.
MSI at the early progenitor cell level was determined in Sca+Kit+lin– clones. As observed earlier, clones from untreated (n = 15) and TMZ-treated wild-type mice exhibited no MSI in primary (n = 17), secondary (n = 64), or tertiary (n = 26) recipients. However, mice that had received transplants of MSH2–/– marrow demonstrated 3% (2 of 64) MSI in clones from secondary recipients. These data suggest that bone marrow failure is associated with MSI acquired in vivo in MSH2–/– cells transplanted after TMZ exposure.
Telomere length
Because telomere shortening results in chromosomal instability and could, therefore, be associated with loss of the stem cell phenotype, we examined the relationship between mismatch repair and telomere maintenance. For these analyses, we used a sensitive flow cytometry FISH method in which an FITC-labeled peptide nucleic acid probe was hybridized to telomere repeat sequences. This method has been shown to correlate (r = 0.9) with the conventional Southern blot method and is advantageous because it allows analysis of different bone marrow subpopulations.19
We found no significant difference in telomere length of low forward and side scatter bone marrow mononuclear cells from wild-type or MSH2–/– mice not receiving transplants. Measured by flow cytometry FISH, the mean fluorescence intensity (MFI) was 6.5 ± 0.77 for wild-type compared with 7.2 ± 0.39 for MSH2–/– cells (n = 2 mice performed in triplicate) (Figure 5A). Furthermore, the average MFI of cells harvested from untreated secondary recipients with transplants of 100% MSH2–/– cells was 28.4 ± 4.5 (n = 3 mice performed in triplicate) compared with 27.9 ± 4.5 in 3 mice recipients of MSH2–/– cells that were then treated with TMZ (Figure 5B). It should be noted that comparisons were performed within each experiment, and the difference in MFI measurements between the 2 separate experiments is not indicative of differences in telomere length, but rather instrument variation. Interestingly, TMZ-treated MSH2–/– mice had a more heterogeneous telomere length distribution than untreated MSH2–/– in all mice tested (n = 4; P = .01, K-S test of data in Figure 5B). Thus, although telomere shortening is not observed in MSH2–/– marrow cells, there is a more subtle redistribution of telomere length after TMZ treatment
Representative histograms demonstrating telomere length in murine bone marrow MNCs. Telomere length was measured by hybridization of a FITC-labeled peptide nucleic acid probe to telomere repeat sequences in bone marrow MNCs from (A) wild-type and MSH2–/– mice not receiving transplants and (B) P2 mice recipients of serially transplanted 100% MSH2–/– cells in the presence or absence of TMZ treatment. In panel A, there was no significant difference in telomere length (mean fluorescence intensity) between wild-type and MSH2–/– mice. In panel B, the average mean fluorescence intensity was similar in treated and untreated mice receiving MSH2–/– cells; however, TMZ-treated mice had a more heterogeneous distribution of telomere length. In 3 mice tested, there was a significant increase in the breadth of telomere length distribution; P = .01 (Kolmogorov-Smirnov [K-S] test that calculates the difference between the summation of 2 curves). Absolute values of telomere length in panels A and B cannot be compared because samples were measured separately.
Representative histograms demonstrating telomere length in murine bone marrow MNCs. Telomere length was measured by hybridization of a FITC-labeled peptide nucleic acid probe to telomere repeat sequences in bone marrow MNCs from (A) wild-type and MSH2–/– mice not receiving transplants and (B) P2 mice recipients of serially transplanted 100% MSH2–/– cells in the presence or absence of TMZ treatment. In panel A, there was no significant difference in telomere length (mean fluorescence intensity) between wild-type and MSH2–/– mice. In panel B, the average mean fluorescence intensity was similar in treated and untreated mice receiving MSH2–/– cells; however, TMZ-treated mice had a more heterogeneous distribution of telomere length. In 3 mice tested, there was a significant increase in the breadth of telomere length distribution; P = .01 (Kolmogorov-Smirnov [K-S] test that calculates the difference between the summation of 2 curves). Absolute values of telomere length in panels A and B cannot be compared because samples were measured separately.
Discussion
These data indicate that mismatch repair–deficient hematopoietic stem cells acquire microsatellite instability after methylating agent exposure and lose repopulating capacity during the stem cell proliferative stress of sequential transplantation. Because we found no clear difference between wild-type and MSH2–/– progenitor cells in cell cycle distribution, homing, or telomere length, we conclude that the stem cell defect is directly attributable to loss of mismatch repair. Thus, long-term stem cell maintenance requires 3 features of mismatch repair: (1) the ability to accurately replicate DNA, (2) the ability to restore DNA to normal after DNA damage, and (3) the ability to remove replication defects in areas of small nucleotide repeats.
A striking observation in our studies was the degree of resistance to methylating agents among MSH2–/– hematopoietic progenitor cells coupled to the finding that the surviving progenitors were at a repopulating disadvantage compared with normal cells. There appears to be 2 DNA repair pathways involved in methylating agent resistance: (1) O6-methylguanine-DNA methyltransferase (MGMT), which removes the methyl adduct from O6-methylguanine after methylating agent exposure, and (2) mismatch repair that attempts repair of the same lesion, generating an aberrant repair process that induces apoptosis signals and cell death. We previously showed that MGMT–/– mice died of marrow failure following methylating agent exposure but could be rescued by transplantation with wild-type marrow cells, despite the absence of MGMT in all other organs.22 This finding indicated that hematopoietic stem cells are very sensitive to methylating agents and rely on MGMT for protection and that marrow is the organ that determines overall sensitivity to methylating agents, not the intestinal mucosa, pulmonary tree, or liver. In contrast, mice defective in any of the mismatch repair proteins are tolerant to methylating agents. Even MLH1/MGMT double knock-out mice, that retain high levels of O6-methylguanine DNA adducts, are resistant to the toxicity of methylating agents.23 These data suggested that the O6-methylguanine lesion is responsible for acute marrow failure after methylating agents because of apoptosis of hematopoietic progenitors. This lesion is also responsible for lymphomatous transformation because overexpression of MGMT in the thymus protects mice from this malignancy.24
The acute drug resistance of MSH2–/– hematopoietic progenitor cells that we observed predicted that there would be a survival advantage for mismatch repair–defective stem cells after methylating agent exposure and transplantation. Instead, we observed that both treated and untreated hematopoietic MSH2–/– cells were at a competitive disadvantage during the repopulating stress of serial transplantation. These cells were lost during cotransplantation with wild-type cells using a direct competitive repopulation model prior to drug treatment, the most efficient way to insure equivalent drug exposure. Although this defect is not noted in primary recipients, it is clearly evident after the stress of serial transplantation.
There are other examples of stem cell failure associated with defects in proliferation signals and DNA repair. Fanconi anemia progenitors are sensitive to DNA damage, and mice die with marrow failure even though other tissues are preserved.25 P27 kip1–/– hematopoietic stem cells have a repopulating advantage and have evidence of increased cells in G0.26 In contrast to the effect of p27 kip1–/–, p21 cip1/waf1–/– hematopoietic stem cells have a repopulating defect and are more sensitive to cytotoxic agents associated with increased cell cycle progression and loss of G0 cells.27 However, we were unable to detect a difference between wild-type and MSH2–/– early progenitors in cell cycle during serial transplantation or drug treatment in the MSH2–/– marrow progenitors. Since Allsopp et al28 reported shortening of telomeres during serial transplantation in hematopoietic stem cells, we asked whether there was a difference in telomeres from MSH2–/– secondary transplant recipients either treated with TMZ or untreated. We did not observe a significant difference in telomere length between TMZ-treated or untreated MSH2–/– cells. Nor did we see a difference in wild-type or MSH2–/– mice not receiving transplants. Therefore, telomere shortening is not an explanation for the stem cell exhaustion that we observed.
The central observation of these studies is that hematopoietic progenitors from MSH2–/– mice accumulate microsatellite instability during serial transplantation after methylating agent exposure. Although it is commonly stated that mice defective in mismatch repair have microsatellite instability, most of these observations come from clonal outgrowth, either from tumors17,29,30 or fibroblast cells lines in culture.21,31 In MSH2 knock-out mice, stimulated B cells in Peyer patches have increased rates of microsatellite instability that precludes proper secondary hypermutation during the generation of high-affinity IgG antibodies.32 Mutation frequencies of up to 2 × 10–4 have been observed in splenic T cells from PMS2–/– mice.31 Our finding that microsatellite instability could be detected in more than 1% of CFUs and Sca+Kit+lin––derived clones from MSH2–/– mice and that this frequency increased with serial transplantation and TMZ treatment indicates that early progenitors have developed evidence of genomic instability after only a few divisions in vivo. The frequencies of microsatellite instability we observed (up to 10.6%) are 10 to 100 times higher than those reported with T cells.31 Because the clones were analyzed after 5 to 6 divisions in culture from the single quiescent cell, replication errors occur early in the expansion of the affected stem cells, at the time of the burst of proliferation during hematopoietic reconstitution.
Thus, the question moves to what effect microsatellite instability may have on stem cells. A recent study identified a series of DNA repair and checkpoint genes with high levels of microsatellite instability in endometrial cancers,33 suggesting that alterations in these genes could be responsible for altered signal pathways and cell cycle regulation and ultimately in loss of the stem cell phenotype. How stem cells die or lose essential stem cell function is not clear but is likely to be due to loss of gene function; altered gene regulation such as loss of the strictly regulated expression of cytokine receptors, signaling molecules, and transcription factors; or loss of cell cycle regulation. As a result, stem cells may simply differentiate in the presence of microsatellite instability. It is tempting to postulate that similar events as a result of defects in DNA repair take place in other marrow failure syndromes such as the myelodysplastic syndromes.
Although a defect in hematopoiesis is not identified in first-generation mismatch repair–deficient mice, there is evidence that hematopoiesis is not normal because these mice develop spontaneous T-cell lymphomas and are more sensitive to methylating agent–induced lymphomas as well.4,17,30,34,35 We have also seen lymphomas in healthy mice receiving transplants of MMR-defective marrow cells (L.L. and S.L.G., unpublished observations, April 1999), although none were observed in the serial transplantations reported here. There have also been reports of human leukemias and lymphomas with defects in mismatch repair. Most human cases have identified acquired mismatch repair deficiency in either primary, secondary, or relapsed hematologic malignancies, but the causative relationship is not clear.10-15,36 However, there are also 6 reported cases of childhood leukemias arising in patients homozygous for either MSH2, MLH1, or PMS2 loss of function mutations.16
Thus, it appears that mismatch repair deficiency results in both a stem cell failure syndrome and increased propensity for malignant transformation. It is even possible that these 2 conditions are linked. Other human leukemias arise in the setting of stem cell loss. Fanconi anemia, paroxysmal nocturnal hemaglobinurea (PNH), and myelodysplastic syndromes are associated with stem cell loss and emergence of malignant stem cell–derived leukemic clones.25 In these diseases, the residual stem cells compete poorly until they emerge with an acquired selective growth advantage leading to malignant transformation. We recently found that the PigA+, presumed normal, CFU in marrow samples of patients with PNH was defective in proliferation compared with the PNH PigA– cells.37 Other stem and progenitor cells of MMR-defective mice have evidence of a proliferation defect and loss of function. Intestinal crypt stem cells of MMR-deficient mice have a defect in cell cycle and altered growth rates.38,39 In addition, a defect in meiosis leads to loss of spermatocytes and sterility in PMS2-30 and MLH1-deficient40,41 mice. Thus, there is ample suggestion that mismatch repair deficiency results in altered growth and survival of stem cells of multiple organ lineages. An increased mutation rate may give rise to stem cell failure, a proliferative advantage, and malignant transformation.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-10-3035.
Supported in part by Public Health Service grants RO1CA63193, RO1CA73062, RO1ES06288, and P30CA43703.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tak Mak of the Amgen Research Institute at University of Toronto for the MSH2 knock-out mice and Mike Sramkoski from the Cancer Center Flow Cytometry Facility for technical assistance.

![Figure 4. Microsatellite instability in CFUs isolated from mice receiving MSH2–/– bone marrow. Individual CFUs were harvested from mice receiving transplants of 100% MSH2–/– cells and subjected to PCR using a 33P-labeled primer specific for the informative locus D3mit 203. PCR products were electrophoresed on a 7% denaturing gel. The representative gel depicts characteristic microsatellite instability in 2 of 13 CFUs (lanes 3 [insertion] and 12 [deletion]) from secondary recipient mice. Similar evaluations of wild-type marrow from primary and secondary recipients failed to show evidence of microsatellite instability.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2002-10-3035/6/m_h81734841004.jpeg?Expires=1769114170&Signature=OoyfepkoKhHf6BR3u8jqKfRBeeu-2KxvyRA90YOwAVN8VBn13U9Z43PweoJIeu-ggfcBRogISU3gGu7mbnDRyO2yinthcKuckpw-Cg7ylghwRTZV0t1GUSmhihbxIZZzh-NHde6rpDhFBHtEnB8w2mkDAMdANSTpQUDl7yKy2aH0gCNKgKCNAQ~GeHE0xNrtEGwtzEhcXTflhTZsVI~I7Ze~M7RiH7dy9E~KRl88irk7ZMr6IwV1iY4lSePRzV027ZnbrHeDkhv1NakZV0TgUirprEltdUfwwGv7A~zCxKvBro0VqlRE7itq-e14I15p7JKbEeuQkPQMovjW7JGyDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Representative histograms demonstrating telomere length in murine bone marrow MNCs. Telomere length was measured by hybridization of a FITC-labeled peptide nucleic acid probe to telomere repeat sequences in bone marrow MNCs from (A) wild-type and MSH2–/– mice not receiving transplants and (B) P2 mice recipients of serially transplanted 100% MSH2–/– cells in the presence or absence of TMZ treatment. In panel A, there was no significant difference in telomere length (mean fluorescence intensity) between wild-type and MSH2–/– mice. In panel B, the average mean fluorescence intensity was similar in treated and untreated mice receiving MSH2–/– cells; however, TMZ-treated mice had a more heterogeneous distribution of telomere length. In 3 mice tested, there was a significant increase in the breadth of telomere length distribution; P = .01 (Kolmogorov-Smirnov [K-S] test that calculates the difference between the summation of 2 curves). Absolute values of telomere length in panels A and B cannot be compared because samples were measured separately.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2002-10-3035/6/m_h81734841005.jpeg?Expires=1769114170&Signature=4jZITeBU4kyONs83C7aYeAx6K0Fl7RXOh-aIWT-L62-UqNiJjU1pbRohPn8~nPSzOVTFqU-UW3TC2khfFOgrWlyK0sRf~HBjRppVuWHtW47fVkYq0d2GYUI0-Zf~9pzRtpxGbNI4nbAc7JY-Wg9gLh0u4976fy2~0crP5~4q5n48bluodcM43jdc-21WKwMJdPriqLg1UYiQKtlUgSM6ctZq1pQWuARldL1iWXugSxtvUx8WATsl6wndcTYQm2Dz0XsByFV2ngGEXrJRSmzzLbiDOjQpBPgK8uLEcrGUI3VgWOOF~Sdi38PGeEFDRHrm4mh2u3KS70FAjlpQqzHp0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal