Abstract
We previously found that some myeloma cell lines express the heparin-binding epidermal growth factor–like growth factor (HB-EGF) gene. As the proteoglycan syndecan-1 is an HB-EGF coreceptor as well as a hallmark of plasma cell differentiation and a marker of myeloma cells, we studied the role of HB-EGF on myeloma cell growth. The HB-EGF gene was expressed by bone marrow mononuclear cells in 8 of 8 patients with myeloma, particularly by monocytes and stromal cells, but not by purified primary myeloma cells. Six of 9 myeloma cell lines and 9 of 9 purified primary myeloma cells expressed ErbB1 or ErbB4 genes coding for HB-EGF receptor. In the presence of a low interleukin-6 (IL-6) concentration, HB-EGF stimulated the proliferation of the 6 ErbB1+ or ErbB4+ cell lines, through the phosphatidylinositol 3-kinase/AKT (PI-3K/AKT) pathway. A pan-ErbB inhibitor blocked the myeloma cell growth factor activity and the signaling induced by HB-EGF. This inhibitor induced apoptosis of patients'myeloma cells cultured with their tumor environment. It also increased patients' myeloma cell apoptosis induced by an anti–IL-6 antibody or dexamethasone. The ErbB inhibitor had no effect on the interaction between multiple myeloma cells and stromal cells. It was not toxic for nonmyeloma cells present in patients' bone marrow cultures or for the growth of hematopoietic progenitors. Altogether, these data identify ErbB receptors as putative therapeutic targets in multiple myeloma.
Introduction
Multiple myeloma (MM) is a B-cell neoplasia characterized by the accumulation of clonal malignant plasma cells in the bone marrow. In most patients, malignant plasma cells require mediators delivered by the tumor environment to survive and proliferate. Interleukin-6 (IL-6) is one of these mediators produced in the bone marrow environment.1,2 However, IL-6 alone can support the growth of myeloma cells in vitro only in patients with extramedullary proliferation.3 In patients with intramedullary MM, additional factors produced by the tumor environment are necessary to promote the survival of primary myeloma cells, together with IL-6.3 Searching for such intercellular communication signals with DNA arrays, we found that some myeloma cell lines expressed the heparin-binding epidermal growth factor–like growth factor (HB-EGF) gene4 and that autocrine HB-EGF was involved in the IL-6–induced survival of 2 myeloma cell lines.5
HB-EGF is one of the 11 members of the epidermal growth factor (EGF)–related peptide growth factor family. This family binds to and induces the homo- or heterodimerization of 3 receptors: the EGF receptor ErbB1, ErbB3, and ErbB4. ErbB2 is a fourth member unable to bind EGF ligands. ErbB2 is a preferred heterodimerization partner for all other ErbB members and increases ErbB receptor signaling.6 The EGF family includes EGF, transforming growth factor α, amphiregulin, and epigen, which bind to ErbB1, and HB-EGF, betacellulin, and epiregulin, which bind to both ErbB1 and ErbB4.7 It also includes the 4 neuregulins. Neuregulin 1 and 2 bind to both ErbB3 and ErbB4 and neuregulin 3 and 4 bind only to ErbB4.8 ErbB receptors can cross-communicate with other receptors, including growth hormone receptor,9 G-protein–coupled receptors,10 gp130 IL-6 transducer,11,12 or IGF-1 receptor.13,14 Recently, interferon α (IFN-α) was shown to trigger ErbB3 phosphorylation in the KAS-6/1 myeloma cell line.15
An abnormal expression of ErbB receptors and/or EGF family ligands has been reported in numerous cancers.16-18 In particular, ErbB1 is expressed or overexpressed in a wide variety of solid human tumors, including non–small cell lung (NSCL), prostate, breast, colorectal, and ovarian cancers.19 The ErbB4 gene is also amplified in cancer and is associated with a poor prognosis.20 The ErbB2 gene is frequently amplified in epithelial cancers, mainly melanoma, breast, and ovarian cancers, leading to abnormal EGF signaling.21 Expression of ErbB3 is seen in many of the same tumor types that overexpress ErbB2.22 The involvement of the EGF/ErbB family in cancer has encouraged the development of 2 families of ErbB inhibitors in clinical studies: monoclonal antibodies and ErbB tyrosine kinase inhibitors. ErbB kinase inhibitors are small molecules that compete with adenosine triphosphate binding to the ErbB kinase domains. They block ErbB kinase activity specifically, but not the activity of other receptors.23 Antibodies to ErbB2 are currently used in the treatment of breast and ovarian cancers. Antibodies to ErbB1 and inhibitors of ErbB kinase activity are under active investigation in phase 1 to phase 3 trials for a variety of tumors.24
In this study, we show that HB-EGF was a growth factor for 6 of 9 myeloma cell lines that expressed ErbB1 and/or ErbB4 HB-EGF receptors, but not for 3 nonresponsive cell lines. HB-EGF activity required a low concentration of IL-6 and was blocked by an anti–IL-6 antibody. HB-EGF triggered the PI-3K/AKT pathway, but not the Janus kinase–signal transducer and activator of transcription (JAK/STAT) and mitogen-activated protein kinase (MAPK) pathways. The biologic activity of HB-EGF was blocked by PD169540, a pan-ErbB kinase inhibitor analog to the CI-1033 clinical-grade inhibitor.25 PD169540 ErbB inhibitor induced primary myeloma cells apoptosis in short-term culture and strongly potentiated dexamethasone (DEX) or anti–IL-6 monoclonal antibody (MoAb)–induced apoptosis. Altogether, these data provide a framework for targeting the ErbB pathway in novel, biologically based therapeutics in multiple myeloma.
Materials and methods
Myeloma cell lines and reagents
The human myeloma cell lines (HMCLs) were obtained in our laboratory and their characteristics have been reported previously.4,26,27 Eight are IL-6–dependent myeloma cell lines (XG-1, XG-2, XG-5, XG-6, XG-7, XG-11, XG-16, XG-19). Their growth is dependent on addition of exogenous IL-6. Upon removal of IL-6, myeloma cells progressively apoptose.3,28 One is autonomously growing (RPMI 8226). The HM-CLs were routinely maintained in RPMI 1640, 10% fetal calf serum (FCS) and 2 ng/mL IL-6. The experiments were performed in RPMI 1640 with 5% FCS.
Bone marrow or peripheral blood samples were obtained from patients with intramedullary myeloma or with plasma cell leukemia (PCL), after they had provided informed consent. Recombinant HB-EGF and IL-6 were purchased from R&D Systems (Minneapolis, MN), the PD153035 and AG1478 ErbB1 inhibitors from Alexis Biochemicals (San Diego, CA), the LY294002 PI-3 kinase inhibitor from Sigma (St Louis, MO), the PD169540 pan-ErbB kinase inhibitor was a generous gift from Pfizer Global Research and Development (Ann Arbor, MI), and the B-E8 anti–IL-6 MoAb was a generous gift from Dr Wijdenes (Diaclone, Besancon, France).
Cell proliferation assay
Cells were cultured for 5 to 7 days (depending on the cell lines) in 96-well flat-bottomed microtiter plates at 104 cells/well in 200 μL RPMI 1640 culture medium and 5% FCS. Various concentrations of cytokines or growth factors or inhibitors of cytokine/growth factor were added at the beginning of the culture in 6 culture wells per group. At the end of the culture, cells were pulsed with tritiated thymidine (Amersham Pharmacia Biotech, Orsay, France) for 12 hours, then harvested and counted as reported previously.29
Detection of apoptotic cells
Myeloma cells were cultured for 3 days in 24-well flat-bottomed microtiter plates at 105 cells/well in 1 mL RPMI 1640 medium with 5% FCS and various cytokines/growth factors or inhibitors. At the end of the culture, cells were washed once in phosphate-buffered saline (PBS) and suspended in annexin V–fluorescein isothiocyanate (FITC) solution (Boehringer, Mannheim, Germany). Fluorescence was analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
We generated cDNA with 2 μg total RNA using the Superscript II reverse transcriptase (Life Technologies) and oligo d(T)12-18 (Amersham Pharmacia Biotech) as primer. Each 25-μL PCR reaction contained 1 μL of the first strand cDNA, 1 μM of each primer (sense and antisense) designed for annealing to different exons, 0.2 mM of each dNTP, 1.5 mM MgCl2, 1X polymerase buffer, and 2 units of Taq polymerase (Life Technologies). The following primers were used: HB-EGF, 5′-TGG TGC TGA AGC TCT TTC TGG (sense) and 5′-GTG GGA ATT AGT CAT GCC CAA (antisense); β actin, 5′-TCC CTG GAG AAG AGC TAC GA (sense) and 5′-AGC ACT GTG TTG GCG TAC AG (antisense); ErbB1, 5′-CAG CGC TAC CTT GTC ATT CAG (sense) and 5′-TCA TAC TAT CCT CCG TGG TCA (antisense); ErbB2, 5′-CGC TTT GTG GTC ATC CAG AAT G (sense) and 5′-TCG TGT TCA CAC TGG CAC GTC (antisense); ErbB3, 5′-TGG CCC GAG ACC CAC CAC GGT ATC TG (sense) and 5′-AGT TAC GTT CTC TGG GCA TTA GCC TT (antisense); ErbB4, 5′-AGT TTT CAA GGA TGG CTC GAG ACC CTC (sense) and 5′-AGC TTA CAC CAC AGT ATT CCG GTG TCT (antisense).
The sizes of the PCR products were as follows: HB-EGF, 605 bp; β actin, 194 bp; ErbB1, 726 bp; ErbB2, 821 bp; ErbB3, 1129 bp; and ErbB4, 1013 bp. The amplification profile was 1 minute at 95°C, 1 minute at 62°C (HB-EGF) or 60°C(β actin, ErbB1, ErbB2, ErbB3, and ErbB4), 1 minute at 72°C, followed by a final extension of 10 minutes at 72°C. The cycle number was 25 for β actin and 30 for HB-EGF, ErbB1, ErbB2, ErbB3, and ErbB4. Reaction products were electrophoresed on a 1.3% agarose gel.
Purification of primary myeloma cells and CD14 monocytes
Malignant plasma cells were purified (> 95% myeloma cells) from 9 patients with MM (5 with intramedullary myeloma and 4 with plasma cell leukemia), with the MI15 anti-CD138 MoAb and antimouse IgG1 MACS microbeads (Miltenyi Biotech, Paris, France) as described.30 Bone marrow CD14 monocytes were purified (> 98% CD14 cells) from the bone marrow of 3 patients with MM with anti-CD14 MACS microbeads (Miltenyi Biotech).
Bone marrow stromal cell (BMSC) culture
BMSCs were obtained from 5 patients with MM. Bone marrow mononuclear cells were obtained after Ficoll-Hypaque density centrifugation and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 20% FCS at a concentration of 106 cells/mL. After 3 days, nonadherent cells were removed and fresh culture medium with 20% FCS was added. The culture medium was substituted with fresh medium and 20% FCS once a week. When a confluent layer of adherent cells was obtained, the cells were trypsinized and cultured in DMEM supplemented with 10% FCS.
Adhesion assay of myeloma cells to BMSCs and IL-6 production
BMSCs obtained from one patient were immortalized with a human telomerase reverse transcriptase gene–green fluorescent protein (hTERT-GFP) retrovirus produced in our laboratory. After selection with G418, 100% of the stromal cells expressed GFP as assayed with fluorescence-activated cell sorting (FACS). Stromal cells were trypsinized and plated in 24-well culture plates at a density of 2.5 × 104 cells/well and incubated overnight at 37°C with or without the PD169540 pan-ErbB inhibitor. XG-11 myeloma cells (1.5 × 105 per well) were added and cultured for 24 hours. Nonadherent cells were counted and adherent cells were trypsinized and counted. In the 2 cell populations, GFP+ stromal cells were quantified using FACS analysis. The IL-6 production by BMSCs after myeloma cell adhesion was assayed as described.31 Briefly, both confluent BMSCs and myeloma cells were preincubated with or without PD169540. Myeloma cells and BMSCs were cocultured for 24 hours at 37°C, with or without PD169540, in 4 different culture wells per group. The culture supernatant was recovered at the end of the culture and assayed for IL-6 with an enzyme-linked immunosorbent assay (ELISA; Beckman-Coulter, Marseilles, France).
Immunoprecipitation
Myeloma cells (20 × 106) were lysed in Tris, pH 7.4, 50 mM, NaCl 150 mM, 5 mM EDTA (ethylenediaminetetraacetic acid), 10 mM sodium pyrophosphate (NaPPi), 1 mM Na3VO4, 160 mM NaF, 2.5 mM PMSF (phenylmethylsulfonyl fluoride), 10 μM leupeptin, 2 μM pepstatin, 10 mg/L aprotinin, 1% NP 40, 5 g/L deoxycholate. The lysates were immunoprecipitated with an anti-ErbB4 (clone H4.72.8; Neomarkers, Union City, CA) or anti-ErbB1 (clone LA-22; Upstate Biotechnology, Lake Placid, NY) antibody. The precipitated material was separated on a 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting with an anti-ErbB4 (clone C-18; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-ErbB1 antibody (clone LA-1; Upstate Biotechnology).
Western blot
Myeloma cells were starved overnight in RPMI 1640 culture medium in the presence of 1% bovine serum albumin (BSA), without cytokines. Cells were then stimulated with HB-EGF (5 μg/mL), insulin-like growth factor 1 (IGF-1; 1 μg/mL), or IL-6 (20 ng/mL) for 15 minutes at 37°C. Cells were lysed in 10 mM Tris-HCl (pH 7.05), 50 mM NaCl, 50 mM NaF, 30 mM NaPPi, 1% Triton X-100, 5 μM ZnCl2, 100 μM Na3VO4, 1 mM dithiothreitol (DTT), 20 mM β-glycerophosphate, 20 mM p-nitrophenolphosphate (PNPP), 20 μg/mL aprotinin, 2.5 μg/mL leupeptin, 0.5 mM PMSF, 0.5 mM benzamidine, 5 μg/mL pepstatin, and 50 nM okadaic acid. Lysates were resolved in 7.5% SDS-PAGE and transferred to a nitrocellulose membrane (Schleicher and Schuell, Kassel, Germany). Membranes were blocked for 2 hours at room temperature in 140 mM NaCl, 3 mM KCl, 25 mM Tris-HCl (pH 7.4), 0.1% tween 20 (TBS-T), 5% nonfat milk, and then immunoblotted with the primary antibodies: anti–phospho-STAT3, anti–phospho-AKT, and anti–phospho-MAPK rabbit polyclonal antibodies from New England Biolabs (Beverly, MA), at a 1:1000 dilution. As a control for protein loading, we used anti-STAT3 mouse monoclonal antibody from Transduction Laboratories (Lexington, KY), anti-AKT rabbit polyclonal antibody from New England Biolabs, and anti-MAPK rabbit polyclonal antibody from Santa Cruz Biotechnology. The primary antibodies were visualized with goat antirabbit (Sigma) or goat antimouse (Biorad, Hercules, CA) peroxidase-conjugated antibodies by an enhanced chemiluminescence detection system.
Mononuclear cell culture
Mononuclear cells from 8 patients with MM were cultured for 5 days at 5 × 105 cells/mL in RPMI 1640 medium, 5% FCS, 1 ng/mL IL-6, without (control) or with the PD169540 pan-ErbB inhibitor (1 μM), the B-E8 anti–IL-6 MoAb (10 μg/mL) or 10–6 M dexamethasone (DEX) used alone or in combination. In each culture group, the viability and cell counts were assayed and myeloma cells were stained with an anti–CD138-PE MoAb (Beckman-Coulter).
Assay of hematopoietic colony-forming cells
Bone marrow mononuclear cells from 5 patients with MM were grown in a semisolid culture medium with hematopoietic cytokines (GF H4434; StemCell Technologies, Vancouver, BC, Canada). Cells were cultured without (control) or with the PD169540 pan-ErbB kinase inhibitor (1 μM), the B-E8 anti–IL-6 MoAb (10 μg/mL), or 10–6 M DEX used alone or in combination. The number of granulocyte-macrophage colonies was counted on day 14 of incubation.
Statistical analysis
Results were compared with a Student t test or a Wilcoxon test for pairs.
Results
Gene expression of EGF receptor family members and HB-EGF in myeloma cell lines
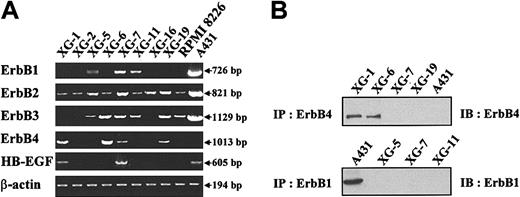
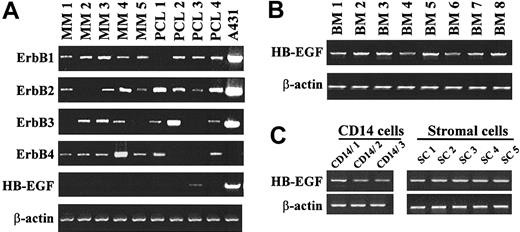
Gene expression of the 4 members of the EGF receptor family was studied in 9 myeloma cell lines. Eight are IL-6–dependent myeloma cell lines (XG-1, XG-2, XG-5, XG-6, XG-7, XG-11, XG-16, XG-19) and one is an autonomously growing cell line (RPMI 8226). The A431 epidermoid carcinoma cell line was used as a control. In agreement with previous studies,32 a high ErbB1, ErbB2, and ErbB3 expression, unlike ErbB4, was found in A431 cells (Figure 1A). ErbB2 was expressed in all cell lines and ErbB3 in 6 of 9 cell lines. The gene expression was always weaker than that found in the A431 cells. Three myeloma cell lines (XG-5, XG-7, and XG-11) expressed ErbB1. Four of 9 myeloma cell lines expressed ErbB4, with a high expression in XG-1 and XG-6 cells and a weak one in XG-7 and XG-19 cells. ErbB4 protein was detected by immunoprecipitation in XG-1 and XG-6 cells and was probably too low to be detected in XG-7 and XG-19 cells (Figure 1B). ErbB1 was detected by immunoprecipitation in A431, but protein was below the detection limit in XG-5, XG-7, and XG-11 cells (Figure 1B). In agreement with our previous report,4 we found HB-EGF expression in 2 of 9 myeloma cell lines (Figure 1A).
Expression of EGF receptor family members in myeloma cell lines. (A) ErbB1, ErbB2, ErbB3, ErbB4, and HB-EGF gene expression was assayed with RT-PCR in 9 myeloma cell lines. The A431 epidermoid carcinoma cell line was used as a control. Amplification of β actin shows the equivalence of the cDNA loading and amplification. Results are from one experiment representative of 3. (B) Myeloma cells or A431 cells were lysed and the lysates were immunoprecipitated with the H4.72.8 anti-ErbB4 or the LA-22 anti-ErbB1 antibody. The precipitated material was separated on a 7.5% SDS-PAGE and analyzed by Western blotting with a second anti-ErbB antibody (anti-ErbB4, clone C-18, or anti-ErbB1, clone LA-1). Results are of one experiment representative of 3.
Expression of EGF receptor family members in myeloma cell lines. (A) ErbB1, ErbB2, ErbB3, ErbB4, and HB-EGF gene expression was assayed with RT-PCR in 9 myeloma cell lines. The A431 epidermoid carcinoma cell line was used as a control. Amplification of β actin shows the equivalence of the cDNA loading and amplification. Results are from one experiment representative of 3. (B) Myeloma cells or A431 cells were lysed and the lysates were immunoprecipitated with the H4.72.8 anti-ErbB4 or the LA-22 anti-ErbB1 antibody. The precipitated material was separated on a 7.5% SDS-PAGE and analyzed by Western blotting with a second anti-ErbB antibody (anti-ErbB4, clone C-18, or anti-ErbB1, clone LA-1). Results are of one experiment representative of 3.
Sensitivity of myeloma cell lines to HB-EGF
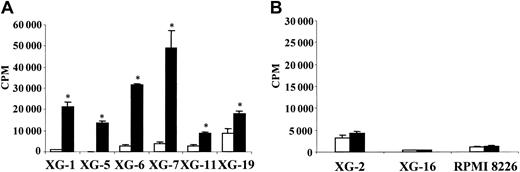
To investigate the effect of HB-EGF on myeloma cell growth, we first used the XG-1 and XG-6 cell lines, which cannot survive and proliferate without adding IL-6 when cultured at a low cell density.26 HB-EGF induced the proliferation of XG-1 cells and had no effect on XG-6 cells (Figure 2). One difference between XG-1 and XG-6 cells is a low production of autocrine IL-6 by XG-1 cells.33 This production of autocrine IL-6 by XG-1 cells is too weak to support the growth of XG-1 cells alone but contributed to the HB-EGF–induced proliferation since it was abrogated by anti–IL-6 MoAb (Figure 2). In the presence of a low IL-6 concentration (5 pg/mL), HB-EGF dramatically increased the IL-6–induced proliferation of XG-1 and XG-6 myeloma cells 14- and 12-fold, respectively. Anti–IL-6 MoAb abrogated this effect (Figure 2). With a high IL-6 concentration, HB-EGF increased the proliferation of XG-1 cells 3-fold and did not increase that of XG-6 cells, which was likely already at its maximum level. A significant effect was found with 100 ng/mL HB-EGF and a maximum effect with 100 ng/mL to 1000 ng/mL, depending on the myeloma cell lines (results not shown). Using annexin V, we observed that HB-EGF protected myeloma cells from apoptosis (Figure 3). Again, the myeloma cell survival activity of HB-EGF required a low concentration of IL-6 and was inhibited by adding an anti–IL-6 antibody. Results obtained with XG-1 and XG-6 cells were extended to the 6 IL-6–dependent myeloma cell lines that expressed the ErbB1 or ErbB4 gene. Their proliferation was highly stimulated by adding HB-EGF (Figure 4A). No effect was found in the other 3 cell lines, which failed to express ErbB1 or ErbB4 (Figure 4B). These data indicate that HB-EGF enhances survival and proliferation of IL-6–dependent MM cell lines.
Sensitivity of myeloma cell lines to HB-EGF. XG-1 and XG-6 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6, or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, or 500 pg/mL IL-6, or 500 pg/mL IL-6 and 1 μg/mL HB-EGF (with or without 10 μg/mL B-E8 anti–IL-6 MoAb). Cells were cultured for 6 days and were pulsed for 12 hours with tritiated thymidine at the end of the culture. Data are means ± SDs of the tritiated thymidine incorporation determined on sixplicate culture wells and are those from one experiment representative of 4.
Sensitivity of myeloma cell lines to HB-EGF. XG-1 and XG-6 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6, or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, or 500 pg/mL IL-6, or 500 pg/mL IL-6 and 1 μg/mL HB-EGF (with or without 10 μg/mL B-E8 anti–IL-6 MoAb). Cells were cultured for 6 days and were pulsed for 12 hours with tritiated thymidine at the end of the culture. Data are means ± SDs of the tritiated thymidine incorporation determined on sixplicate culture wells and are those from one experiment representative of 4.
HB-EGF reduces the apoptosis induced by IL-6 removal. XG-1 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6, or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, or 500 pg/mL IL-6, or 500 pg/mL IL-6 and 1 μg/mL HB-EGF (with or without 10 μg/mL B-E8 anti–IL-6 MoAb). Cells were cultured for 3 days and stained with FITC–annexin V to determine the percentage of apoptotic cells. Results are from one experiment representative of 3.
HB-EGF reduces the apoptosis induced by IL-6 removal. XG-1 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6, or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, or 500 pg/mL IL-6, or 500 pg/mL IL-6 and 1 μg/mL HB-EGF (with or without 10 μg/mL B-E8 anti–IL-6 MoAb). Cells were cultured for 3 days and stained with FITC–annexin V to determine the percentage of apoptotic cells. Results are from one experiment representative of 3.
Correlation between ErbB1/ErbB4 expression and HB-EGF activity on myeloma cells.ErbB1/ErbB4–positive myeloma cells (A) and ErbB1/ErbB4–negative myeloma cells (B) were cultured for 5 to 7 days in RPMI 1640, 5% FCS, and 5 pg/mL IL-6, without (□) or with (▪) HB-EGF and then pulsed with tritiated thymidine for 12 hours at the end of the culture. Data are expressed as means ± SDs of tritiated thymidine incorporation determined on sixplicate culture well. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 alone, using a Student t test (P ≤ .05).
Correlation between ErbB1/ErbB4 expression and HB-EGF activity on myeloma cells.ErbB1/ErbB4–positive myeloma cells (A) and ErbB1/ErbB4–negative myeloma cells (B) were cultured for 5 to 7 days in RPMI 1640, 5% FCS, and 5 pg/mL IL-6, without (□) or with (▪) HB-EGF and then pulsed with tritiated thymidine for 12 hours at the end of the culture. Data are expressed as means ± SDs of tritiated thymidine incorporation determined on sixplicate culture well. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 alone, using a Student t test (P ≤ .05).
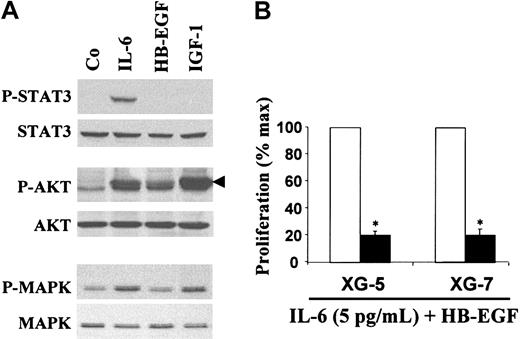
We next looked for signaling molecules activated by HB-EGF. We used the XG-7 myeloma cell line that is the most sensitive to HB-EGF (Figure 4A). HB-EGF induced AKT phosphorylation but not STAT3 phosphorylation (Figure 5A, lane 3). HB-EGF, used alone or with a small amount of IL-6, did not induce MAPK phosphorylation in myeloma cells after a short exposure (15 minutes; Figure 5A, lane 3) or longer exposures (60 minutes, 2 hours, 24 hours; results not shown). As a control, we used IL-6, which activated the JAK/STAT, MAPK, and PI3-K/AKT pathways (Figure 5A, lane 2), and IGF-1, which activated the MAPK and PI3-K/AKT pathways (Figure 5A, lane 4), in agreement with previous reports.29,34,35 Indeed, the PI-3K/AKT pathway was critical for HB-EGF–induced myeloma cell proliferation since the PI-3K inhibitor LY294002 inhibited this proliferation (Figure 5B).
HB-EGF activity is mediated by activation of the PI-3K/AKT pathway. (A) XG-7 myeloma cells were starved overnight in culture medium without serum or cytokines. Cells were then stimulated with either IL-6 (20 ng/mL), IGF-1 (1 μg/mL), or HB-EGF (5 μg/mL) for 15 minutes at 37°C. Lysates from unstimulated (Co) or stimulated myeloma cells were immunoblotted with anti–phospho-specific STAT3 antibody (top panel) and then reprobed with anti-STAT3 antibody (second panel), anti–phospho-specific AKT antibody (third panel) and reprobed with anti-AKT antibody (fourth panel), anti–phospho-specific MAPK antibody (fifth panel) and reprobed with anti-MAPK antibody (bottom panel). (B) XG-5 and XG-7 myeloma cell lines were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with (▪) the PI-3K inhibitor LY294002 (25 μM). Cells were cultured for 6 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05).
HB-EGF activity is mediated by activation of the PI-3K/AKT pathway. (A) XG-7 myeloma cells were starved overnight in culture medium without serum or cytokines. Cells were then stimulated with either IL-6 (20 ng/mL), IGF-1 (1 μg/mL), or HB-EGF (5 μg/mL) for 15 minutes at 37°C. Lysates from unstimulated (Co) or stimulated myeloma cells were immunoblotted with anti–phospho-specific STAT3 antibody (top panel) and then reprobed with anti-STAT3 antibody (second panel), anti–phospho-specific AKT antibody (third panel) and reprobed with anti-AKT antibody (fourth panel), anti–phospho-specific MAPK antibody (fifth panel) and reprobed with anti-MAPK antibody (bottom panel). (B) XG-5 and XG-7 myeloma cell lines were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with (▪) the PI-3K inhibitor LY294002 (25 μM). Cells were cultured for 6 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05).
Inhibition of HB-EGF–induced myeloma cell proliferation by ErbB inhibitors
We then looked for the ability of ErbB inhibitors to block the HB-EGF–induced growth of myeloma cells. We used the PD169540 pan-ErbB kinase inhibitor, an analog of the CI-1033 clinical-grade inhibitor,25 which at a 1-μM concentration is known to inhibit specifically ErbB1, ErbB2, and ErbB4 kinase activities, without affecting a large panel of other kinases (Dr Leopold, Pfizer Laboratories, personal communication, August 2002). Accordingly, PD169540 blocked ErbB1 phosphorylation induced by HB-EGF in A431 cells (Figure 6A). PD169540 reversed the HB-EGF–induced survival of myeloma cells (Figure 6B) and inhibited the HB-EGF–induced proliferation of the 6 myeloma cell lines that were sensitive to HB-EGF (Figure 6C). This inhibitor had no toxic effect on the growth induced by high IL-6 concentration of XG-6 cells, that failed to express the HB-EGF gene (Figure 6D). In the 2 cell lines expressing the HB-EGF gene, XG-1 and XG-7, PD169540 inhibitor partially blocked the proliferation induced by high IL-6 concentration, in agreement with our previous results obtained with anti–HB-EGF antibodies or CRM197 mutated diphtheria toxin (Figure 6D).4,5 These data demonstrate again that autocrine HB-EGF produced by some myeloma cell lines contributes to their growth induced by recombinant IL-6. As expected, the ErbB inhibitor blocked HB-EGF–induced AKT phosphorylation in XG-7 cells (Figure 6E). Its specificity is shown by its lack of effect on IGF-1–induced Akt phosphorylation (Figure 6E). Interestingly, it also inhibited IL-6–induced AKT phosphorylation. This observation is in agreement with the inhibition of IL-6–dependent proliferation of this HB-EGF–producing cell line by ErbB inhibitor (Figure 6D) and indicates a cooperation between gp130 and ErbB transduction pathways.
The myeloma cell growth factor activity of HB-EGF is inhibited by a pan-ErbB kinase inhibitor. (A) A431 cells were stimulated with HB-EGF, with or whithout PD169540, for 15 minutes prior to lysis and immunoprecipitation of ErbB1. Tyrosine phosphorylation of ErbB1 was assayed using an antiphosphotyrosine antibody (4G10; Upstate Biotechnology) and ErbB1 was quantified with an anti-ErB1 antibody (clone LA-22). (B) Myeloma cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6 or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, without or with 1 μM of the PD169540 ErbB inhibitor. Cells were cultured for 3 days and stained with FITC–annexin-V to determine the percentage of apoptotic cells. (C) Myeloma cell lines were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with (▪) the PD169540 ErbB inhibitor (1 μM). Cells were cultured for 5 to 7 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05). (D) XG-1, XG-6, and XG-7 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with a high concentration of IL-6 (500 pg/mL), without (□) or with (▪) the PD169540 ErbB inhibitor (1 μM). Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained without inhibitor, using a Student t test (P ≤ .05). (E) XG-7 myeloma cells were starved overnight in culture medium without serum or cytokines with or without the PD169540 ErbB inhibitor. Cells were then stimulated with either no cytokine (Co), or IL-6 (20 ng/mL), or HB-EGF (5 μg/mL), or IGF-1 (1 μg/mL) for 15 minutes at 37°C. Lysates from unstimulated (Co) or stimulated myeloma cells were immunoblotted with anti–phospho-specific AKT antibody (top panel) and reprobed with anti-AKT antibody (bottom panel).
The myeloma cell growth factor activity of HB-EGF is inhibited by a pan-ErbB kinase inhibitor. (A) A431 cells were stimulated with HB-EGF, with or whithout PD169540, for 15 minutes prior to lysis and immunoprecipitation of ErbB1. Tyrosine phosphorylation of ErbB1 was assayed using an antiphosphotyrosine antibody (4G10; Upstate Biotechnology) and ErbB1 was quantified with an anti-ErB1 antibody (clone LA-22). (B) Myeloma cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with either 5 pg/mL IL-6 or 5 pg/mL IL-6 and 1 μg/mL HB-EGF, without or with 1 μM of the PD169540 ErbB inhibitor. Cells were cultured for 3 days and stained with FITC–annexin-V to determine the percentage of apoptotic cells. (C) Myeloma cell lines were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with (▪) the PD169540 ErbB inhibitor (1 μM). Cells were cultured for 5 to 7 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05). (D) XG-1, XG-6, and XG-7 cells were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with a high concentration of IL-6 (500 pg/mL), without (□) or with (▪) the PD169540 ErbB inhibitor (1 μM). Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained without inhibitor, using a Student t test (P ≤ .05). (E) XG-7 myeloma cells were starved overnight in culture medium without serum or cytokines with or without the PD169540 ErbB inhibitor. Cells were then stimulated with either no cytokine (Co), or IL-6 (20 ng/mL), or HB-EGF (5 μg/mL), or IGF-1 (1 μg/mL) for 15 minutes at 37°C. Lysates from unstimulated (Co) or stimulated myeloma cells were immunoblotted with anti–phospho-specific AKT antibody (top panel) and reprobed with anti-AKT antibody (bottom panel).
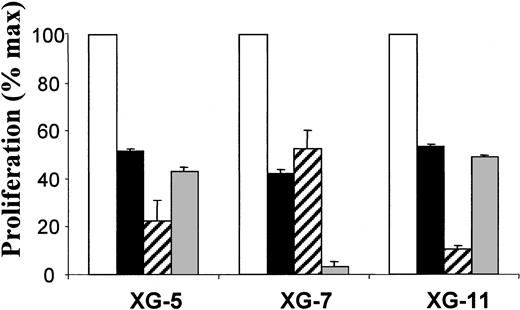
We then tested the other 2 inhibitors known to block ErbB1 tyrosine kinase activation, AG1478 and PD153035, on the 3 ErbB1-positive myeloma cell lines, XG-5, XG-7, and XG-11. These 2 inhibitors also blocked the HB-EGF–induced proliferation of the 3 ErbB1 myeloma cell lines in a manner similar to PD169540 (Figure 7). As all myeloma cell lines express the ErbB2 gene and as ErbB2 can participate in the HB-EGF–induced signaling,36 we also tested the Herceptin anti-ErB2 antibody.37 We found that this antibody did not affect HB-EGF–induced myeloma cell proliferation (results not shown).
The myeloma cell growth factor activity of HB-EGF is inhibited by 2 ErbB1-specific inhibitors.ErbB1–positive myeloma cell lines (XG-5, XG-7, and XG-11) were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with PD169540 (1 μM; ▪), AG1478 (1 μM; ▨), and PD153035 (1 μM; ▦). Cells were cultured for 6 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05).
The myeloma cell growth factor activity of HB-EGF is inhibited by 2 ErbB1-specific inhibitors.ErbB1–positive myeloma cell lines (XG-5, XG-7, and XG-11) were IL-6 starved for 3 hours and cultured in RPMI 1640 culture medium and 5% FCS with 5 pg/mL IL-6 and 1 μg/mL HB-EGF without (□) or with PD169540 (1 μM; ▪), AG1478 (1 μM; ▨), and PD153035 (1 μM; ▦). Cells were cultured for 6 days and pulsed with tritiated thymidine at the end of the culture. Mean tritiated thymidine incorporations were determined in sixplicate culture wells and results are expressed as percentages ± SDs of the mean proliferation without ErbB inhibitor. *Indicates that the mean value is statistically significantly different from that obtained with 5 pg/mL IL-6 and HB-EGF without inhibitor, using a Student t test (P ≤ .05).
The pan-ErbB kinase inhibitor potentiates dexamethasone-induced or anti–IL-6 MoAb-induced apoptosis of patient myeloma cells
We first looked for the expression of ErbB receptors in primary purified myeloma cells from 5 patients with intramedullary myeloma and 4 patients with PCL. As indicated in Figure 8A, 7 of 9 purified primary myeloma cells expressed ErbB4. 8 of 9 expressed ErbB1 and ErbB2, and 6 expressed ErbB3. Thus, 9 of 9 primary myeloma cells expressed either ErbB1 and/or ErbB4 and therefore might be sensitive to HB-EGF. Purified myeloma cells from 8 of 9 patients did not express the HB-EGF gene but the bone marrow mononuclear cells of 8 of 8 patients with MM expressed the HB-EGF gene (Figure 8B). Bone marrow CD14 monocytes were purified from 3 patients and bone marrow stromal cell lines were derived from 5 patients. It is noteworthy that HB-EGF expression was found in 3 of 3 purified bone marrow CD14 cells as well as in 5 of 5 stromal cell lines (Figure 8C).
Expression of the ErbB receptors and HB-EGF in purified primary myeloma cells and in the tumor environment. (A) ErbB1, ErbB2, ErbB3, ErbB4, and HB-EGF expression was assayed by RT-PCR on primary myeloma cells. These cells were purified (> 95%) from 9 patients (5 with intramedullar myeloma and 4 with PCL). The A431 epidermoid carcinoma cell line was used as a control. (B) HB-EGF gene expression was analyzed by RT-PCR in unselected mononuclear cells from 8 patients with MM. (C) HB-EGF gene expression was analyzed by RT-PCR in CD14 purified monocytes (> 98%) from 3 patients and in stromal cells derived from 5 patients. Amplification of β actin shows the equivalence of the cDNA loading and amplification. Results are of one experiment representative of 3.
Expression of the ErbB receptors and HB-EGF in purified primary myeloma cells and in the tumor environment. (A) ErbB1, ErbB2, ErbB3, ErbB4, and HB-EGF expression was assayed by RT-PCR on primary myeloma cells. These cells were purified (> 95%) from 9 patients (5 with intramedullar myeloma and 4 with PCL). The A431 epidermoid carcinoma cell line was used as a control. (B) HB-EGF gene expression was analyzed by RT-PCR in unselected mononuclear cells from 8 patients with MM. (C) HB-EGF gene expression was analyzed by RT-PCR in CD14 purified monocytes (> 98%) from 3 patients and in stromal cells derived from 5 patients. Amplification of β actin shows the equivalence of the cDNA loading and amplification. Results are of one experiment representative of 3.
We then investigated the effect of the pan-ErbB inhibitor on the survival of primary myeloma cells. Detailed results obtained with 8 patients are shown in Table 1. In 6 of 8 patients, the B-E8 anti–IL-6 MoAb or the pan-ErbB kinase inhibitor significantly reduced the number of viable myeloma cells (respectively by 45% and 55%, P ≤ .05; Table 1 and Figure 9A). The effect of DEX was less marked and not significant (P = .07). Of major interest, the pan-ErbB inhibitor dramatically increased both anti–IL-6 MoAb-induced or DEX-induced apoptosis. We observed a 76% reduction of the number of viable myeloma cells with anti–IL-6 MoAb and the ErbB inhibitor (P = .0028) and a 84% reduction with DEX and the ErbB inhibitor (P = .0028) (Figure 9A). In 2 patients, the 3 compounds had no effect on myeloma cell survival, either used alone or in combination (Table 1). Interestingly, the pan-ErbB inhibitor had no effect on the survival of other bone marrow nonmyeloma cells (Figure 9B). We then looked for the effect of the pan-ErbB kinase inhibitor on the growth of hematopoietic precursors present in the bone marrow of 5 patients with MM using a methylcellulose semisolid assay (Figure 10A). None of the 3, pan-ErbB kinase inhibitor, DEX, or anti–IL-6 MoAb, significantly affected it, either used alone or in combination (P > .5).
Effect of the pan-ErbB inhibitor on the survival of primary myeloma cells
. | Control . | Anti-IL-6 MoAb . | DEX . | ErbB inhibitor . | Anti-IL-6 MoAb + ErbB inhibitor . | DEX + ErbB inhibitor . |
|---|---|---|---|---|---|---|
| Myeloma cell count/mL | ||||||
| Responder patients | ||||||
| Patient 1 | 30 338 | 22 880 | 40 763 | 26 460 | 10 075 | 17 193 |
| Patient 2 | 78 990 | 45 090 | 22 640 | 61 938 | 23 088 | 5 750 |
| Patient 3 | 30 653 | 29 952 | 13 486 | 17 790 | 17 460 | 9 984 |
| Patient 4 | 14 280 | 7 560 | 6 800 | 6 820 | 6 528 | 6 125 |
| Patient 5 | 162 316 | 121 856 | 126 990 | 42 420 | 13 900 | 14 820 |
| Patient 6 | 68 544 | 24 795 | 51 696 | 2 415 | 2 080 | 1 260 |
| Median value | 49 599 | 27 374 | 31 702 | 22 125 | 11 988 | 8 055 |
| Nonresponder patients | ||||||
| Patient 7 | 3 824 | 3 043 | 3 477 | 3 040 | 3 056 | 3 690 |
| Patient 8 | 24 922 | 20 812 | 24 552 | 19 560 | 18 260 | 16 629 |
| Nonmyeloma cell count/mL | ||||||
| Responder patients | ||||||
| Patient 1 | 344 663 | 302 120 | 334 238 | 323 540 | 314 925 | 307 808 |
| Patient 2 | 221 010 | 254 910 | 257 360 | 248 062 | 216 912 | 244 250 |
| Patient 3 | 259 347 | 230 048 | 206 514 | 282 210 | 282 540 | 230 016 |
| Patient 4 | 325 720 | 292 440 | 293 200 | 303 180 | 233 472 | 243 875 |
| Patient 5 | 177 684 | 218 144 | 213 010 | 167 580 | 186 100 | 185 180 |
| Patient 6 | 271 456 | 265 205 | 188 304 | 227 585 | 257 920 | 198 740 |
| Median value | 265 402 | 260 058 | 235 185 | 265 136 | 245 696 | 236 946 |
| Nonresponder patients | ||||||
| Patient 7 | 156 176 | 166 957 | 186 523 | 196 960 | 156 944 | 146 310 |
| Patient 8 | 315 078 | 199 188 | 285 448 | 220 440 | 201 740 | 213 371 |
. | Control . | Anti-IL-6 MoAb . | DEX . | ErbB inhibitor . | Anti-IL-6 MoAb + ErbB inhibitor . | DEX + ErbB inhibitor . |
|---|---|---|---|---|---|---|
| Myeloma cell count/mL | ||||||
| Responder patients | ||||||
| Patient 1 | 30 338 | 22 880 | 40 763 | 26 460 | 10 075 | 17 193 |
| Patient 2 | 78 990 | 45 090 | 22 640 | 61 938 | 23 088 | 5 750 |
| Patient 3 | 30 653 | 29 952 | 13 486 | 17 790 | 17 460 | 9 984 |
| Patient 4 | 14 280 | 7 560 | 6 800 | 6 820 | 6 528 | 6 125 |
| Patient 5 | 162 316 | 121 856 | 126 990 | 42 420 | 13 900 | 14 820 |
| Patient 6 | 68 544 | 24 795 | 51 696 | 2 415 | 2 080 | 1 260 |
| Median value | 49 599 | 27 374 | 31 702 | 22 125 | 11 988 | 8 055 |
| Nonresponder patients | ||||||
| Patient 7 | 3 824 | 3 043 | 3 477 | 3 040 | 3 056 | 3 690 |
| Patient 8 | 24 922 | 20 812 | 24 552 | 19 560 | 18 260 | 16 629 |
| Nonmyeloma cell count/mL | ||||||
| Responder patients | ||||||
| Patient 1 | 344 663 | 302 120 | 334 238 | 323 540 | 314 925 | 307 808 |
| Patient 2 | 221 010 | 254 910 | 257 360 | 248 062 | 216 912 | 244 250 |
| Patient 3 | 259 347 | 230 048 | 206 514 | 282 210 | 282 540 | 230 016 |
| Patient 4 | 325 720 | 292 440 | 293 200 | 303 180 | 233 472 | 243 875 |
| Patient 5 | 177 684 | 218 144 | 213 010 | 167 580 | 186 100 | 185 180 |
| Patient 6 | 271 456 | 265 205 | 188 304 | 227 585 | 257 920 | 198 740 |
| Median value | 265 402 | 260 058 | 235 185 | 265 136 | 245 696 | 236 946 |
| Nonresponder patients | ||||||
| Patient 7 | 156 176 | 166 957 | 186 523 | 196 960 | 156 944 | 146 310 |
| Patient 8 | 315 078 | 199 188 | 285 448 | 220 440 | 201 740 | 213 371 |
Mononuclear cells from 8 patients with MM were cultured for 5 days in RPMI 1640 medium, 5% FCS, 1 ng/mL IL-6, with or without the PD169540 ErbB inhibitor (1 μM), B-E8 anti-IL-6 MoAb (10 μg/mL), and DEX (10-6 M). At day 5 of culture, the viability and cell counts were assayed and the percentage of CD138* viable myeloma cells was determined by flow cytometry. Results are expressed as number of myeloma and nonmyeloma cells per milliliter. The median values were calculated from the 6 responder patients. Two patients did not significantly respond to the 3 compounds, either used alone or in combination
The PD169540 ErbB inhibitor potentiates dexamethasone and anti–IL-6–induced apoptosis of primary myeloma cells. Mononuclear cells from 8 patients with MM were cultured at 5 × 105 cells/mL in RPMI 1640 medium and 5% FCS with either the PD169540 ErbB inhibitor (1 μM) or anti–IL-6 MoAb (B-E8; 10 μg/mL) or dexamethasone (DEX, 10–6 M), alone or in combination, for 5 days. As endogenous production of IL-6 is highly variable in short-term culture of patients' bone marrow, 1 ng/mL recombinant IL-6 was added to eliminate this source of variability. In each culture group, viability and cell counts were assayed and myeloma cells were stained with an anti-CD138–PE antibody. Results are median values of the numbers of myeloma (A) or nonmyeloma cells (B), compared with that of the control group, which assigned the value of 100%. Only the 6 responder patients listed in Table 1 are represented there. *Indicates a statistically significant difference of the median value compared with that of the control group using a Wilcoxon test for pairs (P ≤ .05). **Indicates a statistically significant difference of the median value compared with that obtained with the anti–IL-6 MoAb or the DEX groups using a Wilcoxon test for pairs (P ≤ .05).
The PD169540 ErbB inhibitor potentiates dexamethasone and anti–IL-6–induced apoptosis of primary myeloma cells. Mononuclear cells from 8 patients with MM were cultured at 5 × 105 cells/mL in RPMI 1640 medium and 5% FCS with either the PD169540 ErbB inhibitor (1 μM) or anti–IL-6 MoAb (B-E8; 10 μg/mL) or dexamethasone (DEX, 10–6 M), alone or in combination, for 5 days. As endogenous production of IL-6 is highly variable in short-term culture of patients' bone marrow, 1 ng/mL recombinant IL-6 was added to eliminate this source of variability. In each culture group, viability and cell counts were assayed and myeloma cells were stained with an anti-CD138–PE antibody. Results are median values of the numbers of myeloma (A) or nonmyeloma cells (B), compared with that of the control group, which assigned the value of 100%. Only the 6 responder patients listed in Table 1 are represented there. *Indicates a statistically significant difference of the median value compared with that of the control group using a Wilcoxon test for pairs (P ≤ .05). **Indicates a statistically significant difference of the median value compared with that obtained with the anti–IL-6 MoAb or the DEX groups using a Wilcoxon test for pairs (P ≤ .05).
The PD169540 ErbB inhibitor has no effect on the growth of the hematopoietic progenitors or on the adhesion of MM cells to stromal cells. (A) Bone marrow mononuclear cells from 5 patients with MM were grown in a semisolid culture medium with hematopoietic cytokines for 14 days. Cells were treated with either the PD169540 pan-ErbB inhibitor (1μM) or anti–IL-6 MoAb (B-E8; 10 μg/mL) or 10–6 M dexamethasone (DEX), alone or in combination. The number of granulocyte-macrophage colonies was counted on day 14 of incubation. Data are means ± SD of the number of colonies determined on 2 different culture plates for each condition. (B) XG-11 cells were cultured with either BMSC-coated (▪) or noncoated culture plates (□), without or with 1μM PD169540 for 24 hours. Nonadherent cells were counted and adherent cells were trypsinized and counted. In the 2 cell populations, GFP+ stromal cells were quantified with FACS analysis. Results are expressed as percentage of bound plasma cells ± SDs. The percentage was calculated as follows: percentage bound cells = ([input cells] – [nonadherent cells]/[input cells]) × 100. Results are of one experiment representative of 3.
The PD169540 ErbB inhibitor has no effect on the growth of the hematopoietic progenitors or on the adhesion of MM cells to stromal cells. (A) Bone marrow mononuclear cells from 5 patients with MM were grown in a semisolid culture medium with hematopoietic cytokines for 14 days. Cells were treated with either the PD169540 pan-ErbB inhibitor (1μM) or anti–IL-6 MoAb (B-E8; 10 μg/mL) or 10–6 M dexamethasone (DEX), alone or in combination. The number of granulocyte-macrophage colonies was counted on day 14 of incubation. Data are means ± SD of the number of colonies determined on 2 different culture plates for each condition. (B) XG-11 cells were cultured with either BMSC-coated (▪) or noncoated culture plates (□), without or with 1μM PD169540 for 24 hours. Nonadherent cells were counted and adherent cells were trypsinized and counted. In the 2 cell populations, GFP+ stromal cells were quantified with FACS analysis. Results are expressed as percentage of bound plasma cells ± SDs. The percentage was calculated as follows: percentage bound cells = ([input cells] – [nonadherent cells]/[input cells]) × 100. Results are of one experiment representative of 3.
Effect of HB-EGF inhibitor on adhesion of myeloma cells to BMSCs and IL-6 production
As HB-EGF is expressed by MM patients' stromal cells and can be involved in juxtacrine intercellular communication,38 we investigated whether the PD169540 could affect the adhesion of myeloma cell to BMSCs. About 20% myeloma cells adhered to BMSCs and pretreatment of both myeloma cells and BMSCs with the pan-ErbB inhibitor did not affect myeloma cell adhesion to stromal cells (Figure 10B). Given that binding of myeloma cells to BMSCs had been proven important to promote IL-6 production by stromal cells and myeloma cell survival,31,39 we next investigated whether the PD169540 could affect the secretion of IL-6 by BMSCs cocultured with myeloma cells. IL-6 secretion was 889 pg/mL ± 17 pg/mL without ErbB inhibitor and 810 pg/mL ± 43 pg/mL with ErbB inhibitor. No IL-6 was detected in the supernatant of myeloma cells (results not shown). Therefore, the pan-ErbB inhibitor did not significantly affect the IL-6 production by stromal cells cultured with myeloma cells.
Discussion
As we initially found that the HB-EGF gene was expressed in some myeloma cell lines and could stimulate their growth,4,5 our aim was to investigate whether this observation was a common feature in multiple myeloma and whether ErbB inhibitors could be potentially useful in treating this disease.
First, we show here that HB-EGF expression is not a salient feature of primary myeloma cells. There is no HB-EGF gene expression in the purified myeloma cells of 8 of 9 patients with MM. HB-EGF expression was found in the myeloma cells of only one patient with PCL. An autocrine production of HB-EGF by myeloma cells could eventually make it possible to obtain a myeloma cell line in vitro, explaining why 2 myeloma cell lines of 9 express the HB-EGF gene. Interestingly, the bone marrow tumor environment of all 8 patients with MM expressed the HB-EGF gene. Monocytes were purified from 3 patients with MM and were found to express the HB-EGF gene. This was expected because stimulated monocytes are known to produce HB-EGF40 and monocytes are highly activated in MM patients' bone marrow, in particular producing high levels of IL-6.41 HB-EGF expression was also found in the 5 stromal cell lines derived from the bone marrow of patients with MM. We could not directly assay for a production of HB-EGF protein because a sensitive HB-EGF ELISA is not commercially available. Thus, these current data emphasize that the bone marrow environment of patients with MM produces HB-EGF, in particular monocytes and stromal cells and that primary myeloma cells rarely express the HB-EGF gene.
Second, we found that myeloma cells from 6 of 9 myeloma cell lines and myeloma cells from 9 of 9 patients expressed the 2 HB-EGF receptor genes, ErbB1 and/or ErbB4. Using RT-PCR, we did not detect ErbB expression in polyclonal plasmablasts generated from normal peripheral blood B cells using the methodology we recently reported.42 In agreement with this study, we found that an ErbB inhibitor did not affect the generation of plasmablasts (results not shown). These results are intriguing and suggest that ErbB is either expressed on bone marrow plasmocytes, unlike plasmablasts, or involved in plasma cell oncogenesis. We are now trying to obtain purified bone marrow plasma cells to investigate this point, but these cells are rare cells (0.25% of bone marrow cells) and for ethical reasons, it is difficult to obtain enough normal bone marrow to purify them. The level of ErbB gene expression on myeloma cell lines was low compared with A431 cells. In particular, ErbB1 protein was not detectable by immunoprecipitation in 2 myeloma cell lines, unlike in A431 cells. ErbB4 was detected in 2 of the 4 cell lines expressing the ErbB4 gene. Despite this low ErbB expression, recombinant HB-EGF stimulated the growth of the 6 ErbB1+ and/or ErbB4+ cell lines, unlike the 3 ErbB1–/ErbB4– cell lines. We found that recombinant HB-EGF activity required a low concentration of IL-6 and was blocked by an antibody to IL-6. Recombinant HB-EGF alone stimulated the proliferation of XG-1 cells but this was due to their previously reported ability to produce a small amount of autocrine IL-6,33 since HB-EGF–induced growth was blocked by an anti–IL-6 MoAb. These data are in agreement with recent data showing the ability of distinct cell surface receptors to cross-communicate. This cooperation between HB-EGF/ErbB and IL-6/gp130 pathways to trigger myeloma cell growth might be explained by the interaction of ErbB transducing elements with gp130, as it was reported previously in epithelial cancer cells. In a prostate carcinoma cell line, Qiu et al first demonstrated receptor crosstalk between the gp130 and ErbB3 via a gp130/ErbB2/ErbB3 complex.11 Badache et al showed that IL-6–induced MAPK/PI3K activity depends on the cooperation with an EGFR autocrine loop in T47D breast cancer cells. In response to IL-6 treatment, the protein phosphatase Shp-2 and the docking molecule Gab1, which are constitutively associated with ErbB1, are recruited to gp130/JAK complexes and subsequently phosphorylated.12 In addition, EGF was shown to enhance oncostatin M (OSM)–induced inhibition of cellular proliferation in the MCF-7 breast carcinoma cell line, where gp130 is constitutively associated with ErbB2 and ErbB3.43 Recently, several examples of receptor crosstalk have been reported in multiple myeloma. IFN-α stimulation can induce tyrosine phosphorylation of gp13044 or ErbB315 in a myeloma cell line. Podar et al showed that gp130 and IGF-1 receptor are colocalized in caveolae, which are cholesterol-rich plasma membrane microdomains.45 These data suggest that these caveolin pits might play an important role in receptor interactions in multiple myeloma, as gp130 IL-6 transducer, IGF1-R, and eventually ErbB receptors and coreceptors might be colocalized in these membrane structures. Thus, the present study shows that HB-EGF is a third major myeloma cell growth factor, as are IL-6 and IGF-1.46 Further studies are necessary to clarify how a low concentration of IL-6 and HB-EGF work together to promote myeloma cell growth.
The 2 known myeloma cell growth factors, IL-6 and IGF-1, activate 3 major transduction pathways in myeloma cells: the JAK/STAT pathway induced by IL-6, the MAPK, and the PI3-K/AKT pathway induced by both IL-6 and IGF-1.29,34,35,46,47 An activation of these 3 pathways in our myeloma cells by IL-6 or IGF-1 was confirmed here. In agreement with the known activation of PI-3K/AKT by the ErbB receptor family in various cells,16 HB-EGF induced AKT phosphorylation in myeloma cells. It did not activate STAT3 or MAPK phosphorylation. MAPK phosphorylation was looked for from 15 minutes to 24 hours after HB-EGF triggering, excluding a delayed activation. This lack of MAPK activation by HB-EGF was unexpected. Indeed, the ras/MAPK pathway is frequently activated by EGF ligands in various cells.16 We will explore whether other signaling pathways known to be activated by HB-EGF in various cells (PCLγ, PKC, Sarc kinase)48 could be involved in HB-EGF–induced myeloma cell growth.
A major point was to determine whether inhibitors of ErbB kinases could promote apoptosis of primary myeloma cells and block the HB-EGF myeloma cell growth activity. As myeloma cells expressed ErbB4 and/or ErbB1, we used the PD169540 inhibitor, which inhibits kinase activity of ErbB1, ErbB2, and ErbB4, without affecting a large panel of other kinases. This inhibitor is close to the CI-1033 investigated in patients with neck, breast, and NSCL cancers.23 This pan-ErbB kinase inhibitor blocked the HB-EGF–induced proliferation of the 6 sensitive cell lines at a 1-μM concentration, which ensures specificity on ErbB members (Dr Leopold, Pfizer Laboratories, personal communication, August 2002). Two other ErbB1 inhibitors (AG1478 and PD153035) also blocked the HB-EGF–induced stimulation of the 3 ErbB1 myeloma cell lines. Despite ErbB2 expression in myeloma cells, we found no inhibition of HB-EGF–induced myeloma cell proliferation by the Herceptin anti-ErbB2 MoAb. As ErbB2 participated in HB-EGF response in various cells,36 this suggests that ErbB2 expression in myeloma cells is too weak to be functional. The PD169540 pan-ErbB inhibitor did not affect the IL-6–induced proliferation of the XG-6 myeloma cells that did not express the HB-EGF gene. It had no toxicity on the 14-day growth of bone marrow hematopoietic progenitors. As HB-EGF is a membrane protein that can be involved in juxtacrine stimulation and is produced by bone marrow stromal cells, we looked for a putative role of PD169540 on the interaction of myeloma cells and BMSCs in accordance with the model reported by Uchimaya et al.31 We found that the ErbB inhibitor had no effect on myeloma cell adhesion to stromal cells or on IL-6 production by stromal cells cocultured with myeloma cells.
It is of major interest that the pan-ErbB kinase inhibitor induced a 55% reduction in the number of viable primary myeloma cells cultured for several days together with their bone marrow environment, which expresses the HB-EGF gene. In association with DEX, it induced an 84% reduction in myeloma cell number, whereas DEX alone induced only a 36% reduction. A similar additive effect was observed with an anti–IL-6 MoAb. Interestingly, this toxicity was specific to myeloma cells and no reduction in the number of nonmyeloma cells was observed. Several ErbB inhibitors are in various stages of clinical development and some are producing significant clinical responses. The compound that is at the most advanced stage of development is IRESSA (ZD1839), which targets EGFR. This compound has shown an acceptable tolerability and promising clinical efficacy in phase 1 trials and is currently in phase 3 trials in advanced NSCL and breast cancers.49 CI-1033 is another clinical-grade irreversible tyrosine kinase inhibitor that is unique because it targets the 3 ErbB members with kinase activity.25 It has potent and long-lasting in vivo activity in a range of tumor models. For example, it has demonstrated antitumor activity against A431 and H125 tumors in vivo.50 Data from 3 phase 1 trials in patients with head and neck, breast, and NSCL cancers showed that CI-1033 is generally well tolerated.51 A phase 2 study in patients with advanced ovarian cancer and a randomized phase 2 trial with patients with NSCL after a first or second line of chemotherapy are being done.52
In conclusion, our data are very encouraging and provide a framework for clinical evaluation of ErbB inhibitors in patients with MM, alone and coupled with dexamethasone or anti–IL-6 MoAb, now available for clinical use.53 A first step will be to investigate in preclinical studies whether ErbB inhibitors can block the growth of myeloma cell lines and primary myeloma cells with SCID mice models of human multiple myeloma.27,54 These current data are in agreement with the recent development of novel biologically based treatment approaches that target mechanisms whereby MM cells grow and survive in their bone marrow environment. The most relevant example is the proteasome inhibitor PS341, which was identified as a promising new therapeutic agent in multiple myeloma.55
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-05-1510.
Supported by grants from the Ligue Nationale Contre le Cancer (équipe labellisée), Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Leopold (Pfizer Global Research and Development, AnnArbor, MI) for providing us with the PD169540 pan-ErbB inhibitor.





![Figure 10. The PD169540 ErbB inhibitor has no effect on the growth of the hematopoietic progenitors or on the adhesion of MM cells to stromal cells. (A) Bone marrow mononuclear cells from 5 patients with MM were grown in a semisolid culture medium with hematopoietic cytokines for 14 days. Cells were treated with either the PD169540 pan-ErbB inhibitor (1μM) or anti–IL-6 MoAb (B-E8; 10 μg/mL) or 10–6 M dexamethasone (DEX), alone or in combination. The number of granulocyte-macrophage colonies was counted on day 14 of incubation. Data are means ± SD of the number of colonies determined on 2 different culture plates for each condition. (B) XG-11 cells were cultured with either BMSC-coated (▪) or noncoated culture plates (□), without or with 1μM PD169540 for 24 hours. Nonadherent cells were counted and adherent cells were trypsinized and counted. In the 2 cell populations, GFP+ stromal cells were quantified with FACS analysis. Results are expressed as percentage of bound plasma cells ± SDs. The percentage was calculated as follows: percentage bound cells = ([input cells] – [nonadherent cells]/[input cells]) × 100. Results are of one experiment representative of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2003-05-1510/6/m_zh80050457790010.jpeg?Expires=1764972831&Signature=ecjwc7KWvIN7K91Y9HY~5WB0muU0SiHK3eBpWcpky5UE0Sd0FFaBgW2CvEoCLMd3t2IipFP05-jWCIXkEbqe7Rk-kNLshszO2Pr9Xnyb8J4M93IhiPTM1c-y8kMVZJ2FCZjVZkZOLcy-z02HQCyAqFipbGm9ahYGK~NTTDSwZ4141nrYoMFz7fN7X2krD~xEBT5c~5YlFBPr1HEYwhr41z1fB8E-EPx03c5vCZg~0e9Mqx3V3tYHDJuZj2S0Y4ee6Y~Wrdx~UaWLhPBONlMYbwNoyELkRkajCVzgas3UJzM8kz0TI3cy2Y7iRzDVn-xa0x9pdt5fdxw1POia6JtZ0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal