Abstract
The most abundant intracellular proteins, heat shock proteins (HSPs), serve as molecular chaperones for regulatory and maturation pathways. Diverse families of HSPs have been shown to bind antigenic peptides and to play major roles in innate and adaptive immune responses through the common HSP receptor, CD91. HIV-1+ patients with Kaposi sarcoma (KS) were matched for CD4 count and HIV-1 RNA viral load to HIV-1+ patients without Kaposi sarcoma (and negative for Kaposisarcoma–associated herpesvirus). We then investigated the pathways used by tumor lysates, viral lysates, and viral particles in their activation. In particular, we observed immune responses after HSP depletion using antitumor antibiotics and blockade of the common HSP receptor, CD91. Despite the impaired functional capacity of dendritic cells (DCs) derived from patients with KS, DCs retain the ability to prime the adaptive arm of the immune system through the common HSP receptor, leading to phenotypic activation and stimulation of tetramer-positive CD8+ cytotoxic T cells. We also show that interferon-producing plasmacytoid DCs are selectively depleted in KS-positive compared with matched KS-negative HIV-1–infected patients. Functionally impaired DCs can effectively cross-present immune responses through the common HSP receptor. These results have important implications for the etiopathogenesis of KS and for the development and design of any compounds, including vaccines, derived from cellular lysates.
Introduction
The pivotal role played by dendritic cells (DCs) in antigen detection and T-cell activation makes them an attractive adjuvant for use in cancer vaccines1-3 and for therapies that target the latent reservoir of HIV.4-6 Typically, DCs can present exogenous antigens on major histocompatibility complex (MHC) class 2 molecules and endogenously synthesized antigens on MHC class 1.7 DCs can also take up exogenous peptides chaperoned by heat shock proteins (HSPs) and re-present them through the classical proteosome/transporter-associated antigen processing (TAP)–dependent endogenous pathway complexed with their MHC class 1 molecules.8
The basis for the immunologic capabilities of HSPs is thought to reflect 2 intrinsic properties. First, they act as general adjuvants to the innate immune system, promoting maturation and activation of DCs.9,10 Second, they are able to deliver nonnative bound peptides to antigen-presenting cells such as DCs to yield peptide-specific T-cell stimulation.11 The high efficiency observed here has been attributed in many cases to direct binding to and internalization through the CD91 molecule (also called α2-macroglobulin receptor or the low-density lipoprotein-related protein), which has been identified as a common receptor for the HSPs hsp70, hsp90 (the 2 most abundant HSPs), gp96, hsp110, and calreticulin.8,12 Subsequent activation of DCs is thought to lead to migration to lymphoid tissues with the loss of CD1a expression and the up-regulation of MHC and the costimulatory molecules CD40, CD80, CD83, and CD86.13 HSP cannot, however, directly activate T cells, and it is able to elicit T-cell responses only in the presence of antigen-presenting cells, usually DCs.14 Despite this, immunization with autologous HSPs isolated from cancer cells or virally infected cells elicits protective cellular immune responses in mice,15 a strategy that also appears safe and feasible in humans.16
Although HSPs are extremely abundant, particularly at times of cellular stress, extremely small quantities of HSP-peptide complexes are required to elicit CD8+ cytotoxic T lymphocyte (CTL) responses in vivo.17,18 Such immunization, however, is sensitive to the abrogation of DCs in the host19,20 (and also to the inhibition of HSP activity). At least 2 distinct human DC subsets, myeloid DCs (MDCs) and plasmacytoid DCs (PDCs, also known as interferon-producing cells), have been characterized based on their expression of the β-integrin CD11c.21,22 Given the efficiency of DCs in stimulating T-cell–dependent immune responses, we investigated whether alterations in DC subset number, primary DC function, or both could be a factor in the development of T-cell function impairment in patients with AIDS-related Kaposi sarcoma (KS).
Manipulating the buffering capacity of HSPs by mutation or pharmacologic blockade23-25 has provided an invaluable tool for elucidating the role of these ancient, conserved molecules and has even suggested a role in adaptive evolution.26,27 The specific range of client proteins to which HSPs bind allowed us an opportunity to inhibit the function of certain HSPs and to investigate subsequent immune activation in DCs obtained from patients with and without KS. Monocyte-derived DCs, known to express receptors that bind and internalize common HSPs,28 were used as a model of primary DC function, and pulsed DCs were used to stimulate secondary T-cell responses. Because it has been suggested that various cell surface proteins on cells of the innate immune system bind HSPs and play a role in the induction of cellular responses, we specifically studied the role of the common HSP receptor CD91 in the responses of allogeneic tumor and viral-associated antigens not traditionally thought to use HSP-peptide complexes in the priming of DCs. The results presented establish a role for the innate immune system and for HSP-related pathways in the etiopathogenesis of KS.
Patients, materials, and methods
Patients and generation of DCs
HIV-seropositive patients receiving highly active antiretroviral therapy (HAART) and with KS (n = 12) were matched to HIV-seropositive patients without KS (control group, n = 6). Patients were matched on a 2:1 ratio based on CD4 count (within 50 cells/mm3) and HIV viral load (within 0.5 log10). The first 6 KS-negative (and KS herpesvirus [KSHV]–negative) consecutive patients attending during the same period who satisfied these criteria and who agreed to participate were enrolled. Because of the requirement for large (60 mL on each occasion), repetitive, and fresh blood draws, we selected 1 control subject to 2 KS patients; not all the experiments were performed on all the patients (described in figure legends). Patients receiving chemotherapy or interferon were excluded. All patients were enrolled between January and August 2002, the study received appropriate ethical approval, and all volunteers were from the Chelsea and Westminster Hospital, London. Of patients offered participation in the study, 6 refused. Informed consent was provided according to the Declaration of Helsinki.
Blood was processed within 2 hours of drawing, no freezing occurred, and all reagents were selected for their low levels of endotoxin contamination. Peripheral blood mononuclear cells (PBMCs) were separated on a Ficoll-Histopaque density gradient and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), l-glutamine, and antibiotics. PBMCs used for DC subset separation or for production of monocyte-derived DCs were further separated by centrifugation over 50% Percoll.
Monocyte-derived DCs were propagated from PBMCs using a previously described protocol.29 Briefly, PBMCs were incubated on gelatin-coated flasks for 2 hours at 37°C, nonadherent cells were removed, and fresh medium was supplemented with 800 U/mL recombinant human granulocyte macrophage–colony-stimulating factor (rhGM-CSF), and 800 U/mL recombinant human interleukin-4 (rhIL-4) was added. Cells were incubated for 7 days at 37°C in 5% CO2. Half the volume of the medium was removed on alternate days and was replaced with fresh medium supplemented with the GM-CSF and IL-4.
Quantitation of DCs, phenotypic analysis, and tetramers
Three-color flow cytometric analysis was performed on 5 × 105 PBMCs labeled with phycoerythrin (PE)–conjugated anti-CD3, -CD14, -CD16 (all from PharMingen, Oxford, United Kingdom), -CD19, PercP-conjugated anti–HLA-DR (BD Biosciences, Oxford, United Kingdom), and fluorescein isothiocyanate (FITC)–conjugated anti-CD11c (DAKO, Ely, United Kingdom). At least 100 000 cells were acquired in the live gate and were analyzed on a Becton Dickinson FACScalibur using CellQuest software. DCs were defined as HLA-DR+, lineage cocktail negative (CD3–, CD14–, CD16–, CD19–)–negative, and CD11c+ (myeloid) or CD11c– (plasmacytoid). The concentration of DCs in blood was estimated by using the percentages generated by fluorescence-activated cell sorter (FACS) analysis, original PBMC count, and volume of blood collected. Phenotypic analysis of monocyte-derived DCs was performed using 5 × 105 cells stained with FITC-conjugated anti-CD1a and anti-CD80, PE-conjugated anti-CD83, PercP-conjugated anti–HLA-DR, antigen-presenting cell (APC)–conjugated anti-CD40 (all from PharMingen), and FITC-conjugated anti-CD91 (Biomac, Leipzig, Germany). Positive staining for each marker was determined by comparison with appropriate isotype-matched negative controls and unstimulated samples. Labeling with 5-(and 6-) carboxyfluorescein diacetate succimidyl ester (CFSE; Molecular Probes, Eugene, OR) occurred by suspending a final concentration of 10 μM with 2 × 107 PBMCs/mL. After incubation at 37°C for 10 minutes, the staining reaction was quenched by the addition of a large volume of ice-cold phosphate-buffered saline followed by 2 washes in the same medium. Analysis of cell division was performed after 5-day mixed-lymphocyte reactions (MLRs), as described.30,31
An HLA-A*201–specific tetramer containing the KSHV K12 peptide sequence LLNGWRWRL (Proimmune, Oxford, United Kingdom) was performed using staining with PE-conjugated anti-CD8 (BD Biosciences). KSHV-negative patients and patients lacking HLA-A2 were used as controls and showed no tetramer-positive staining.
HLA class 1 and 2 typing were performed by the Anthony Nolan Trust (Royal Free Hospital) using amplification refractory mutation system polymerase chain reaction (PCR) with sequence-specific primers.32 Plasma HIV-1 viral loads were determined by the Bayer HIV-1 RNA 3.0 (bDNA) Assay (Berkshire, United Kingdom) or by PCR assay (Cobas Amplicor HIV-1 Monitor test version 1.5; Roche Diagnostics, United Kingdom) with a lower level of detection of 50 HIV-1 RNA copies per milliliter. Absolute CD4 and CD8 counts (cells/mm3) were obtained by flow cytometry (Beckman Coulter, Marseille, France).
Pulsing of monocyte-derived DCs, CD91 blocking, and HSP depletion
Day 6 DCs were cultured overnight with antigens. Tumor lysate was prepared from freshly excised KS biopsy samples obtained from patients participating in this study, and it was prepared as described using liquid nitrogen to room temperature freeze-thaw cycles.33,34 UV-inactivated KSHV and Epstein-Barr virus (EBV)–purified viral particles were added at a final concentration of 2 × 109 vp/mL, and KSHV viral lysate was added giving a final protein concentration of 0.03 mg/mL (all from Advanced Biotechnologies, Columbia, MD). Addition of tumor necrosis factor-α (TNF-α; Sigma, St Louis, MO) at 10 ng/mL occurred when described, 8 hours after pulsing with antigen. Anti–CD91 α-chain monoclonal antibodies (BioMac) were incubated with DCs for 30 minutes at 4°C before pulsing with antigens. Azide-free anti-CD3 (an immunoglobulin G1 [IgG1] molecule as for anti-CD91; produced from an in-house hybridoma) was used as an isotype control. All DCs were washed twice before syngeneic leukocyte reaction (SLR) on day 7 (using 2 × 105/well fresh autologous PBMCs as responders). This was performed using DCs, as described in “Mixed lymphocyte reaction” below. Background wells included DCs with autologous PBMCs, DCs with anti-CD91 antibody and autologous PBMCs, DCs with TNF-α, DCs with radicicol, and PBMCs alone. CD8 depletion experiments used CD8 Dynabeads. The anti-HSP antibiotic radicicol (monorden; Sigma), which binds and inhibits the adenosine triphosphate/adenosine diphosphate (ATP/ADP) domain of Hsp90 with nanomolar affinity (thereby inhibiting its activity), was added to SLRs at a final concentration of 0.25 μg/mL.24,26
Mixed lymphocyte reaction
MLR was performed as described35 following the same procedure used for SLR. Fresh PBMCs were isolated from healthy HIV-seronegative control subjects or from patients for use as responders, and these were cultured in triplicate at 105 cells/well for 5 days with a maximum of 1 × 104 stimulator cells/well (irradiated monocyte-derived DCs) in 96-well, round-bottomed plates in 200 μL medium. Five doubling dilutions of stimulators were made, and cultures were pulsed with [3H]thymidine (1 μCi [0.037 MBq]/well; Amersham, Freiberg, Germany) for 16 hours. Nuclei were harvested onto fiberglass filter paper (Packard Instrument, Meriden, CT), and radioactivity in the filter paper was quantified as counts per minute (cpm) with the use of a direct beta counter 9600 (Packard Instrument).
Results were expressed as stimulation index (SI), determined as mean cpm in cultures containing responding and stimulator cells divided by mean cpm in culture-containing responder cells alone, or as δ-cpm, determined as mean cpm minus the appropriate background (ie, PBMC alone, DC with autologous PBMC, DC with TNF-α, or CD91 and autologous DC).
Statistical analysis
Comparisons of DC subset numbers between patients with and without KS were assessed using the Wilcoxon test. Correlations between CD4/CD8 count and DC number were undertaken using Spearman rank correlation coefficient, separately for the patients with and without KS. For allogeneic stimulatory capacity, for each number of stimulating DCs between patients with KS and matched controls, comparisons were made using the Wilcoxon test. Because it was deemed necessary to perform comparisons at each stimulator number, P values presented have been adjusted using Bonferroni correction. We did not perform statistical analyses on phenotypic comparisons of monocyte-derived DCs before and after tumor lysate because of low sample size (n = 3, matched subjects and controls). All P values presented are 2 sided.
Results
Depletion of plasmacytoid in patients with KS
We present a study of 18 HIV-1–infected, antiretroviral-experienced patients, 12 of whom have active KS, which remains the commonest neoplasm in HIV-infected patients, particularly in sub-Saharan Africa.36,37 Although the overall decline in the incidence of KS in the West can be attributed to the use of HAART,38-41 we now observe KS developing in patients on treatment with an HIV-1 RNA viral load below the conventional limit of detection.
Table 1 shows the immunologic and virologic parameters in the patients in the present study. After PBMC isolation, we quantitated human DC subsets (myeloid [MDC]; plasmacytoid [PDC]) in our patients by multiparametric flow cytometry based on their expression of the β-integrin CD11c21,22 (MDCs are CD11c+ and PDCs are CD11c–). Previous studies have shown that MDC and PDC are reduced in HIV infection,43,44 but we now find that PDCs are more severely and selectively depleted in patients with KS than in matched HIV-1+ controls without KS (Table 1).
Characteristics for 18 HIV-1—infected patients
. | CD4 cells/mm3 . | CD8 cells/mm3 . | . | . | . | DCs in PBMCs, % . | . | No. DCs/mL blood . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | . | HIV VL copies/mL . | HAART . | ACTG stage . | MDC . | PDC . | MDC . | PDC . | ||
| Patients with KS | |||||||||||
| 1 | 576 | 1146 | < 50 | 3TC, aba, lop, ten | T0I0 | 0.33 | 0.08 | 2438 | 600 | ||
| 2 | 484 | 1449 | < 50 | 3TC, ind, D4T | T0I0 | 0.23 | 0.01 | 2901 | 139 | ||
| 3 | 392 | 756 | < 50 | rit, ind, AZT, 3TC | T0I0 | 0.21 | 0.01 | 1608 | 112 | ||
| 4 | 363 | 663 | < 50 | 3TC, aba, nev | T0I0 | 0.31 | 0.04 | 1248 | 164 | ||
| 5 | 147 | 705 | < 50 | comb, nev | T1I1 | 0.28 | 0.03 | 1175 | 137 | ||
| 6 | 169 | 406 | < 50 | comb, efa | T0I1 | 0.24 | 0.05 | 1673 | 357 | ||
| 7 | 104 | 317 | 374 490 | AZT, 3TC, efa | T1I0 | 0.15 | 0.01 | 980 | 67 | ||
| 8 | 115 | 1273 | 450 614 | AZT, 3TC, efa | T1I0 | 0.17 | 0.02 | 717 | 100 | ||
| 9 | 128 | 251 | 5 311 | Trizivir, efa | T1I0 | 0.3 | 0.02 | 602 | 42 | ||
| 10 | 132 | 617 | < 50 | lop, ten, DDI | T1I0 | 0.16 | 0.07 | 80 | 34 | ||
| 11 | 28 | 329 | < 50 | comb, efa | T1I0 | 0.16 | 0.02 | 161 | 20 | ||
| 12 | 52 | 188 | < 50 | Trizivir, efa | T1I0 | — | — | — | |||
| Patients without KS | |||||||||||
| 13 | 526 | 930 | < 50 | Trizivir | NA | 0.22 | 0.19 | 1980 | 1760 | ||
| 14 | 364 | 369 | < 50 | comb, efa | NA | 0.26 | 0.25 | 1813 | 1729 | ||
| 15 | 168 | 274 | < 50 | comb, rit, ind | NA | 0.29 | 0.27 | 1698 | 1581 | ||
| 16 | 126 | 758 | 176 555 | D4T, 3TC, aba | NA | 0.28 | 0.19 | 1321 | 882 | ||
| 17 | 148 | 236 | 3 658 | comb, efa | NA | 0.29 | 0.25 | 488 | 417 | ||
| 18 | 64 | 197 | < 50 | DDI, 3TC, efa | NA | 0.18 | 0.22 | 175 | 221 | ||
. | CD4 cells/mm3 . | CD8 cells/mm3 . | . | . | . | DCs in PBMCs, % . | . | No. DCs/mL blood . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | . | HIV VL copies/mL . | HAART . | ACTG stage . | MDC . | PDC . | MDC . | PDC . | ||
| Patients with KS | |||||||||||
| 1 | 576 | 1146 | < 50 | 3TC, aba, lop, ten | T0I0 | 0.33 | 0.08 | 2438 | 600 | ||
| 2 | 484 | 1449 | < 50 | 3TC, ind, D4T | T0I0 | 0.23 | 0.01 | 2901 | 139 | ||
| 3 | 392 | 756 | < 50 | rit, ind, AZT, 3TC | T0I0 | 0.21 | 0.01 | 1608 | 112 | ||
| 4 | 363 | 663 | < 50 | 3TC, aba, nev | T0I0 | 0.31 | 0.04 | 1248 | 164 | ||
| 5 | 147 | 705 | < 50 | comb, nev | T1I1 | 0.28 | 0.03 | 1175 | 137 | ||
| 6 | 169 | 406 | < 50 | comb, efa | T0I1 | 0.24 | 0.05 | 1673 | 357 | ||
| 7 | 104 | 317 | 374 490 | AZT, 3TC, efa | T1I0 | 0.15 | 0.01 | 980 | 67 | ||
| 8 | 115 | 1273 | 450 614 | AZT, 3TC, efa | T1I0 | 0.17 | 0.02 | 717 | 100 | ||
| 9 | 128 | 251 | 5 311 | Trizivir, efa | T1I0 | 0.3 | 0.02 | 602 | 42 | ||
| 10 | 132 | 617 | < 50 | lop, ten, DDI | T1I0 | 0.16 | 0.07 | 80 | 34 | ||
| 11 | 28 | 329 | < 50 | comb, efa | T1I0 | 0.16 | 0.02 | 161 | 20 | ||
| 12 | 52 | 188 | < 50 | Trizivir, efa | T1I0 | — | — | — | |||
| Patients without KS | |||||||||||
| 13 | 526 | 930 | < 50 | Trizivir | NA | 0.22 | 0.19 | 1980 | 1760 | ||
| 14 | 364 | 369 | < 50 | comb, efa | NA | 0.26 | 0.25 | 1813 | 1729 | ||
| 15 | 168 | 274 | < 50 | comb, rit, ind | NA | 0.29 | 0.27 | 1698 | 1581 | ||
| 16 | 126 | 758 | 176 555 | D4T, 3TC, aba | NA | 0.28 | 0.19 | 1321 | 882 | ||
| 17 | 148 | 236 | 3 658 | comb, efa | NA | 0.29 | 0.25 | 488 | 417 | ||
| 18 | 64 | 197 | < 50 | DDI, 3TC, efa | NA | 0.18 | 0.22 | 175 | 221 | ||
Twelve patients had histologically confirmed active KS; 6 HIV-1—infected patients were matched to these for CD4 count (within 50 cells/mm3) and HIV-1 RNA viral load (within 0.5 Log10) such that patients 1 and 2 (KS negative) were matched to patient 13 (KS positive), patients 3 and 4 were matched to patient 14, and so on. All patients were male, aged 25 to 40 years. The 6 patients without KS were confirmed as KSHV negative by PCR to 2 sections of the unique KSHV gene K1. The percentage and numbers of myeloid (CD11c+) and plasmacytoid (CD11c-) DCs are shown. Antiretrovirals include abacavir (aba), combivir (comb), efavirenz (efa), indinivir (ind), lopinivir (lop), nevirapine (nev), and ritonavir (rit). Median length of time on HAART was 3 years (range, 1-7 years). There were no statistically significant differences between the 2 groups for the median length of time on HAART, number of previous regimens, or CD8 counts (none of these criteria were included in the matching of KS-positive to -negative patients). DC subsets were not counted for 1 patient with KS. ACTG indicates AIDS Clinical Trials Group Oncology Committee,42 where (briefly) tumor status (0 indicates cutaneous only; 1, more than cutaneous disease) and immunologic status (0 indicates a CD4 count < 150; 1, CD4 count > 150 cells/mm3) are used to stage KS; NA indicates not applicable.
The median MDC for the KS-positive group was 1175/mL blood (interquartile range, 660-1641/mL) compared with 1509/mL (696-1784/mL) for the KS-negative group. The median PDC for the KS-positive group measured 112/mL (55-112/mL) compared with 1232/mL (533-1692/mL) for the KS-negative group. Both the number and the percentage of PDCs were significantly decreased in patients with KS compared with matched controls with CD4 and HIV-1 viral load confirmed by PCR as negative for Kaposi sarcoma–associated herpesvirus (KSHV) (P = .009 for absolute numbers; P = .005 for percentage of PDC; Wilcoxon). MDC numbers in KS patients, though reduced compared with those in uninfected controls, were not significantly different from those in KS-negative, HIV-infected patients (P = .657 for numbers; P = .554 for percentages).
There were significant correlations between CD4 count and MDC (KS and non-KS groups) and CD4 count and PDC (non-KS group). For the non-KS group, CD8 count correlated significantly with absolute numbers of MDC and PDC. The correlations were assessed separately for matched patients and controls (Table 2).
Spearman rank correlation coefficients and 95% Cl for the absolute numbers of MDCs and PDC (mL blood) versus absolute CD4 or CD8 counts (cells/mm3)
. | P (95% Cl) . |
|---|---|
| CD4 correlations | |
| KS group | |
| CD4 vs MDC | .92 (.71-.98)* |
| CD4 vs PDC | .56 (-.05-.87) |
| Non-KS group | |
| CD4 vs MDC | .94 (.56-.99)* |
| CD4 vs PDC | .94 (.56-1.00)* |
| CD8 correlations | |
| KS group | |
| CD8 vs MDC | .573 (-.04-.87) |
| CD8 vs PDC | .509 (-.13-.85) |
| Non-KS group | |
| CD8 vs MDC | .829 (.0519-.98)* |
| CD8 vs PDC | .829 (.0519-.98)* |
. | P (95% Cl) . |
|---|---|
| CD4 correlations | |
| KS group | |
| CD4 vs MDC | .92 (.71-.98)* |
| CD4 vs PDC | .56 (-.05-.87) |
| Non-KS group | |
| CD4 vs MDC | .94 (.56-.99)* |
| CD4 vs PDC | .94 (.56-1.00)* |
| CD8 correlations | |
| KS group | |
| CD8 vs MDC | .573 (-.04-.87) |
| CD8 vs PDC | .509 (-.13-.85) |
| Non-KS group | |
| CD8 vs MDC | .829 (.0519-.98)* |
| CD8 vs PDC | .829 (.0519-.98)* |
Correlations were assessed separately for each of the matched KS patients and controls. For one KS patient, PDC and MDC numbers were unavailable.
Significant at P < .05.
After the demonstration that PDCs are quantitatively reduced in patients with KS, we investigated whether DC from KS patients are functionally impaired (ie, qualitatively different).
Monocyte-derived DCs from KS patients are functionally impaired
Because of the difficulty in performing functional studies on the low numbers of DCs obtained by magnetic bead selection and the finding that HSPs share a common receptor that is expressed on human monocyte-derived DCs,28 we used in vitro–generated DCs as a model of DC function for further study. These were derived by culturing blood monocytes with GM-CSF and IL-4 for 6 to 7 days.29 To assess DC function in patients with KS compared with controls, we observed their potentials for stimulating the proliferation of allogeneic T cells obtained from 3 healthy HIV-seronegative, HLA-mismatched laboratory controls. We ensured that patients were negative for hepatitis C antibody because it has been previously shown that DCs recovered from patients with hepatitis C infection have impaired allostimulatory capacity.45,46
Five doubling dilutions of ex vivo monocyte-derived DCs, commencing with 10 000 stimulators, were used. In allogeneic MLR, DCs exhibited significantly more stimulatory capacity than freshly prepared monocytes for the proliferation of allogeneic T cells. However, the degree of T-cell proliferation with DCs derived from patients with KS was significantly lower than with the HIV-1+/KSHV– matched controls (Figure 1A). This was also confirmed by following proliferation as a function of cell division by using allogeneic T cells labeled with CFSE. The CFSE label was lost at a slower rate, and fewer divisions occurred in response to DCs derived from patients with KS than with controls (Figure 1B). To establish that the defect resided in the DCs and not in responding T cells, we performed 4 MLRs using monocyte-derived DCs from control subjects with T cells obtained from patients with KS. Results showed no differences to MLRs using T cells from healthy responders.
Impaired allostimulatory activity by DCs from KS patients. (A) [3H]-thymidine incorporation by allogeneic T cells cultured with DCs from KS-positive or -negative patients in MLR. Allogeneic responses for 10 patients with KS (patients 1-10) and for 6 controls without are shown (median and interquartile ranges [IQR]). The Bonferroni-corrected P values at each DC/responder ratio is labeled below the number of stimulators on the x-axis. (B) CFSE staining of allogeneic T cells followed by stimulation with monocyte-derived DCs from a representative patient with KS (patient 6; black histogram) and a matched patient without (patient 15; gray histogram in foreground). CFSE staining enables the number of cell divisions to be observed. At least 3 divisions of T cells occur in response to DCs derived from patient 15 (1-2 divisions with patient 6).
Impaired allostimulatory activity by DCs from KS patients. (A) [3H]-thymidine incorporation by allogeneic T cells cultured with DCs from KS-positive or -negative patients in MLR. Allogeneic responses for 10 patients with KS (patients 1-10) and for 6 controls without are shown (median and interquartile ranges [IQR]). The Bonferroni-corrected P values at each DC/responder ratio is labeled below the number of stimulators on the x-axis. (B) CFSE staining of allogeneic T cells followed by stimulation with monocyte-derived DCs from a representative patient with KS (patient 6; black histogram) and a matched patient without (patient 15; gray histogram in foreground). CFSE staining enables the number of cell divisions to be observed. At least 3 divisions of T cells occur in response to DCs derived from patient 15 (1-2 divisions with patient 6).
KS antigen priming through the HSP pathway is preserved
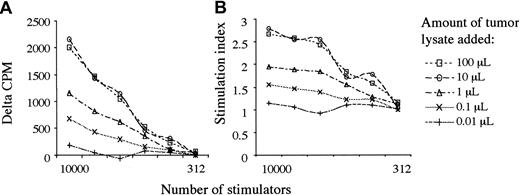
To observe the function of patients' monocyte-derived DCs in response to KS antigens, we performed syngeneic leukocyte reactions (SLRs) after overnight pulsing of 6-day-old monocyte-derived DCs with tumor lysate that was produced from 3 different patients' KS lesions. Remarkably, we observed that despite an impaired ability to stimulate responses against allogeneic T cells, lysate-pulsed DCs stimulated the proliferation of syngeneic lymphocytes. Titrations of tumor lysate showed a saturable response in patients with KS (Figure 2) and no effect in KS-negative controls after 5 days of coculture with autologous T cells. Allogeneic and autologous tumor lysates showed no significant differences in immunogenicity. The addition of TNF-α 8 hours after pulsing with tumor lysate resulted in increased responses compatible with its role in switching DCs from an antigen-capturing to an antigen-presenting function.47
Tumor lysate stimulates a saturable proliferative response. Graphs show response to Log10 changes in tumor lysate, from 100 μL to 0.01 μL. (A) δ-cpm represents counts per minute minus background. (B) The graph shows the cpm/background = stimulation index. DC pulsing with tumor lysate was tested in all 12 patients, and saturability of this pathway was tested in patient 2. Tumor lysates were made from patients 1, 6, and 7, and results showed no differences using different autologous or allogeneic tumor lysates in the same or different patients.
Tumor lysate stimulates a saturable proliferative response. Graphs show response to Log10 changes in tumor lysate, from 100 μL to 0.01 μL. (A) δ-cpm represents counts per minute minus background. (B) The graph shows the cpm/background = stimulation index. DC pulsing with tumor lysate was tested in all 12 patients, and saturability of this pathway was tested in patient 2. Tumor lysates were made from patients 1, 6, and 7, and results showed no differences using different autologous or allogeneic tumor lysates in the same or different patients.
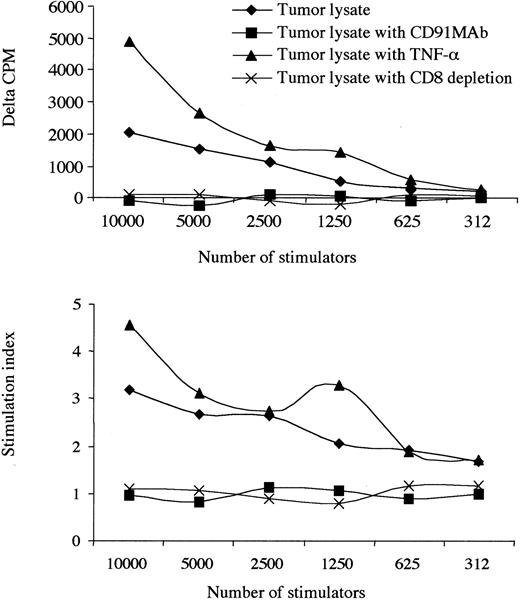
Most important, the blockade of CD91 receptors on monocyte-derived DCs abrogated immunogenicity. This was not reversed with the addition of TNF-α, suggesting specific use of the common HSP receptor, CD91, in response to tumor lysate (Figure 3). Proliferating cells were identified as CD8 lymphocytes based on the observation that the depletion of CD8 cells reduced the proliferative response to background levels. Furthermore, by tetramer staining, we show that there is a progressive increase in the number of KSHV-specific CD8 T cells during the culture period, suggesting that the responding cells are antigen specific (Figure 4). The increase occurred over each of the 5 days of the SLR, and no tetramer staining was observed in KSHV-negative or HLA-A2–negative patients.
Presentation of KS tumor lysate is CD91 dependent. The effect of an allogeneic tumor lysate, TNF-α administration, at 10 ng/mL and blockade of CD91 receptors with or without TNF-α. Results from 1 patient with a low CD4 count (patient 12) are shown. CD8 depletion abrogated responses, and no responses to KS lysate were observed in 6 HIV+/KSHV–controls. Where δ-cpm < 0 and SI < 1.0 indicates that the background cpm was greater than the observed experimental cpm. Experiments using tumor lysate and monocyte-derived DCs labeled with an isotype antibody (anti-CD3) did not change the observed results when compared with tumor lysate alone.
Presentation of KS tumor lysate is CD91 dependent. The effect of an allogeneic tumor lysate, TNF-α administration, at 10 ng/mL and blockade of CD91 receptors with or without TNF-α. Results from 1 patient with a low CD4 count (patient 12) are shown. CD8 depletion abrogated responses, and no responses to KS lysate were observed in 6 HIV+/KSHV–controls. Where δ-cpm < 0 and SI < 1.0 indicates that the background cpm was greater than the observed experimental cpm. Experiments using tumor lysate and monocyte-derived DCs labeled with an isotype antibody (anti-CD3) did not change the observed results when compared with tumor lysate alone.
KS tumor lysate stimulates KSHV-specific CD8 T cells. KSHV–tetramer-positive, CD8+ CTLs increased in number during each day of an SLR (in an HLA-A*201–positive patient, patient 2). In this experiment, monocyte-derived DCs were pulsed with tumor lysate before pulsing of autologous T cells. The double-positive population increased from 0.02% on day 1 to 38% on day 5.
KS tumor lysate stimulates KSHV-specific CD8 T cells. KSHV–tetramer-positive, CD8+ CTLs increased in number during each day of an SLR (in an HLA-A*201–positive patient, patient 2). In this experiment, monocyte-derived DCs were pulsed with tumor lysate before pulsing of autologous T cells. The double-positive population increased from 0.02% on day 1 to 38% on day 5.
Tumor lysate stimulates maturation of DCs through CD91
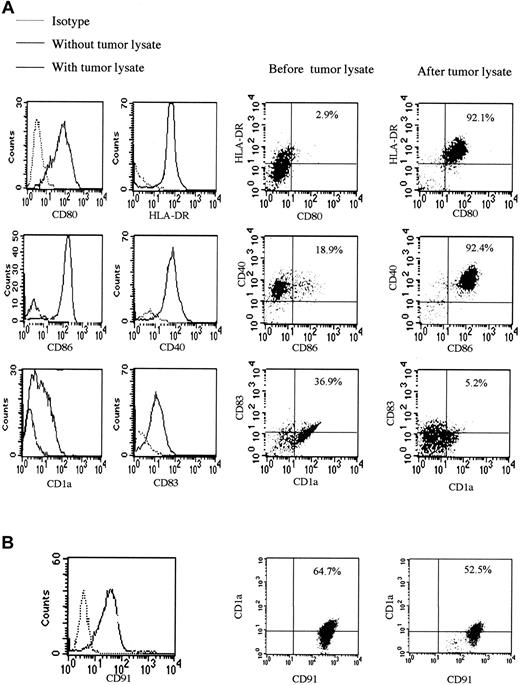
Direct contact with many pathogens leads to the maturation of DCs, so we studied the effect of tumor lysate on the expression of surface molecules of monocyte-derived DCs. Up-regulation of the known important DC maturation and activation markers occurred after overnight incubation with saturating amounts of tumor lysate, apart from CD1a expression, which decreased. This is a situation observed in activated DCs, reported to lose CD1a on migration to lymph nodes13 (Figure 5A). CD91 receptor blocking antibody prevented changes in expression of surface markers induced by tumor lysate; this, therefore, blocked DC maturation and activation. Levels of CD91, which is expressed in large amounts on the surfaces of monocyte-derived DCs, decreased after exposure to tumor lysate (Figure 5B). There were no significant differences in levels of CD91 (either percentage staining or mean fluorescence intensity) between matched KS patients and controls.
Tumor lysate stimulates DC maturation and activation. (A) Surface expression of CD1a, CD40, CD80, CD83, and CD86, known DC and activation markers, is shown after exposure of monocyte-derived DCs to tumor lysate. This maturation and activation was partially blocked by coating of monocyte-derived DCs with anti-CD91. There were no statistically significant differences in phenotypic markers of KS and non-KS monocyte-derived DCs. (B) Surface expression of CD91 before and after the addition of tumor lysate was compatible with the presence of HSPs in the preparation. Percentages indicate percentage of cells in the upper right quadrant of each plot.
Tumor lysate stimulates DC maturation and activation. (A) Surface expression of CD1a, CD40, CD80, CD83, and CD86, known DC and activation markers, is shown after exposure of monocyte-derived DCs to tumor lysate. This maturation and activation was partially blocked by coating of monocyte-derived DCs with anti-CD91. There were no statistically significant differences in phenotypic markers of KS and non-KS monocyte-derived DCs. (B) Surface expression of CD91 before and after the addition of tumor lysate was compatible with the presence of HSPs in the preparation. Percentages indicate percentage of cells in the upper right quadrant of each plot.
A control experiment was performed with the anti-CD91 antibody (and another with the isotype) by incubating it overnight with monocyte-derived DCs alone. Surface marker expression remained unchanged compared with monocyte-derived DCs without antibody.
Purified virus particles and viral lysates are presented through the CD91 pathway
As we demonstrated that tumor-associated antigens can activate functionally impaired DCs through CD91, we observed the effect of purified viral antigens in SLRs. We found that KSHV whole viral particles and KSHV viral lysate were presented by DCs (Figure 6) through the CD91 pathway in patients with KS. Whole Epstein-Barr viral particles used as a control in 3 patients resulted in similar stimulation, and CD8+ T-cell depletion abrogated responses. As for the tumor lysate, CD91 blockade of these monocyte-derived DCs reduced cpm levels to background amounts and stimulation indices to 1.
Purified KSHV antigens stimulate T-cell proliferation. Responses to KSHV whole viral particles (A-B) and KSHV lysate (C-D). Each patient tested is shown by a different mark at each stimulator-responder ratio (the points for each patient are not connected for clarity). Isotype antibody had no effect on results; anti-CD91 reduced responses. Results of δ-cpm (A,C) and the stimulation index (B,D) are shown.
Purified KSHV antigens stimulate T-cell proliferation. Responses to KSHV whole viral particles (A-B) and KSHV lysate (C-D). Each patient tested is shown by a different mark at each stimulator-responder ratio (the points for each patient are not connected for clarity). Isotype antibody had no effect on results; anti-CD91 reduced responses. Results of δ-cpm (A,C) and the stimulation index (B,D) are shown.
Stimulation indices of between 0.8 and 1.9 were observed with the use of overlapping HIV-1 gag and KSHV peptides known to cause direct T-cell activation.48 This confirms previous data demonstrating that peptide-HSP complexes are immunogenic but that HSPs or peptides alone are not.18,49 There were no correlations between CD4 count, CD8 count, HIV-1 viral load, and the counts observed (either δ-cpm or stimulation index). Because these patients were already receiving HAART, these data suggest that the changes in DC function are not readily reversible.
HSP depletion has an effect similar to CD91 blocking
Radicicol (monorden) disrupts the ATPase activity and peptide binding of Hsp90,24 the most abundant HSP, and a recent study suggests it binds and inhibits other HSPs, notably GRP94.25 Given that CD91 blockade abrogated responses to antigens, we observed whether the inhibition of HSP peptide binding had similar effects in this system. For all 3 pulsing and subsequent T-cell priming systems that were tested (tumor lysate, viral particles, viral lysate), the addition of radicicol to SLRs led to a reduction in responses (Figure 7). Responses, however, were not substantially reduced, suggesting that HSPs other than those inhibited by radicicol may have important roles in activating immune responses through CD91. The different preferences of different HSPs for distinct peptides suggests that each chaperone may be used to augment specific T-cell responses and that effective presentation of an entire peptide repertoire (by way of the common HSP receptor) to the immune system may require multiple chaperones. It is likely that each system here contains different HSP-peptide complexes—only some of which are inhibited by radicicol.
HSP depletion with radicicol decreases responses. Results from 3 patients (patients 2, 4, 10) in whom radicicol was used are shown. Median and range are shown for experiments in 3 KS patients using 10 000 stimulator monocyte-derived DCs (titration results with a changing number of stimulators, as for Figure 6, are not shown). δ-cpm is shown at the top, and stimulation indices are shown on the lower graph. These results are not included in Figure 6. KS lysates in all 3 patients were allogeneic.
HSP depletion with radicicol decreases responses. Results from 3 patients (patients 2, 4, 10) in whom radicicol was used are shown. Median and range are shown for experiments in 3 KS patients using 10 000 stimulator monocyte-derived DCs (titration results with a changing number of stimulators, as for Figure 6, are not shown). δ-cpm is shown at the top, and stimulation indices are shown on the lower graph. These results are not included in Figure 6. KS lysates in all 3 patients were allogeneic.
Discussion
The pathogenesis of Kaposi sarcoma has been debated for many years. The observation that the withdrawal of immunosuppression after transplantation50,51 or that the treatment of HIV-seropositive patients with HAART39,40 caused regression of this tumor has suggested that loss of immunologic control may be a mechanism in the genesis of this condition.
In this study we have shown that plasmacytoid DCs disappear preferentially from the circulation in HIV-1–infected patients with KS compared with KS-negative controls (P = .009). It is known that PDCs are highly susceptible to infection with HIV-1 and that they release infectious virus.52 The selective reduction of PDCs observed in KS is likely to have important implications in KS because these cells are the major source of type 1 interferons. An important part of the innate defense against viruses (and tumor cells) is the production of the type 1 interferons IFN-α and IFN-β.43,53 These not only inhibit viral replication,54 they also have important adjuvant effects on a variety of immune cell types, such as monocytes, natural killer cells, and T cells.55
In addition to a quantitative reduction, we also identified a second immunosuppressive mechanism associated with infection by KSHV, namely the impaired functional capacity of monocyte-derived DCs. A similar DC defect has been observed in monocyte-derived DCs obtained from patients with hepatitis C virus infection.45,46 The reduced ability of DCs from patients with KS or hepatitis C to stimulate naive T cells suggests the discovery of a common viral immune evasion strategy, shared by a small RNA virus (hepatitis C) and a large DNA virus (KSHV). Interestingly, current therapies for KS and HCV include interferon-α. Despite this defect, DCs from KS patients were able to cross-present viral and tumor-specific antigens to KSHV-specific CD8 T cells (Figure 4).
Several hypotheses can be proposed to explain these findings. First, mainly naive T cells are stimulated in the allogeneic mixed-leukocyte reaction, whereas memory T cells, which are more easily activated than naive cells, were stimulated in the tumor lysate (or viral lysate/viral particle) experiments. Second, the antigen presentation defect may be associated with impaired ability to stimulate CD4 rather than CD8 cells, as shown by the preserved HSP pathways in DCs that are impaired in their ability to activate CD4+ T cells (Figure 1A).
HSP-CD91 interactions have been confirmed by several studies, and it remains the only HSP receptor that has been independently verified.56,57 After the demonstration that all HSPs use CD91 for uptake before re-presentation by MHC class 1,8,12 other proteins have also been proposed to act as extracellular receptors for HSPs, including CD1458 and the toll-like receptors.59 However, we and others28 have been unable to show toll-like receptor-4 expression on monocyte-derived DCs, and CD14 expression is lost completely 1 to 3 days into culture (we use pulsed monocyte-derived DCs on day 6). Moreover, pretreatment of DCs with anti–toll receptor antibodies or anti-CD14 does not affect HSP uptake.28 One group has suggested CD91-independent cross-presentation of HSP-peptide complexes, particularly because CD91 is expressed on a variety of cell types, including fibroblasts, platelets, and hepatocytes, which do not function in antigen presentation60 (this clearly does not exclude a role for MHC class 1 in presentation, a molecule expressed on all nucleated cells). However, CD91 is expressed heavily on monocyte-derived DCs, and independent cross-presentation has only been shown for one particular HSP (GRP94)60 (manuscript submitted). Recent data indicate that CD91 is gained by DCs in sites of antigen uptake.61
Because different HSPs bind distinct peptides, each complex may activate specific DC–T-cell responses, and only certain HSPs may be important in a particular system. This has been shown here by the inhibition of Hsp90 using radicicol, which reduced the responses observed, albeit to a lesser degree than CD91 blockade, suggesting a role of other HSPs that were not inhibited (Figure 7). Interestingly, in a different experimental system that also used radicicol (or its functional analog, geldanamycin), the phenotype of self-fertilizing plants was disrupted to such an extent that the authors proposed that the effects of Hsp90 alone were able to influence morphogenetic responses to environmental cues and to buffer normal development from destabilizing effects of stochastic processes.26,27 HSPs are essential for life; hence, we were unable to use knockout mice or cells lacking HSPs because of their nonviability. The pharmacologic evidence we present is in agreement with previous data.
The importance of CD91 has also been shown by its ability to mediate the uptake of HIV-1 Tat protein, apolipoprotein E4, amyloid precursor protein, and amyloid β-protein.62 CD91 has recently been demonstrated to bind and internalize α-defensins in a saturable and dose-dependent manner.63 These small molecules are secreted from stimulated CD8+ T cells in the rare minority of patients who are infected with HIV-1 for more than 10 years with no evidence of progressive disease while remaining off treatment (termed long-term nonprogressors).64 We have now found that the expression of CD91 is increased in such patients, whereas differences between nonprogressors and other patients in the expression of a large range of DC activation and maturation markers remain insignificant, both before and after antigenic challenge.65 It is increasingly clear that CD91, termed here the common HSP receptor, represents an important route for stimulating a CD8+ T-cell responses by MHC class 1–restricted presentation. This may also be a fairly general mechanism by which the innate immune system can stimulate the adaptive arm in viral infections. Up-regulation of CD91 may, therefore, represent a useful therapeutic, and perhaps a preventive, antiviral or antitumor strategy.
Although DCs show extensive plasticity,66,67 it appears that disease-specific antigens can stimulate them through a common pathway leading to a common activated phenotype. The CD91 receptor appears critical in directing the immunogenicity of an array of antigens, which may not be surprising considering its ability to bind different families of HSPs with no structural homology. It has recently been shown that specific incorporation of HSPs into lipid rafts68 and virion envelopes,69 including HIV-1, does occur, and the presence of HSPs in viral coats may explain the observation that viral particle/lysate-induced DC stimulation and presentation are blocked by CD91 antibodies.
Alternatively, it appears that reagents and products generated from lysed cells, prokaryotic and eukaryotic, contain amounts of HSP sufficient to be immunogenic. Considering the ubiquity of HSPs, their intraspecies and interspecies phylogenetic conservation, variety within cells and organelles, promiscuity of binding, and abundance—especially in times of stress (including, we presume, viral infection)—this will be an important issue in any cellular system or in situations in which products derived from cells are used. Furthermore, the ability of allogeneic or autologous tumor lysates and purified disrupted virus preparations to stimulate specific antiviral responses through the CD91 pathway has implications for vaccine design and therapy. This work also points to a potential advantage of using monocyte-derived DCs and allogeneic HSP-peptide preparations (only autologous preparations have been clinically studied thus far16 ) in human immunotherapies, particularly because large amounts of both can be generated.
Although recent research has concentrated on the development of an effective T-lymphocyte response in response to HAART, this is unlikely to be the mechanism for tumor regression in patients with AIDS-related KS. We believe that more extensive study may indicate that the restoration of innate immune responses may have a more important role in controlling this tumor. The present explosive increase in knowledge of the innate immune system provides an opportunity to investigate the precise determinants of the initial events in viral infection, in particular why some are successful in establishing chronic infection while others are cleared.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-03-0891.
Supported by grants from the Medical Research Council (G84/5631), the Junior Research Committee (Chelsea and Westminster Hospital), and the Pathological Society of Great Britain and Ireland.
Pramod Srivastava and Steve Patterson are joint last authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Deborah Ruddy and Adam Sanitt for their help with the manuscript. J.S. is a Medical Research Council PhD student.

![Figure 1. Impaired allostimulatory activity by DCs from KS patients. (A) [3H]-thymidine incorporation by allogeneic T cells cultured with DCs from KS-positive or -negative patients in MLR. Allogeneic responses for 10 patients with KS (patients 1-10) and for 6 controls without are shown (median and interquartile ranges [IQR]). The Bonferroni-corrected P values at each DC/responder ratio is labeled below the number of stimulators on the x-axis. (B) CFSE staining of allogeneic T cells followed by stimulation with monocyte-derived DCs from a representative patient with KS (patient 6; black histogram) and a matched patient without (patient 15; gray histogram in foreground). CFSE staining enables the number of cell divisions to be observed. At least 3 divisions of T cells occur in response to DCs derived from patient 15 (1-2 divisions with patient 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-03-0891/6/m_h81734853001.jpeg?Expires=1763618100&Signature=3GQEgl3kvVSKoT2kCR69G4IkEZozfgocJpKsXPXEfLPw9NwHcRyEmp7YnbLWQEHFD2ZeKE0zT6fZP8Np6R9kctNg0270W4p6tEuk4jvXt2Z7hDF9d6Vx2Fh3xgTh~3rASzQSij2mDGJumlKmWSHF1jAiJ4MANx7DyiFNIlMVrYTrM9Jez65R4biofenwz~0oJypM1GcgKsmeUIrR6kvR-qzWbNSTXQr-1euEIAUrFueRgKI0d1u8odWogwyVulQuIeZbEEdpCzHZPvlMU9oLdAe1-gGRoLkLcvVgz7KQgtsOFqTpo5Z9t62Xvv2eOXiratDQdbNsvbu~wK5GJ~bcqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal